|
Biodiversity Data Journal :
Taxonomic Paper
|
|
Corresponding author:
Academic editor: Pavel Stoev
Received: 17 Sep 2016 | Accepted: 15 Nov 2016 | Published: 17 Nov 2016
© 2016 Xiaosheng Chen, Xiufeng Xie, Shunxiang Ren, Xingmin Wang
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Chen X, Xie X, Ren S, Wang X (2016) Discovery of a New World ladybird beetle Nephaspis indus Gordon, 1996 (Coleoptera: Coccinellidae: Scymnini) on the Island of Taiwan. Biodiversity Data Journal 4: e10537. https://doi.org/10.3897/BDJ.4.e10537
|
|
Abstract
Background
Nephaspis indus Gordon, 1996 was imported into Taiwan from Hawaii in 1990 as a biological control agent for the spiralling whitefly, Aleurodicus dispersus Russell, 1965 (Hemiptera: Aleyrodidae). However, its establishment was not known prior to this study.
New information
Nephaspis indus Gordon, 1996, a natural enemy of Aleurodicus dispersus Russell (Hemiptera: Aleyrodidae) native to the Neotropical region, is recorded as established in Taiwan for the first time. The present paper provides a detailed further description and illustrations of the adult. Diagnostic characters for the genus and species are given and the nomenclature of this species is also discussed.
Keywords
Coccinelloidea, taxonomy, morphology, spiralling whitefly predator, biological control
Introduction
Nephaspis Casey, 1899 (Coleoptera: Coccinellidae: Scymnini) is a New World genus and currently includes 43 species distributed from southern United States to Argentina (
Members of the genus Nephaspis are predators of whiteflies (Hemiptera: Aleyrodidae) (
In this paper, Nephaspis indus Gordon, 1996 is recorded as being established in Taiwan for the first time. The detailed further description and illustrations of the adult are provided. Diagnostic characters for the genus and species are given and the nomenclature of this species is also discussed.
Materials and methods
Specimens examined were collected from the Island of Taiwan and deposited in the Department of Entomology, South China Agricultural University, Guangzhou, China (SCAU). The morphological terms follow
Measurements were taken using a micrometer attached to a SteREO Discovery V20 dissecting stereoscope and are defined as follows: (TW) total width, across both elytra at widest part; (TH) total height, at highest part of elytra in lateral view; (TL) total length, from apical margin of clypeus to apex of elytra; (PL) pronotal length, from the middle of anterior margin to the base of pronotum; (PW) pronotal width at widest part; (EW) elytral width, equal to TW; (EL) elytral length, along suture from base to apex including scutellum; (HW) head width, at widest part including eyes.
Male and female genitalia were dissected, cleared in a 10% solution of NaOH by boiling for several minutes and placed on slides for further study. Photographs of the whole beetles and their genitalia were taken using digital cameras (AxioCamHRc and Coolsnap-Procf& CRI Micro*Color) attached to the microscope. The final plates were laid out with Adobe Photoshop CS 8.0.
Taxon treatments
Nephaspis
Nomenclature
Nephaspis Casey, 1899: 168. Type species: Nephaspis gorhami Casey, 1899, by subsequent designation of
Nephasis:
Type species
Description
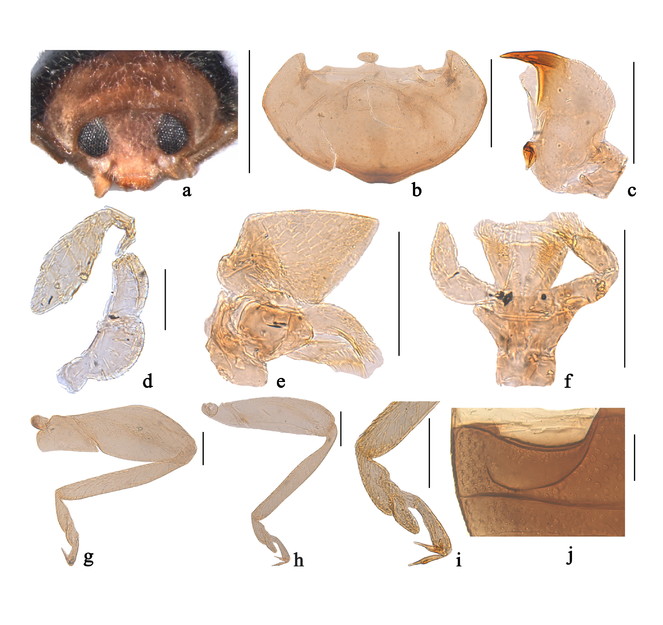
Body elongate oval, moderately convex, with dense pubescence, widest around middle of elytra.
Head with mouthparts directed posteroventrally in repose (Fig.
Pronotum convex, hind margin wider than anterior one (Fig.
Diagnosis
Nephaspis Casey is similar to the Old World genus Clitostethus Weise, 1885 in general appearance and shares the following characters with the latter: body small, length less than 2 mm; antennae composed of 11 antennomeres (Fig.
Distribution
This genus is apparently endemic to Neotropical region with a natural geographic range extending from southern United States (Florida, Louisiana and Texas) and Mexico to Argentina (
Nephaspis indus
Nomenclature
Nephaspis indus Gordon, 1996: 43;
Nephaspis amnicola:
-
scientificName: Nephaspis indus; island:Taiwan; locality:Zhiben, Taidong county; verbatimElevation:140 m; locationRemarks:label transliteration: "Taiwan, Taidong, Zhiben, 2012.08.20, Ren Shunxiang"; [台湾台东知本 140 m, 22°41.63'N 120°59.94'E, 2012.08.20, sweeping, 任顺祥]; verbatimCoordinates:22°41.63'N 120°59.94'E; decimalLatitude:22.6938; decimalLongitude:120.999; georeferenceProtocol:label; samplingProtocol:sweeping; eventDate:2012-8-20; individualCount:8; sex:male; lifeStage:adult; catalogNumber:SCAU (E) 11661; recordedBy:Ren Shun-Xiang; identifiedBy:Xiaosheng Chen; dateIdentified:2016; modified:2016-05-13T10:24:32Z; language:en; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Nephaspis indus; island:Taiwan; locality:Zhiben, Taidong county; verbatimElevation:140 m; locationRemarks:label transliteration: "Taiwan, Taidong, Zhiben, 2012.08.20, Ren Shunxiang"; [台湾台东知本 140 m, 22°41.63'N 120°59.94'E, 2012.08.21, sweeping, 任顺祥]; verbatimCoordinates:22°41.63'N 120°59.94'E; decimalLatitude:22.6938; decimalLongitude:120.999; georeferenceProtocol:label; samplingProtocol:sweeping; eventDate:2012-8-20; individualCount:7; sex:female; lifeStage:adult; catalogNumber:SCAU (E) 11670; recordedBy:Ren Shun-Xiang; identifiedBy:Xiaosheng Chen; dateIdentified:2016; modified:2016-05-13T10:24:32Z; language:en; collectionCode:Insects; basisOfRecord:PreservedSpecimen
Description
TL: 1.23–1.32 mm, TW: 0.87–0.92mm, TH: 0.67–0.69mm, TL/TW: 1.41–1.43, PL/PW: 0.52–0.53, EL/EW: 1.05–1.07, HW/PW: 0.61–0.62, PW/EW: 0.84–0.85.
Body rounded oval, moderately convex, dorsum covered with white pubescence (Fig.
Head with fine frontal punctures, as large as eye facets, 0.5 diameter apart. Eyes densely faceted, interocular distance 0.44 times head width. Pronotal punctures smaller than those on frons, 1.0–1.5 diameters apart. Surface of elytra with punctures larger than those on pronotum, separated by 1.0–2.0 diameters. Prosternal process very short, transversely oval (Fig.
Male genitalia. Penis long, strongly curved at basal 1/2 length (Fig.
Female externally similar to male but with black pronotum (Fig.
Diagnosis
This species is similar to Nephaspis bicolor Gordon, 1982 in general appearance, but can be distinguished from the latter by details of male genitalia, particularly the stout penis guide with a dorsal keel at basal 1/3 length in lateral view (Fig.
Distribution
Taiwan, Hawaii, Trinidad, Honduras.
Notes
There was some confusion about the taxonomy and nomenclature of N. indus. This species was introduced from Honduras, Trinidad and the West Indies into Hawaii as N. amnicola in 1979–1980 where it became effective in biological control of the spiralling whitefly (
Acknowledgements
We are grateful to Dr. Shaukat Ali (SCAU), who helped to check the English text. Our sincere thanks are extended to the editor and two reviewers for their valuable and constructive comments and suggestions on our manuscript. This study was supported by the National Natural Science Foundation of China (31601878) and the Guangdong Natural Science Foundation (2014A030310493), the Science and Technology Program of Guangzhou (201509010023) and the International Cooperation Project of Guangzhou Science and Technology Innovation Committee.
Author contributions
X. Chen conceived the study and wrote the manuscript. X. Xie and X. Wang performed the laboratory work. S. Ren collected the specimens.
References
- Checklist of the coleopterous insects of Mexico, Central America, the West Indies, and South America. Part 3.United States National Museum Bulletin185:343‑550. [InEnglish]. https://doi.org/10.5479/si.03629236.185.3
- A revision of the American Coccinellidae.Journal of the New York Entomological Society7(2):71‑169. [InEnglish].
- A new Carabus and Cychrus, with miscellaneous notes on Coleoptera.The Canadian Entomologist37:160‑164. [InEnglish]. https://doi.org/10.4039/ent37160-5
- Occurrence and biological control of Aleurodicus dispersus.Formosan Entomologist (Special Publication)3:93‑109. [InChinese with English summary].
- Nephaspis maesi nouvelle espèce de Scymnini du Nicaragua (Coleoptera, Coccinellidae).Revue Françaised’Entomologie (Nouvelle Serie)8(4):167‑169. [InFrench].
- A review of the genus Nephaspis Casey and a comparison with the genus Clitostethus Weise (Coleoptera: Coccinellidae).Revista de Agricultura Piracicaba47:145‑154. [InEnglish].
- West Indian Coccinellidae (Coleoptera): some scale predators with keys to genera and species.The Coleopterists Bulletin32(3):205‑218. [InEnglish].
- Two new species of Nephaspis Casey (Coleoptera: Coccinellidae) from Trinidad and Colombia.Proceedings of the Entomological Society of Washington84(2):332‑336. [InEnglish].
- The Coccinellidae (Coleoptera) of America north of Mexico.Journal of the New York Entomological Society93(1):1‑912. [InEnglish].
- Additions to the genus Nephaspis Casey (Coleoptera: Coccinellidae).Acta Zoologica Lilloana39(2):23‑26. [InEnglish].
- South American Coccinellidae (Coleoptera). Part V: A taxonomic revision of the genus Nephaspis Casey.Frustula Entomologica19:1‑50. [InEnglish].
- Coleopterorum Catologus. Pars 118. Coccinellidae. I.W. Junk,Berlin,224pp.
- Efficacy of Nephaspis amnicola and Encarsia ?haitiensis in controlling Aleurodicus dispersus in Hawaii.Proceedings of the Hawaiian Entomological Society24:261‑269. [InEnglish].
- Management of invasive alien species in Thailand. http://www.agnet.org/htmlarea_file/library/20110718163847/eb544.pdf. Accessed on: 2016-7-25.
- LVIII.— Description d'espèces nouvelles de Coccinellidæ.Journal of Natural History Series 104(23):515‑524. https://doi.org/10.1080/00222932908673088
- Australian ladybird beetles (Coleoptera:Coccinellidae): their biology and classification.ABRS,Canberra,286pp. [InEnglish].
- Ślipiński A, Tomaszewska W (2010) Coccinellidae Latreille, 1802. In: Leschen RAB, Beutel RG, Lawrence JF (Eds) Handbook of Zoology, Vol. 2, Coleoptera.Walter de Gruyter GmbH & Co. KG,Berlin/New York,454–472pp. [InEnglish].
- Biological control: Pacific prospects—Supplement 1.Australian Centre for International Agricultural Research12:11‑12. [InEnglish].
- Wen HC (1995) Bionomics and control of spiralling whitefly (Aleurodicus dispersus Russell) in Taiwan.Doctoral dissertation of the Graduate Institute of Plant Pathology and Entomology. National Taiwan University,194pp.
- The Coccinellidae (Coleoptera) of the upper Mississippi basin.Iowa State College Journal of Science27:15‑53. [InEnglish].
- Life history and feeding behaviour of Naphaspis amnicola Wingo.Proceedings of the Hawaiian Entomological Society25:155‑160. [InEnglish].
- Spiralling whitefly and its natural insect enemies.Science Press,Beijing,211pp. [InChinese with English summary].