|
Biodiversity Data Journal :
Research Article
|
|
Corresponding author:
Academic editor: Vladimir Blagoderov
Received: 15 Aug 2016 | Accepted: 28 Nov 2016 | Published: 09 Dec 2016
© 2016 Hironori Iwai, Daiki Horikawa, Kazuharu Arakawa, Masaru Tomita, Takashi Komatsu, Munetoshi Maruyama
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Iwai H, Horikawa D, Arakawa K, Tomita M, Komatsu T (2016) Rearing and observation of immature stages of the hoverfly Microdon katsurai(Diptera, Syrphidae). Biodiversity Data Journal 4: e10185. https://doi.org/10.3897/BDJ.4.e10185
|
|
Abstract
Background
The hoverfly Microdon (Chymophila) katsurai Maruyama et Hironaga 2004 was speculated to be a myrmecophilous species associated with the ant Polyrhachis lamellidens based on observations of adults near the ant nest. However, there have been no reports regarding the observation of immature stages of this species in association with P. lamellidens.
New information
For the first time, we found three M. katsurai larvae inside a P. lamellidens nest and conducted rearing experiments on the larval M. katsurai. P. lamellidens workers did not show any inspection or attack behavior against the M. katsurai larvae under rearing conditions, suggesting that M. katsurai larvae can survive inside a P. lamellidens nest. Although no predatory behavior by the M. katsurai larvae was observed, all the M. katsurai larvae pupated and emerged in a rearing environment. The dorsal surface of the larval M. katsurai has a distinct pale green color with a uniform reticular structure. The puparium of M. katsurai shows several morphological features that are characteristic of the subgenus Chymophila. We conclude that M. katsurai is likely a myrmecophilous species that utilizes P. lamellidens as a specific host and that classification of M. katsurai based on puparium morphology is concordant with that based on adult morphology.
Keywords
Hover fly, Microdontinae, host record, Polyrhachis lamellidens, myrmecophily, puparium
Introduction
It is well known that some species of Microdontinae (Diptera, Syrphidae) spend their larval and pupal periods in ant nests. Several papers reported that larvae of some microdontine species prey on ant eggs, larvae, and pupae (
The microdontine species Microdon katsurai was originally described from Japan (
In the present study, to elucidate whether M. katsurai utilizes P. lamellidens, we tried to collect larvae of M. katsurai from a P. lamellidens nest and rear them until they developed into adults.
Materials and Methods
Collecting samples
We conducted field research on 5 March, 6 March, 15 March, and 16 March 2015 in Hosaka Natural Park (
Three larvae and four puparia of a microdontine were found in the P. lamellidens nest (Figs
Rearing animals
Due to the difficulty of identifying microdontine species by using a larva or pupa, we reared the larvae collected until adults emerged under laboratory conditions. After collecting these larvae, they were reared in the transparent plastic container used for collecting the larval samples for the first 2 d. Thereafter, the larvae were numbered 1 to 3 and reared individually in a new plastic container (130 mm in diameter and 59 mm in height), and wood flakes taken from the P. lamellidens nest were placed on the bottom. Small holes were made in the lid of each container for ventilation. At 2 d after the rearing experiments began on 15 March 2015, several P. lamellidens larvae brought from their nest were introduced to each container as a potential food source for the microdontine larvae. Additionally, 80 adult workers were introduced to each container 5 d after rearing started. Furthermore, maple syrup diluted in water at a ratio of 1:1 and placed on a small piece of aluminium foil was supplied in each rearing container every 3 d.
Each rearing container was kept at approximately 25ºC in a darkroom. Observations of the animals were conducted at intervals of 1-3 d until they became adults. During this period, interaction behaviors between the microdontine larvae and the ants were recorded as well. Additionally, to better observe the ventral morphology of the larva, we detached one larva (No. 2) from a piece of bark flake and placed it to the side wall of the transparent container. The ventral surface of the larva is sticky enough to attach onto the container wall. P. lamellidens workers were removed before the microdontine adults emerged to avoid any attack behavior of ant workers against adult hover flies (
Morphological studies
We conducted observations on the morphology of the microdontines at the larval and adult stages. The body color and morphology of the dorsal surface were recorded in individual larvae, prepupae (this stage lies between the moment when the cuticle of the larva hardens and the appearance of the anterior spiracles (
Results
Development of a microdontine species under rearing conditions
The microdontine larvae did not show any predatory behavior against P. lamellidens larvae throughout the rearing experiments. Neither was any attack behavior of P. lamellidens workers against the microdontine larvae and pupae observed. One of the larvae (No. 2) started pupation 24 d after collection in the field, and the other two (No. 1 and 3) began pupation 16 d after that (Table
Days observed for each larva to reach pupal and adult stages after collection of the specimens from their habitat.
| Individuals | Pupation | Appearance of anterior spiracle | Emergence of adult |
| No. 1 | 16 | 24 | 41 |
| No. 2 | 24 | 27 | 48 |
| No. 3 | 16 | 24 | 41 |
Individuals No. 1 and 3 emerged as adults 25 d after the beginning of pupation (Table
We set a ladder-like scaffold made of a egg paper tray inside a corner of the container for individual No. 2 to mount on 17 d after pupation. In addition, the lid was removed from the container since we noticed that there was condensed water due to the high relative humidity on the inner surface of the container. Then, individual No. 2 emerged with fully expanded wings. The adult of individual No. 2 was transferred into a small glass vial and killed by subjecting it to a temperature of -20ºC in a freezer 6 d after emergence.
Morphology of larval, prepupal, pupal, puparial, and adult Microdontinae
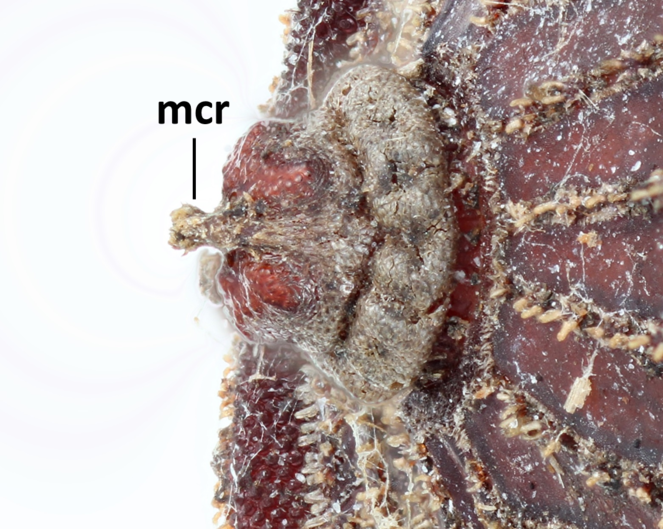
Morphological observations of the microdontine species were conducted at different developmental stages. All the microdontine larvae had a dark brown reticulated structure with a pale green color on the overall dorsal surface (Figs
Three pieces were made from the puparium shown on the left side in Fig.
Concerning the adult, observation was conducted on the specimen casted by individual No. 2 only, which successfully emerged (Fig.
Discussion
Based on observations of the appearance and ovipositional behavior of adult M. katsurai near P. lamellidens nests (
P. lamellidens workers were not observed to inspect or attack the M. katsurai larvae in the rearing environment, suggesting that M. katsurai larvae can survive inside a P. lamellidens nest.
Having been collected in March, the stage of all the larvae was likely the last instar because they did not molt before becoming pupae. Adult M. katsurai appear from the end of May until the beginning of July (
The color of larvae of microdontine species is white or brown (
Based on morphological studies of adult specimens, M. katsurai is classified into the subgenus Chymophila (
The two individuals that failed to emerge, No. 1 and No. 3, could not be identified using adults, but they are considered to be M. katsurai based on the morphology of their puparia.
In Japan, thus far, M. katsurai has been recorded in the Nagano, Ibaraki, Tochigi, Mie, Osaka, Hyogo, Yamaguchi, Kagawa and Kagoshima prefectures (
M. katsurai is a rare species that is designated as Vulnerable (VU) in Japan (
Acknowledgements
The authors thank Mr. T. Isoda from Tokyo University of Foreign Studies and Mr. Y. Takeshita from Nihon University for helping to collect samples. We also thank Dr. N. Kono from Keio University for providing useful advice on this study. This research was supported in part by research funds from the Yamagata Prefectural Government and Tsuruoka City, Japan.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- Life cycle and behavior of Microdon cothurnatus in Washington (Diptera: Syrphidae).Journal of the Kansas Entomological Society46 (3):327‑338.
- Biology and behavior of Microdon piperi in the Pacific Northwest (Diptera: Syrphidae).Journal of the Kansas Entomological Society61 (4):441‑452.
- Key to and descriptions of the third instar larvae of some species of Syrphidae (Diptera) occurring in Britain.Transactions of the Royal Entomological Society of London112 (13):345‑379. https://doi.org/10.1111/j.1365-2311.1960.tb00491.x
- Biology of Microdon fuscipennis (Diptera: Syrphidae) with interpretations of the reproductive strategies of Microdon species found north of Mexico.Proceedings Entomological Society of Washington83 (4):716‑724.
- Cocoon mimicry and predation by myrmecophilous Diptera (Diptera: Syrphidae).The Florida Entomologist68 (4):615‑621. https://doi.org/10.2307/3494863
- Record of Microdon katsurai Maruyama et Hironaga, 2004 from Hyogo pref., Japan.Hana Abu29:48‑49. [InJapanese].
- Larvae and pupae of the genera Microdon and Mixogaster (Diptera, Syrphidae).Transactions of the American Entomological Society81 (1):1‑20.
- Combine ZP software, new version [WWW document]. Release date:2010-6-06. URL: http://www.hadleyweb.pwp.blueyonder.co.uk/
- New knowledge on “Ogon-arinosuabu” from Tochigi prefecture.Hana Abu6:17‑18. [InJapanese].
- Biosynthesis and chemical mimicry of cuticular hydrocarbons from the obligate predator, Microdon albicomatus Novak (Diptera: Syrphidae) and its ant prey, Myrmica incompleta Provancher (Hymenoptera: Formicidae).Journal of the Kansas Entomological Society63 (3):437‑443.
- A record of “Kenran-arinosuabu” from Ibaraki prefecture.Hana Abu29:49. [InJapanese].
- “Ogon-arinosuabu” collected in Nabari city, Mie prefecture.Hana Abu8:62. [InJapanese].
- Ecology of “Ogon-arinosuabu” –research and observation record–.Nature Study44 (1):2‑4. [InJapanese].
- Dipteras collected from Niigata and Nagano prefectures in May and June in 2004.Yosegaki115:1‑9. [InJapanese].
- Microdon katsurai, a new species of myrmecophilous hoverfly (Diptera, Syrphidae) from Japan, associated with Polyrhachis lamellidens (Hymenoptera, Formicidae).Bulletin of the National Science Museum, Tokyo30 (4):173‑179.
- The guests of japanese ants.Tokai University Press,208pp.
- Red data book 2014. –Threatened wildlife of japan– volume 5, Insecta.Gyosei Corporation,509pp. [InJapanese].
- A record of “Kenran-arisuabu” from Yakushima.Gekkan-Mushi526:5‑6. [InJapanese].
- Review of arinosuabu.Hana Abu2:35‑55. [InJapanese].
- An unusual, but not unexpected, evolutionary step taken by syrphid flies: the first record of true primary parasitoidism of ants by Microdontinae.Biological Journal of the Linnean Society111:462‑472. https://doi.org/10.1111/bij.12220
- Review and phylogenetic evaluation of associations between Microdontinae (Diptera: Syrphidae) and ants (Hymenoptera: Formicidae).Psyche: A Journal of Entomology2013:1‑9. https://doi.org/10.1155/2013/538316
- Generic revision and species classification of the Microdontinae (Diptera, Syrphidae).ZooKeys288:1‑213. https://doi.org/10.3897/zookeys.288.4095
- Larval stages of 17 rare and poorly known British hoverflies (Diptera: Syrphidae).Journal of Natural History25 (4):945‑969. https://doi.org/10.1080/00222939100770621
- Colour guide to hoverfly larvae (Diptera, Syrphidae) in Britain and Europe.Dipterists Digest9:1‑155.
- A record of Microdon Katsurai (Diptera, Syrphidae, Microdontinae) from Kagawa Prefecture, Shikoku, Japan.Hana Abu35:27. [InJapanese].
- Natural history and morphology of the hoverfly Pseudomicrodon biluminiferus and its parasitic relationship with ants nesting in bromeliads.Journal of Insect Science14 (38):1‑21. https://doi.org/10.1673/031.014.38
- Three little known hoverflies from Yamaguchi-Pref., Honshu, Japan. (Syrphidae).Hana Abu14:8. [InJapanese].
- Revisionary notes on Nearctic Microdon flies (Diptera: Syrphidae) [Microdon abditus, Microdon abstrusus, Microdon adventitus, morphology, new taxa, North America].Proceedings Entomological Society of Washington83 (4):725‑758.
- Microdon (Diptera: Syrphidae) in nests of Monomorium (Hymenoptera: Formicidae) in Texas.Annals of the Entomological Society of America65 (4):977‑979. https://doi.org/10.1093/aesa/65.4.977
- Studies on myrmecophiles. III. Microdon.Journal of the New York Entomological Society16 (4):202‑213.
- Two extraordinary larval myrmecophiles from Panama.Proceedings of the National Academy of Sciences10 (6):237‑244. https://doi.org/10.1073/pnas.10.6.237