|
Biodiversity Data Journal :
Research Article
|
|
Corresponding author:
Academic editor: Ben Price
Received: 05 Oct 2016 | Accepted: 09 Nov 2016 | Published: 16 Nov 2016
© 2016 R. DeWalt, Scott Grubbs, Brian Armitage, Richard Baumann, Shawn Clark, Michael Bolton
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
DeWalt R, Grubbs S, Armitage B, Baumann R, Clark S, Bolton M (2016) Atlas of Ohio Aquatic Insects: Volume II, Plecoptera. Biodiversity Data Journal 4: e10723. https://doi.org/10.3897/BDJ.4.e10723
|
|
Abstract
Background
We provide volume II of a distributional atlas of aquatic insects for the eastern USA state of Ohio. This treatment of stoneflies (Plecoptera) is companion to
New information
This work is based on a total of 7797 specimen records gathered from 21 regional museums, agency data, personal collections, and from the literature Table
Specimen source, institutional coden, the number of specimen records for each source, and the total number of specimens recorded. *OEPA number of specimens is a severe underestimate since most of the data inadvertently lacked numbers of individuals. Numbers of OEPA specimens reflect only those specimens loaned to RED by OEPA.
| Institution | Coden | #Records | #Specimens |
| Illinois Natural History Survey, Champaign | INHS | 2072 | 9623 |
| Ohio Environmental Protection Agency, Grove City | OEPA | 1744 | 142* |
| Bean Museum, Brigham Young University, Provo, Utah | BYUC | 1168 | 18863 |
| Literature | 892 | 6517 | |
| Ohio Biological Survey (from DeWalt et al. 2012) | OBS-INHS | 573 | 2690 |
| Ohio State University, Columbus | OSUC | 468 | 668 |
| Crane Hollow Preserve Collection, Athens, Ohio | CHPC | 287 | 830 |
| Western Kentucky University, Bowling Green | WKUC | 170 | 873 |
| Fred Kirchner, Huntington, West Virginia | RFKC | 164 | 857 |
| Cleveland Museum of Natural History, Cleveland, Ohio | CLEV | 67 | 172 |
| Canadian National Collection, Ottawa | CNC | 46 | 252 |
| Ohio Historical Society Collection, Columbus | OHSC | 17 | 17 |
| Field Museum of Natural History, Chicago, Illinois | FMNH | 13 | 40 |
| Michigan State University, East Lansing | MSUC | 10 | 62 |
| Purdue University Ent. Res. Coll., West Lafayette, Indiana | PERC | 7 | 18 |
| Bill P. Stark Collection, Clinton, Mississippi | BPSC | 6 | 81 |
| Iowa State University, Ames | ISUC | 4 | 6 |
| Royal Ontario Museum, Toronto, Canada | ROME | 3 | 15 |
| University of Michigan Museum of Zoology, Ann Arbor | UMMZ | 3 | 3 |
| University of Minnesota, St. Paul | UMSP | 6 | 18 |
| Cincinnati Museum of Natural History, Ohio | CNHM | 2 | 2 |
| Southern Illinois University, Carbondale | SIUC | 1 | 5 |
| Total | 7797 | 41828 |
Stonefly names associated with Ohio since
| All names associated with Ohio | Comment | Current |
| Capniidae | ||
| Allocapnia forbesi Frison | 1 | |
| Allocapnia frisoni Ross & Ricker | 1 | |
| Allocapnia granulata Claassen | 1 | |
| Allocapnia illinoensis Frison | 1 | |
| Allocapnia indianae Ricker | 1 | |
| Allocapnia mystica Frison | 1 | |
| Allocapnia nivicola (Fitch) | 1 | |
| Allocapnia ohioensis Ross & Ricker | 1 | |
| Allocapnia pechumani Ross & Ricker | 1 | |
| Allocapnia pygmaea (Burmeister) | 1 | |
| Allocapnia recta (Claassen) | 1 | |
| Allocapnia rickeri Frison | 1 | |
| Allocapnia smithi Ross & Ricker | 1 | |
| Allocapnia vivipara (Claassen) | 1 | |
| Allocapnia zola Ricker | 1 | |
| Capnia vernalis (Newport) | misidentified P. angulata? | |
| Paracapnia angulata Hanson | 1 | |
| Leuctridae | ||
| Leuctra alexanderi Hanson | 1 | |
| Leuctra duplicata Claassen | 1 | |
| Leuctra ferruginea (Walker) | 1 | |
| Leuctra monticola Hanson | misidentified L. alexanderi? | |
| Leuctra rickeri James | 1 | |
| Leuctra sibleyi Claassen | 1 | |
| Leuctra tenella Provancher | 1 | |
| Leuctra tenuis (Pictet) | 1 | |
| Paraleuctra sara (Claassen) | 1 | |
| Zealeuctra claasseni (Frison) | 1 | |
| Zealeuctra fraxina Ricker & Ross | 1 | |
| Nemouridae | ||
| Amphinemura delosa (Ricker) | 1 | |
| Amphinemura nigritta (Provancher) | 1 | |
| Amphinemura varshava (Ricker) | 1 | |
| Nemoura trispinosa Claassen | 1 | |
| Ostrocerca albidipennis (Walker) | 1 | |
| Ostrocerca truncata (Claassen) | 1 | |
| Prostoia completa (Walker) | 1 | |
| Prostoia similis (Hagen) | 1 | |
| Soyedina vallicularia (Wu) | 1 | |
| Taeniopterygidae | ||
| Strophopteryx fasciata (Burmeister) | 1 | |
| Taenionema atlanticum Ricker & Ross | not present | |
| Taeniopteryx burksi Ricker & Ross | 1 | |
| Taeniopteryx lita Frison | 1 | |
| Taeniopteryx maura (Pictet) | 1 | |
| Taeniopteryx metequi Ricker & Ross | 1 | |
| Taeniopteryx nivalis Fitch | 1 | |
| Taeniopteryx parvula Banks | 1 | |
| Peltoperlidae | ||
| Peltoperla arcuata Needham | 1 | |
| Pteronarcyidae | ||
| Pteronarcys cf. biloba Newman | nymphs only | 1 |
| Pteronarcys dorsata (Say) | confirmed | 1 |
| Pteronarcys pictetii Hagen | not confirmed | |
| Chloroperlidae | ||
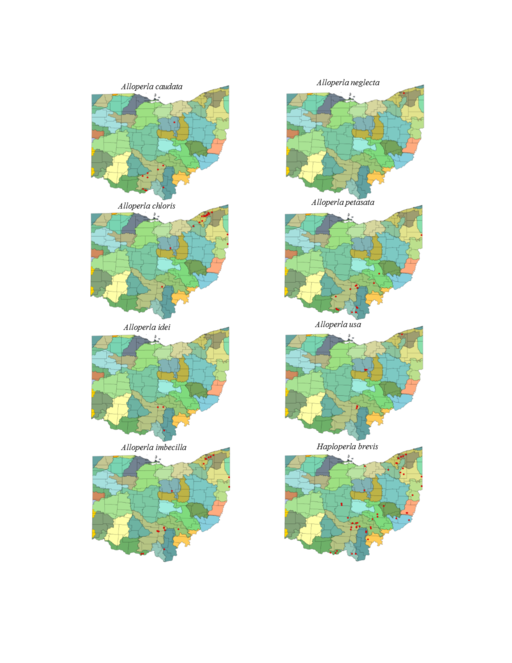
| Alloperla caudata Frison | 1 | |
| Alloperla chloris Frison | 1 | |
| Alloperla idei Ricker | 1 | |
| Alloperla imbecilla (Say) | 1 | |
| Alloperla neglecta Frison | continued uncertainty | 1 |
| Alloperla petasata Surdick | 1 | |
| Alloperla usa Ricker | 1 | |
| Haploperla brevis (Banks) | 1 | |
| Sweltsa mediana Banks | misidentified S. hoffmani | |
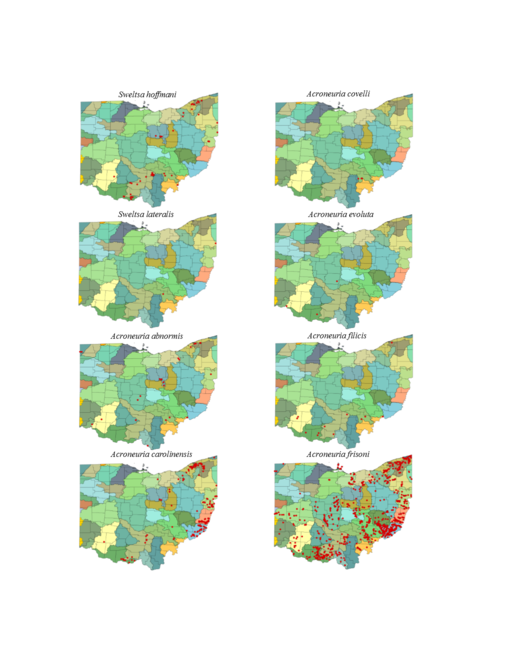
| Sweltsa hoffmani Kondratieff & Kirchner | 1 | |
| Sweltsa lateralis (Banks) | 1 | |
| Sweltsa onkos (Ricker) | misidentified S. hoffmani | |
| Perlidae | ||
| Acroneuria abnormis (Newman) | 1 | |
| Acroneuria carolinensis (Banks) | 1 | |
| Acroneuria covelli Grubbs & Stark | 1 | |
| Acroneuria evoluta Klapálek | 1 | |
| Acroneuria filicis Frison | 1 | |
| Acroneuria frisoni Stark & Brown | 1 | |
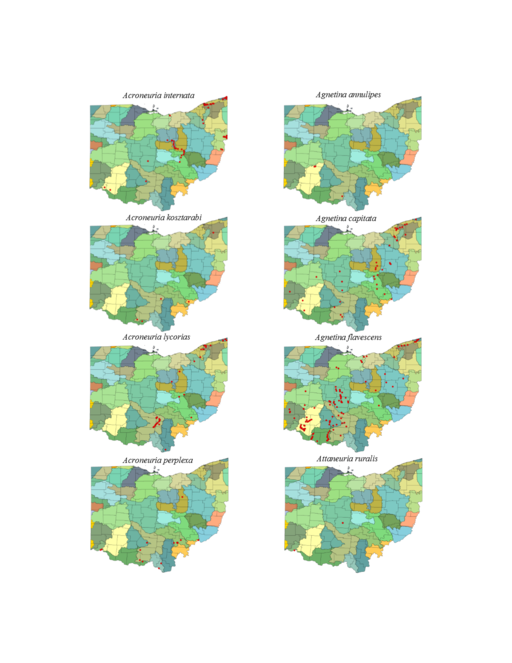
| Acroneuria internata (Walker) | 1 | |
| Acroneuria kirchneri Stark & Kondratieff | 1 | |
| Acroneuria kosztarabi Kondratieff & Kirchner | misidentified A. kirchneri | |
| Acroneuria lycorias (Newman) | 1 | |
| Acroneuria perplexa Frison | 1 | |
| Agnetina annulipes (Hagen) | 1 | |
| Agnetina capitata (Pictet) | 1 | |
| Agnetina flavescens (Walsh) | 1 | |
| Attaneuria ruralis (Hagen) | 1 | |
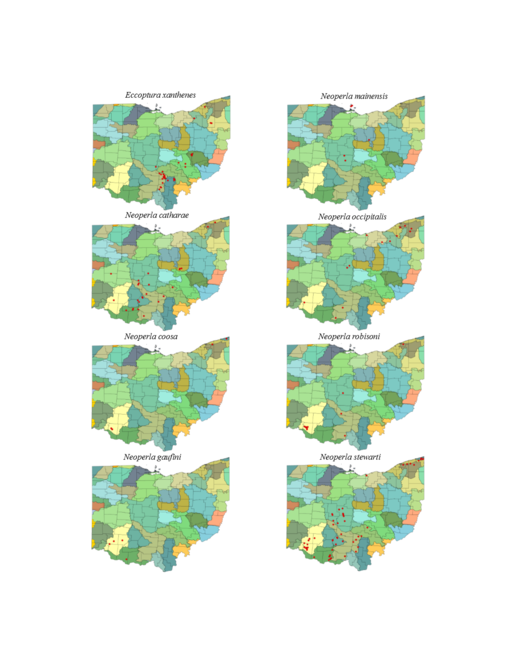
| Eccoptura xanthenes (Newman) | 1 | |
| Neoperla catharae Stark & Baumann | 1 | |
| Neoperla clymene (Newman) | nymphs only, removed from list | |
| Neoperla coosa Stark & Smith | 1 | |
| Neoperla gaufini Stark & Baumann | 1 | |
| Neoperla mainensis Banks | 1 | |
| Neoperla occipitalis (Pictet) | 1 | |
| Neoperla robisoni Poulton & Stewart | 1 | |
| Neoperla stewarti Stark & Baumann | 1 | |
| Paragnetina media (Walker) | 1 | |
| Perlesta adena Stark | 1 | |
| Perlesta cinctipes (Banks) | referable to Perlesta I-4 | |
| Perlesta decipiens (Walsh) | 1 | |
| Perlesta ephelida Grubbs & DeWalt | 1 | |
| Perlesta golconda DeWalt & Stark | removed from list | |
| Perlesta lagoi Stark | lagoi & nitida may be a cline | 1 |
| Perlesta nitida Banks | lagoi & nitida may be a cline | |
| Perlesta placida (Hagen) | any one of 7 spp. possible | |
| Perlesta teaysia Kirchner & Kondratieff | 1 | |
| Perlesta xube Stark & Rhodes | 1 | |
| Perlesta I–4 | new, dark species | 1 |
| Perlinella drymo (Newman) | 1 | |
| Perlinella ephyre (Newman) | 1 | |
| Perlodidae | ||
| Clioperla clio (Newman) | 1 | |
| Cultus decisus (Walker) | uncertain specific/subspecific identity | 1 |
| Diploperla robusta Stark & Gaufin | 1 | |
| Isoperla bilineata (Say) | 1 | |
| Isoperla burksi Frison | 1 | |
| Isoperla decepta Frison | 1 | |
| Isoperla dicala Frison | 1 | |
| Isoperla holochlora (Klapálek) | 1 | |
| Isoperla montana (Banks) | 1 | |
| Isoperla namata Frison | referable to I. montana | |
| Isoperla nana (Walsh) | 1 | |
| Isoperla orata Frison | new state record | 1 |
| Isoperla richardsoni Frison | new state record | 1 |
| Isoperla signata (Banks) | 1 | |
| Isoperla transmarina (Newman) | 1 | |
| Malirekus iroquois Stark & Szczytko | identity confirmed from Ashland Co. | 1 |
| Total | 102 |
Keywords
Ohio, U.S.A., Plecoptera, stoneflies, museum data, distribution, emergence, stream size
Introduction
Stoneflies (Insecta: Plecoptera) are one of many faunal groups that reflect the historical geography of Ohio. The presence and distribution of stoneflies in Ohio demonstrate not only the results of the terraforming effects of Quaternary glaciation, but also the various invasion routes available in preglacial epochs. For example, the preglacial (and pre-Ohio River) Teays River drainage, originated in western North Carolina and provided access to Ohio, Indiana and Illinois (
Properly maintained natural history (museum) collections provide a permanent record of life on Earth (
Given that stoneflies are one of the most sensitive indicators of change in habitat and water quality (
Prior to
A much needed update of the Ohio fauna was begun in the 1980s and continued through the 1990s, conducted by RWB, SMC, BJA, and Ralph F. Kirchner (Wheeling, West Virginia). These efforts did not result in publication, but their thousands of specimens form the basis of this work. Beginning in 2005, RED and SAG borrowed material from individuals and institutions, identified the specimens, digitized the label data for 4,080 vials and pins of stoneflies, and georeferenced all locations, resulting in
Other specimens that improved our coverage include a substantial number of records from Edge of Appalachia Preserve (Adams County, Ohio Brush Creek drainage) collected by RED and specimens collected by Gary A. Coovert since 2004 from Crane Hollow Nature Preserve (Hocking County, Queer Creek drainage). Both locations added new locations for several rare species and confirmed the presence of another. All total, 7,723 specimen records now exist for Ohio stoneflies. This dramatic increase in specimens makes an update desirable, provides an opportunity to present a complete historical accounting of stonefly research conducted in Ohio, explore some relationships of species richness to drainage characteristics, add range maps, conduct analyses of stream widths used by species, and present an analysis of the succession of adult presence throughout the year. None of these analyses were present in
This publication is volume II in a series of atlases of aquatic insects inhabiting Ohio and complements volume I on caddisflies (
Materials and Methods
Digitization of specimen data. Data presented in this work represents a combination of verified specimens, specimen data from the OEPA, and trusted literature. We verified identifications of many of the most difficult to identify species among the OEPA specimens, strongly supporting their inclusion in this study. The specimen data source and number of records (# of vials or pins) are provided for each institution and colleague who provided specimens/data. The methodology for preparing specimens is available in
Most location labels printed prior to 2000 did not contain geographic coordinates. We georeferenced these locations using Acme Mapper 2.1 (
Succession of species. Adults of stonefly species succeed each other as they emerge throughout the year (
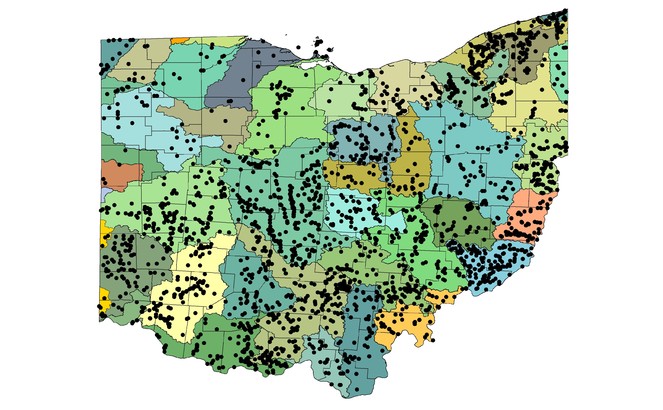
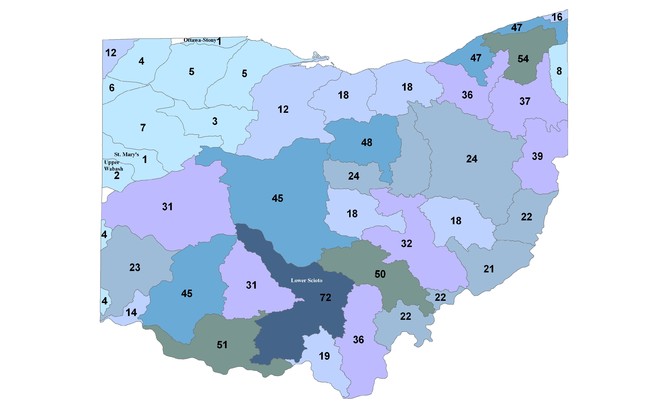
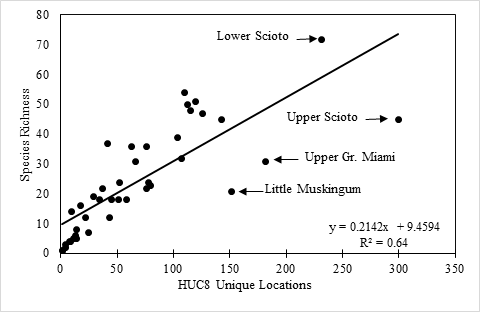
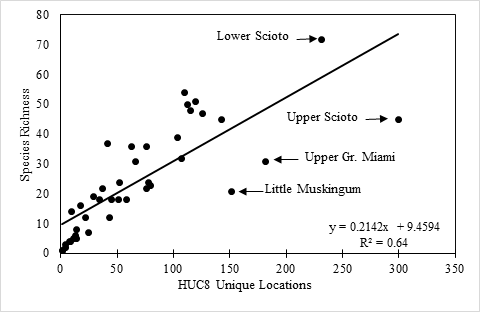
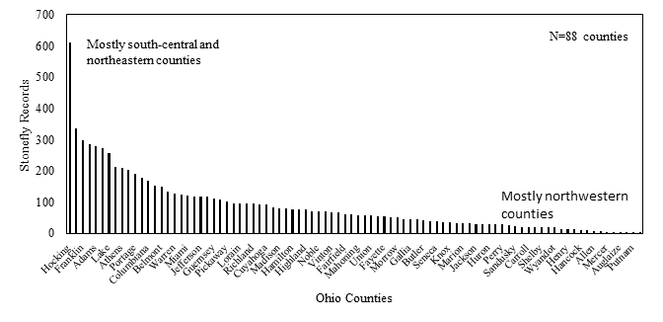
Species richness vs. county and watershed relationships. All georeferenced specimen records were associated with HUC8 coverage in GIS and the drainage numbers and names were returned to the data. The total species richness and number of unique locations within a HUC8 drainage were compiled. A map depicting of the number of species vs. HUC8 drainage was constructed so that drainages with similar species tallies were similarly color-coded. Scatterplots were constructed of species richness versus HUC8 area in km2 and the number of unique locations within a HUC8 to determine if these variables were important to species richness. Deviations from trend lines produced from simple linear regression analyses were noted. Ohio counties, of which there are 88, are geopolitical units for local government (
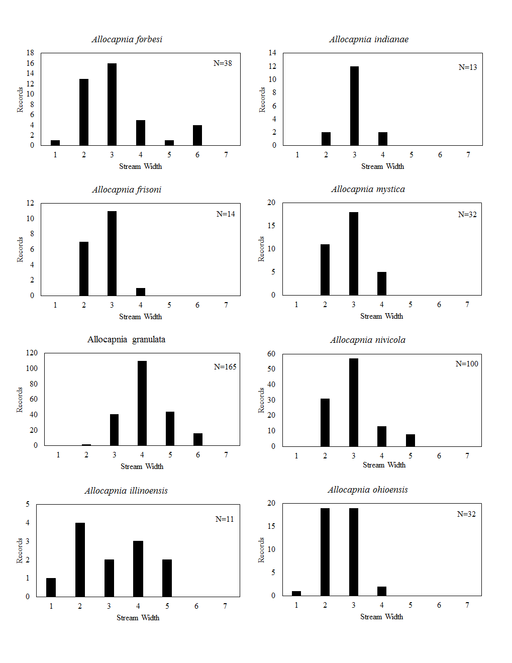
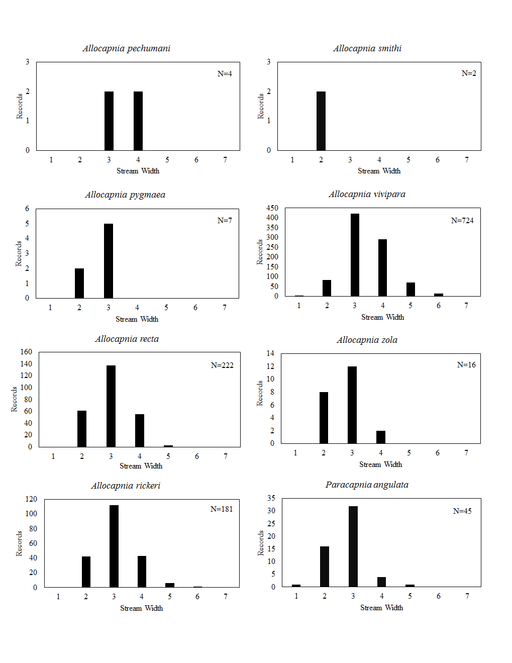
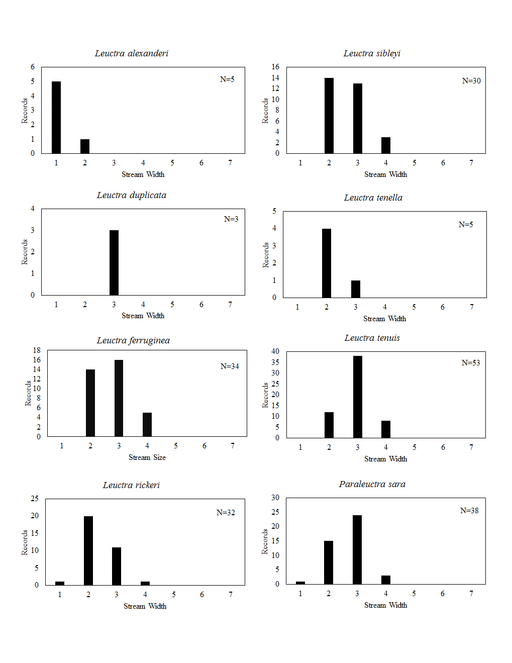
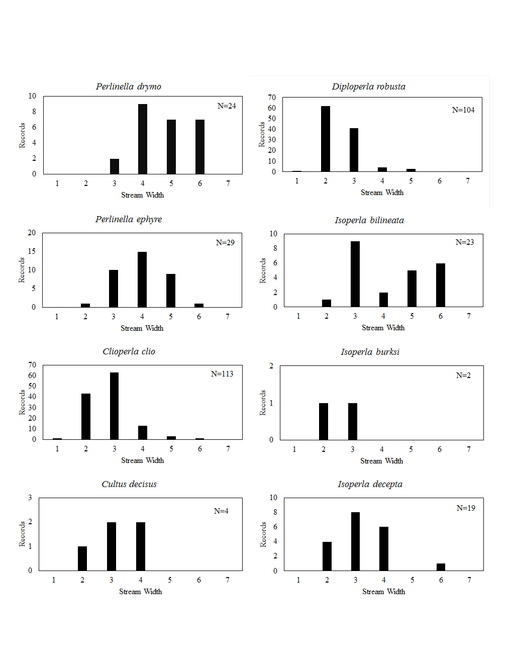
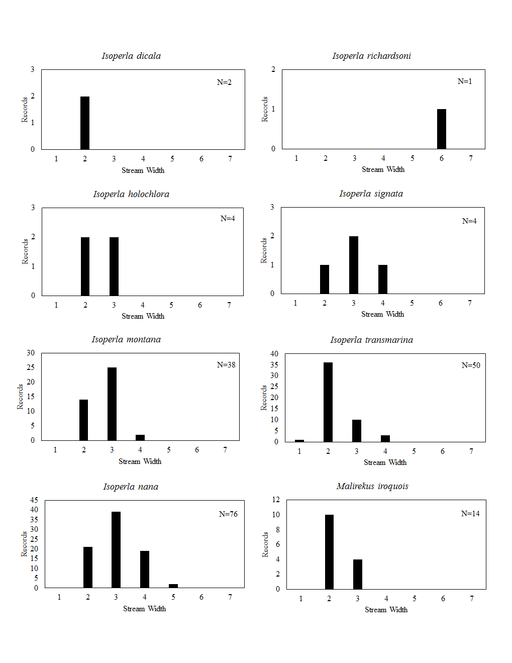
Distribution of species in stream size/type categories. Stoneflies live in a wide range of waterbody sizes, even in large lakes. Drainage area and perhaps the number of links (tributaries) are the best measures of stream size and may often be recovered from Geographic Information Systems data layers. However, these data sets often lack data for the smallest streams. To account for this streams were categorize by stream wetted width (1=seep, 2=1-2 m wide stream, 3=3-10 m wide, 4=11-30 m wide, 5=31-60 m wide, 6=>61 m wide, 7=large lake (Lake Erie specifically). These estimates were made from Acme Mapper (2016) satellite coverages using the scale provided by the program. A histogram of the frequency of site/date events within each stream width or lake category was constructed for each species for all sites that could be georeferenced to a stream or lake (91.2% of 7,723 records).
Access to the data. All specimen data used in this study are archived as a Darwin Core Archive file supported by Pensoft's Integrated Publishing Toolkit (
Results
A total of 7,797 records were gathered from 21 institutional, government, personal collection sources, and from literature sources (Table
At least 53 papers have appeared in print that reference Ohio stoneflies (Suppl. material
Species present and those dismissed from the state tally
In total, 102 species are known to occur in Ohio, though many more names have been associated with the state from previous publications (Table
Larvae of the Pteronarcys scotti Ricker, 1952 species group, what was once considered the subgenus Allonarcys Needham & Claassen, 1925, have spine-like, paired lateral projections on each abdominal segment (
Three publications listed Isoperla namata Frison, 1942 from Ohio (
Species richness vs. watershed and county relationships
Stonefly species richness varied tremendously with HUC8 affiliation (Fig.
Surface area of HUC8 drainages appears to be an unimportant predictor of stonefly species richness (Fig.
At least one stonefly record is available for each of Ohio's 88 counties (Fig.
Succession of adult presence
Ohio stonefly adults may be obtained in nearly every month of the year, but are most frequently collected from January to July (Table
Succession of adult presence of Ohio stonefly species. Darkest shade of gray indicates weeks with at least one collecting events with ≥ 3 adults. Lighter gray indicates weeks with events containing ≤ 2 adults. Lightest gray is suggestive of when emergence would take place since no adult specimens were obtained. Events = number of site/date collecting events (date+location). Family abbreviations: CA=Capniidae, CH=Chloroperlidae, L=Leuctridae, N=Nemouridae, P=Perlidae, PE=Perlodidae, PL=Peltoperlidae, PT=Pteronarcyidae, T=Taeniopterygidae.
| Taxon | Fam. | XI | XII | I | II | III | IV | V | VI | VII | VIII | IX | X | Events | ||||||||||||||||||||||||||||||||||||
| Allocapnia recta | CA | 221 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Allocapnia nivicola | CA | 90 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Allocapnia frisoni | CA | 17 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Allocapnia forbesi | CA | 38 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Allocapnia rickeri | CA | 151 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Allocapnia vivipara | CA | 566 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Allocapnia illinoensis | CA | 12 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Allocapnia mystica | CA | 32 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Allocapnia granulata | CA | 28 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Allocapnia indianae | CA | 14 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Allocapnia ohioensis | CA | 35 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Allocapnia smithi | CA | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Allocapnia pygmaea | CA | 8 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Allocapnia pechumani | CA | 4 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Allocapnia zola | CA | 19 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Paracapnia angulata | CA | 41 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Taeniopteryx burksi | T | 197 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Taeniopteryx maura | T | 31 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Taeniopteryx metequi | T | 14 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Soyedina vallicularia | N | 37 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Taeniopteryx nivalis | T | 10 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Taeniopteryx parvula | T | 7 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Strophopteryx fasciata | T | 15 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Taeniopteryx lita | T | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Prostoia similis | N | 19 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Prostoia completa | N | 9 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Zealeuctra fraxina | L | 5 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Zealeuctra claasseni | L | 16 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Paraleuctra sara | L | 37 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Leuctra sibleyi | L | 41 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Ostrocerca truncata | N | 11 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Ostrocerca albidipennis | N | 19 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Nemoura trispinosa | N | 14 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Amphinemura delosa | N | 111 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Perlinella drymo | P | 4 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Sweltsa hoffmani | CH | 21 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Isoperla bilineata | PE | 24 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Diploperla robusta | PE | 34 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Clioperla clio | PE | 23 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Amphinemura varshava | N | 51 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Amphinemura nigritta | N | 27 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Isoperla nana | PE | 52 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Isoperla signata | PE | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Malirekus iroquois | PE | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Pteronarcys cf. biloba | PT | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Pteronarcys dorsata | PT | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Leuctra tenella | L | 7 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Isoperla richardsoni | PE | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Acroneuria evoluta | P | 5 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Leuctra alexanderi | L | 5 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Leuctra duplicata | L | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Cultus decisus | PE | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Isoperla burksi | PE | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Isoperla dicala | PE | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Isoperla holochlora | PE | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Isoperla orata | PE | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Sweltsa lateralis | CH | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Alloperla neglecta | CH | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Alloperla idei | CH | 3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Isoperla transmarina | PE | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Peltoperla arcuata | PL | 6 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Paragnetina media | P | 3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Isoperla decepta | PE | 10 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Isoperla montana | PE | 19 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Alloperla caudata | CH | 10 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Haploperla brevis | CH | 55 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Alloperla chloris | CH | 26 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Acroneuria frisoni | P | 140 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Acroneuria carolinensis | P | 13 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Acroneuria filicis | P | 35 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Neoperla gaufini | P | 7 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Perlinella ephyre | P | 33 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Acroneuria perplexa | P | 25 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Agnetina capitata | P | 16 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Agnetina flavescens | P | 66 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Neoperla mainensis | P | 8 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Neoperla stewarti | P | 74 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Perlesta decipiens | P | 131 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Acroneuria internata | P | 5 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Alloperla imbecilla | CH | 25 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Alloperla petasata | CH | 21 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Alloperla usa | CH | 13 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Attaneuria ruralis | P | 3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Leuctra ferruginea | L | 34 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Leuctra rickeri | L | 39 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Perlesta adena | P | 61 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Perlesta lagoi | P | 281 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Neoperla robisoni | P | 16 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Perlesta sp. I–4 | P | 17 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Acroneuria abnormis | P | 33 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Perlesta ephelida | P | 53 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Perlesta teaysia | P | 73 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Perlesta xube | P | 6 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Agnetina annulipes | P | 4 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Acroneuria covelli | P | 3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Acroneuria kosztarabi | P | 5 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Acroneuria lycorias | P | 3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Eccoptura xanthenes | P | 11 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Neoperla occipitalis | P | 13 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Neoperla coosa | P | 7 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Neoperla catharae | P | 37 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Leuctra tenuis | L | 79 | ||||||||||||||||||||||||||||||||||||||||||||||||
The superfamilies Perloidea (Chloroperlidae, Perlidae, Perlodidae) and Pteronarcyoidea (Peltoperlidae, Pteronarcyidae) contain spring and summer emerging species. Chloroperlidae, such as Sweltsa hoffmani Kondratieff & Kirchner, 2009, often begin emerging in late April; other "sallflies" follow through early July. Perlodidae are commonly known as "spring stoneflies" since most of their members emerge before summer. Isoperla bilineata (Say, 1823) is the earliest emerging perlodid species with some records beginning in late March, particularly from larger rivers in the southern part of the state. The rest of the species in the family are present primarily in May and early June. Adult presence of I. signata (Banks, 1902) and I. transmarina (Newman, 1838) is inferred (see light gray of Table
Perlidae adults are present from early spring until late summer. The females of perlids live a comparatively long life, hence their adult presence spans up to three months for some species. The single Peltoperlidae species, the roachfly Peltoperla arcuata Needham, 1905, is present in late May through mid-June. The adult presence of Pteronarcyidae, or salmonflies, in Ohio is rather a mystery since only a single adult of one species, Pteronarcys dorsata (Say, 1823), has been collected. The adult presence of P. cf. biloba Newman, 1838 is inferred from larval records and professional judgement.
The bias in this data set for the protracted presence of spent (all or most eggs expelled, but still alive) females should be accounted for by future researchers of stonefly adults. Consulting the dataset associated with this work will improve a researcher's ability to find adult stoneflies. Paying particular attention to whether a year is above or below average in air temperature is also important, as will be future changes in climate that shift emergence of all species to earlier weeks. Some shifting has already undoubtedly occurred.
Species distributions, stream size affiliation, and Adult Presence Phenology
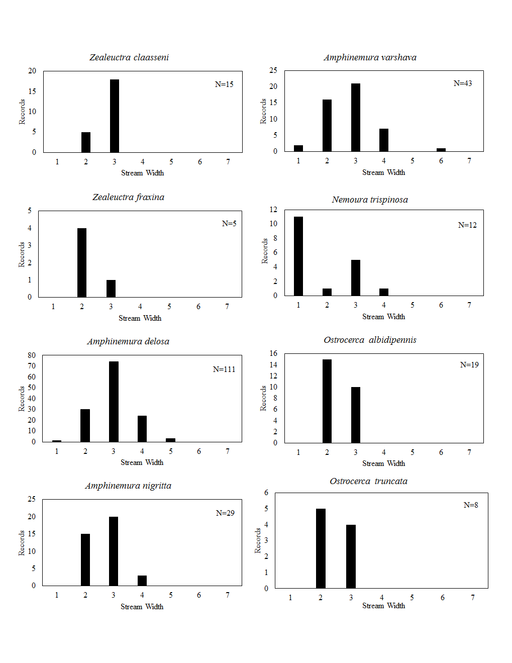
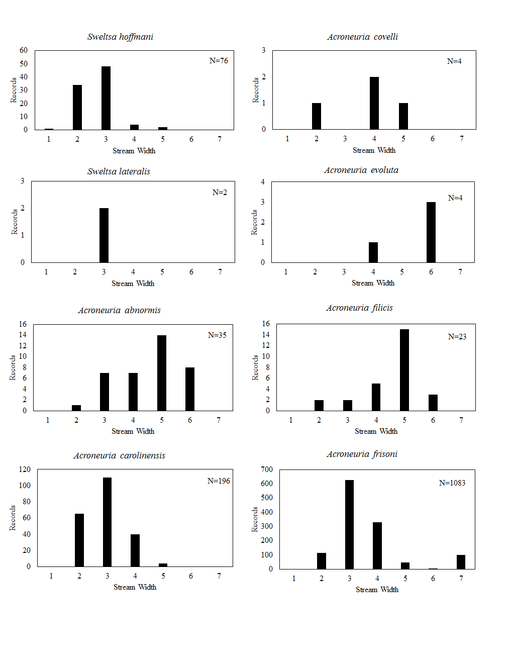
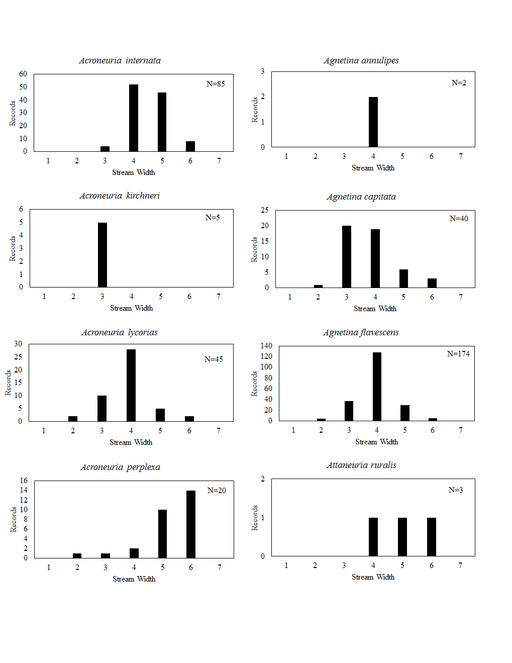
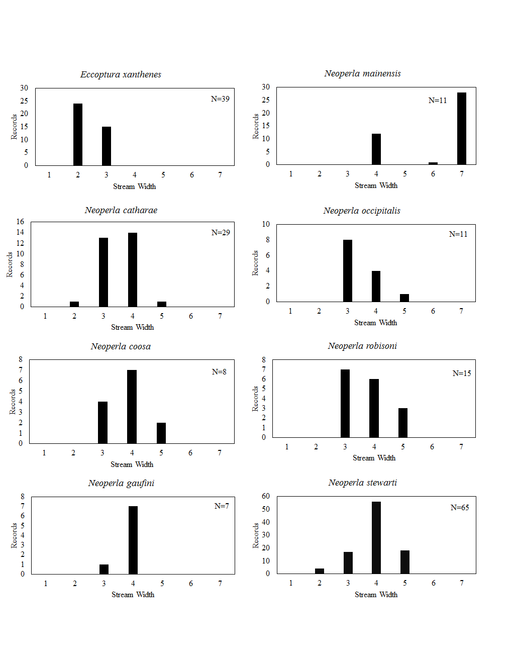
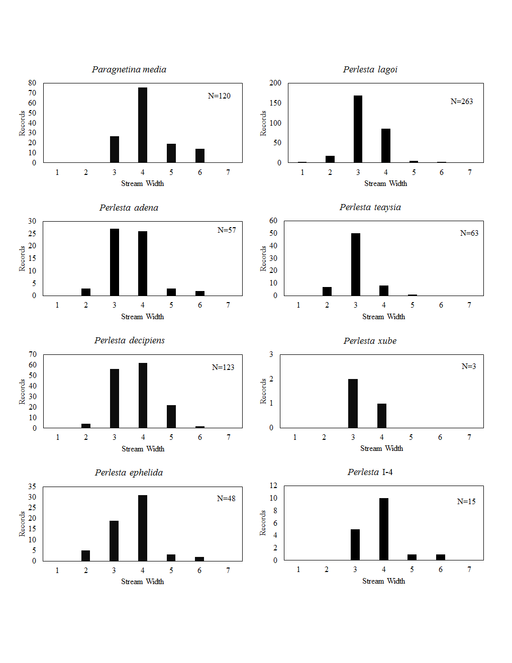
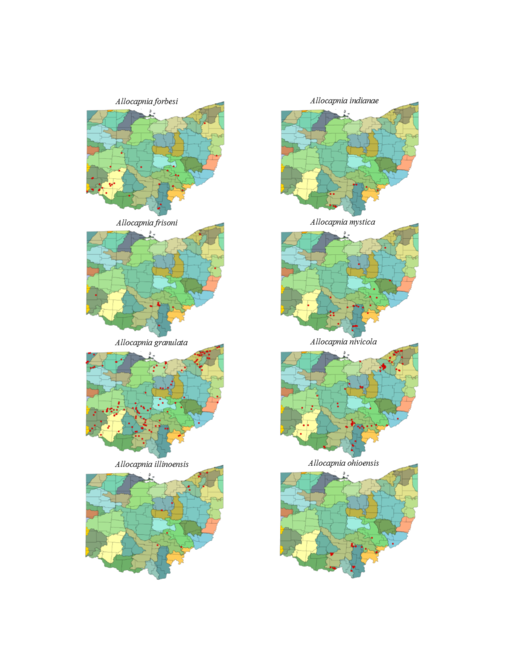
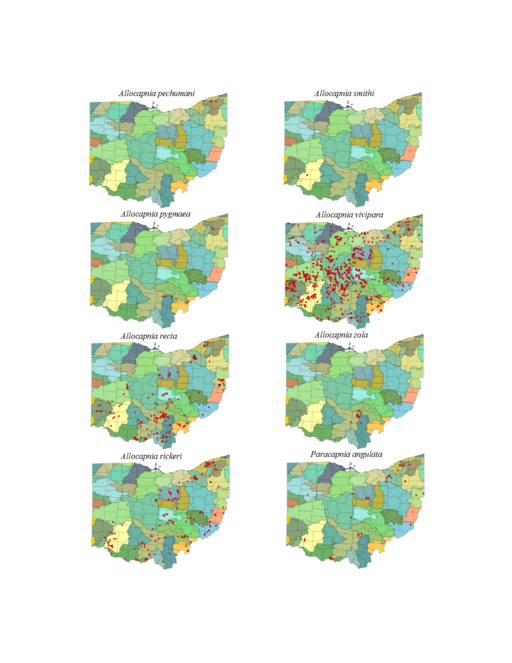
This section documents the relative stream size occupied (Figs
Capniidae. Snowflies
Allocapnia forbesi Frison, 1929. This species is collected most frequently in headwater streams, but may be taken from larger ones as well (Fig.
Allocapnia frisoni Ross & Ricker, 1964. This species occurs in headwater streams (Fig.
Allocapnia granulata (Claassen, 1924). This species inhabits comparatively larger streams and rivers than A. forbesi and A. frisoni (Fig.
Allocapnia illinoensis Frison, 1935. This uncommon species lives in small streams (Fig.
Allocapnia indianae Ricker, 1952. This species occupies small streams (Fig.
Allocapnia mystica Frison, 1929. This species occurs mainly in small streams (Fig.
Allocapnia nivicola (Fitch, 1847). This common species occupies a broad range of stream sizes (Fig.
Allocapnia ohioensis Ross & Ricker, 1964. This species occurs mostly in small streams (Fig.
Allocapnia pechumani Ross & Ricker, 1964. Our records demonstrate this rare species to inhabit medium sized streams (Fig.
Allocapnia pygmaea (Burmeister, 1839). This species occurs in seven small streams (Fig.
Allocapnia recta (Claassen, 1924). This species inhabits small streams (Fig.
Allocapnia rickeri Frison, 1942. This species inhabits small streams (Fig.
Allocapnia smithi Ross & Ricker, 1971. This is one of the rarest stonefly species inhabiting eastern North America. One male and one female are known from two small ravine streams in Warren County (Figs
Allocapnia vivipara (Claassen, 1924). This species occurs in a broad range of stream sizes (Fig.
Allocapnia zola Ricker, 1952. This species occurs in small streams (Fig.
Paracapnia angulata Hanson, 1961. This species inhabits mainly small, cold streams (Fig.
Leuctridae. Needleflies
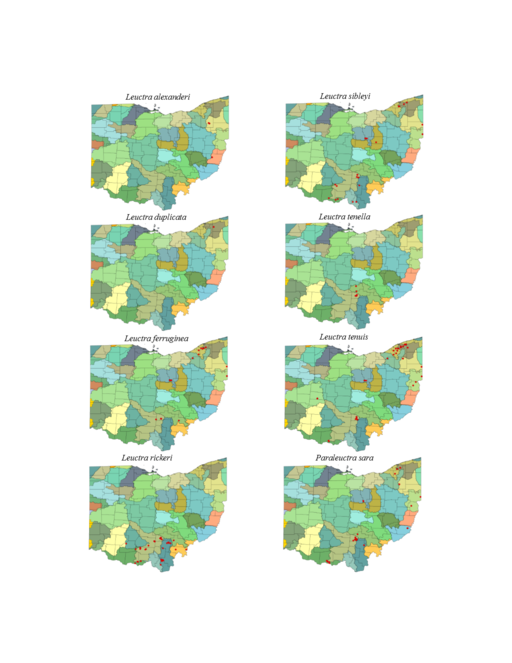
Leuctra alexanderi Hanson, 1941. This species is rare, occurring in only three small streams (Fig.
Leuctra duplicata Claassen, 1923. This species occurs in two small (Fig.
Leuctra ferruginea (Walker, 1852). This species occurs in small streams (Fig.
Leuctra rickeri James, 1976. This species is extremely common in the south-central region of the state (Fig.
Leuctra sibleyi Claassen, 1923. This species occurs in small streams (Fig.
Leuctra tenella Provancher, 1878. This species resides in small streams (Fig.
Leuctra tenuis (Pictet, 1841). This species is most prevalent in small streams (Fig.
Paraleuctra sara (Claassen, 1937). This species occurs in smaller streams (Fig.
Zealeuctra claasseni (Frison, 1929). Collections are from small streams (Fig.
Zealeuctra fraxina. This rarely collected species inhabits headwater streams (Fig.
Nemouridae. Forestflies
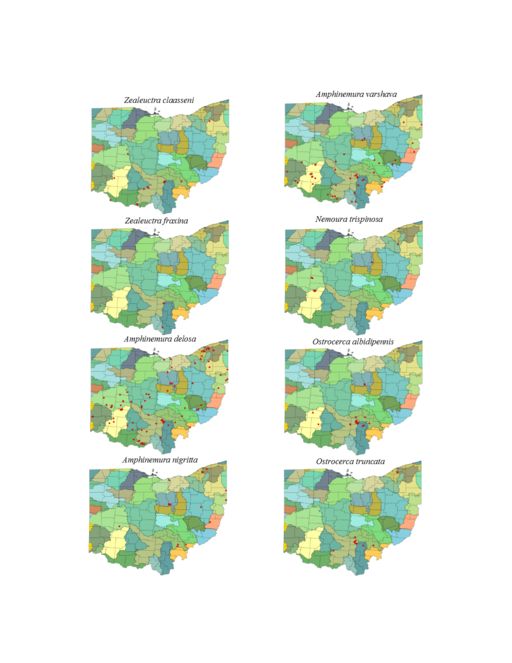
Amphinemura delosa (Ricker, 1952). This common species inhabits a broad range of stream sizes (Fig.
Amphinemura nigritta (Provancher, 1876). This species inhabits small streams (Fig.
Amphinemura varshava (Ricker, 1952). This species inhabits a broad range of stream sizes in Ohio (Fig.
Nemoura trispinosa Claassen, 1923. Several widely-disjunct localities provide habitat for this uncommon species (Fig.
Ostrocerca albidipennis (Walker, 1852). This headwater species (Fig.
Ostrocerca truncata (Claassen, 1923). This is also a headwater species (Fig.
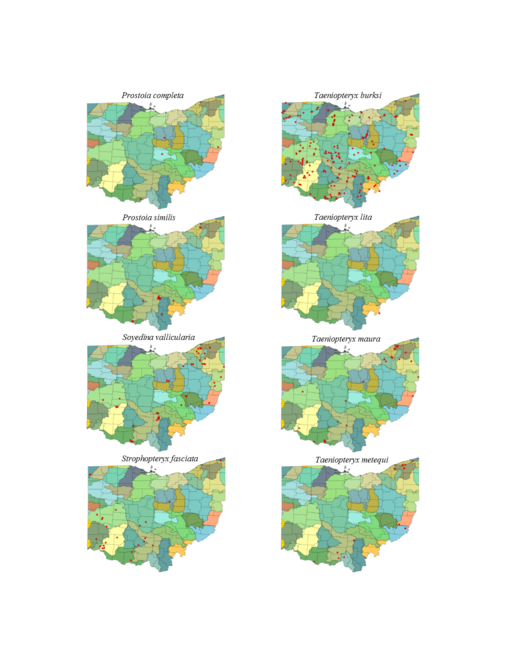
Prostoia completa (Walker, 1852). This species is rarely collected in Ohio, though we believe it should be more abundant (Fig.
Prostoia similis (Hagen, 1861). This species is more widely distributed in Ohio and more abundant where found than P. completa (Fig.
Soyedina vallicularia (Wu, 1923). This common headwater species (Fig.
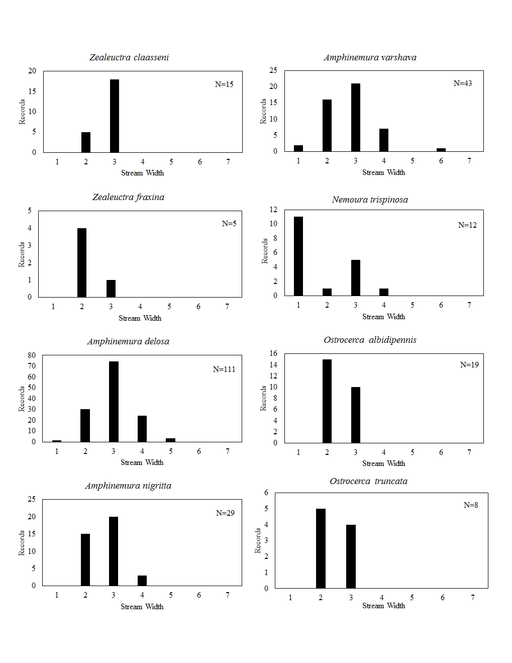
Taeniopterygidae. Willowflies
Strophopteryx fasciata (Burmeister, 1839). This species inhabits larger streams and rivers (Fig.
Taeniopteryx burksi Ricker & Ross, 1968. This species inhabits a large range of stream sizes (Fig.
Taeniopteryx lita Frison, 1942. Adults of this species have yet to be collected in Ohio, the sole specimen being a mature larva taken from the Ohio River in southeastern Ohio (Figs
Taeniopteryx maura (Pictet, 1841). Large streams and small rivers support this species in Ohio (Fig.
Taeniopteryx metequi Ricker & Ross, 1968. This species typically inhabits smaller streams and rivers (Fig.
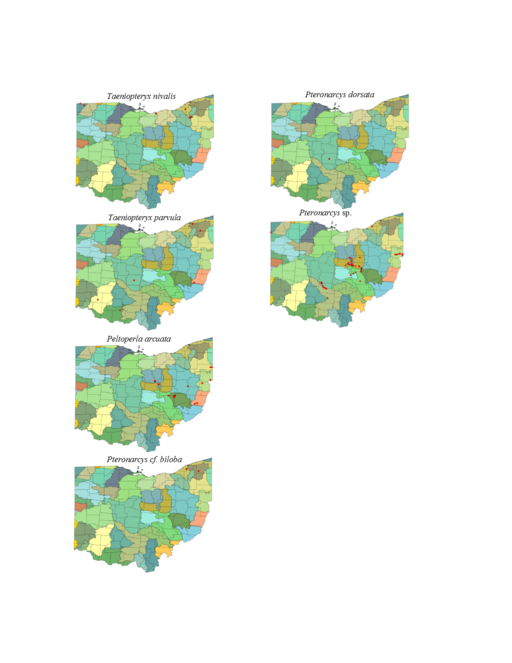
Taeniopteryx nivalis Fitch, 1847. This species inhabits mid-order streams and small rivers (Fig.
Taeniopteryx parvula Banks, 1918. This species typically inhabits mid-order streams and small rivers (Fig.
Peltoperlidae. Roachflies
Peltoperla arcuata Needham, 1905. This is the only representative of the family in Ohio. It is a headwater species (Fig.
Pteronarcyidae. Salmonflies
Pteronarcys cf. biloba Newman, 1838. The identity of this species is uncertain since no adults have been collected in Ohio. The species occurs in two small streams (Fig.
Pteronarcys dorsata (Say, 1823). One adult female exists that validates the occurrence of this species in Ohio (Fig.
Pteronarcys sp. All Pteronarcys larvae inhabiting eastern North America that lack lateral abdominal appendages belong to the P. dorsata species group (
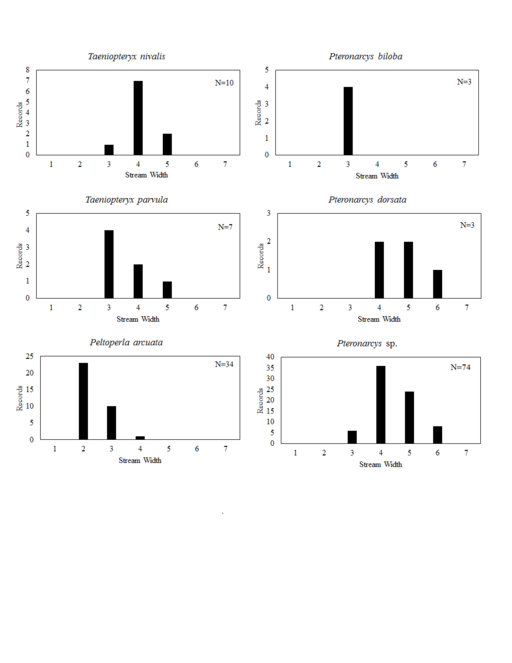
Chloroperlidae. Sallflies
Alloperla caudata Frison, 1934. Small to medium sized streams (Fig.
Alloperla chloris Frison, 1934. This too is a small stream Alloperla (Fig.
Alloperla idei (Ricker, 1935). This species is rarely collected in Ohio with all three records being assigned to streams between 3 and 10 m width (Fig.
Alloperla imbecilla (Say, 1823). The species occurs in mainly small streams (Fig.
Alloperla neglecta Frison, 1935.
Alloperla petasata Surdick, 2004. The species occurs in small streams (Fig.
Alloperla usa Ricker, 1952. This species resides in three widely separated areas of central and northeastern Ohio (Fig.
Haploperla brevis (Banks, 1895). This common species inhabits mainly small streams (Fig.
Sweltsa hoffmani Kondratieff & Kirchner, 2009. Our analysis demonstrates that this common species most often inhabits small, cool, ravine streams, though some have been reported from medium to large rivers (Fig.
Sweltsa lateralis (Banks, 1911). This is another rare species in Ohio. It occurs in small streams (Fig.
Perlidae. Summer Stoneflies
Acroneuria abnormis (Newman, 1838). This species uses a wide range of stream sizes with the greatest frequency of records coming from streams 31-60 m wide (Fig.
Acroneuria carolinensis (Banks, 1905). This common species generally inhabits smaller streams than A. abnormis (Fig.
Acroneuria covelli Grubbs & Stark, 2004. This species is rare in Ohio, being known from only three locations in Athens County (Fig.
Acroneuria evoluta Klapálek, 1909. Only three adult records of this species exist for Ohio, one from a non-specific location in Adams County, another from Black Lick Creek in Franklin County, and another location, "Catonbads", that cannot be placed (
Acroneuria filicis Frison, 1942. This species once occurred in a wide variety of stream sizes (Fig.
Acroneuria frisoni Stark & Brown 1991. This species occurs widely across Ohio (Fig.
Acroneuria internata (Walker, 1852). This species inhabits small and medium sized rivers (Fig.
Acroneuria kirchneri Stark & Kondratieff, 2004. This rare species presumably inhabits only small streams (Fig.
Acroneuria lycorias. This species utilizes a wide range of stream sizes (Fig.
Acroneuria perplexa Frison, 1937. This species is considered extirpated from Ohio since all records span the years 1899 to 1948 (
Agnetina annulipes. Data for this species are scanty with only two of four records capable of being georeferenced. These two records place it in the Little Miami River near Clifton Falls, a medium sized river in that location (Fig.
Agnetina capitata (Pictet, 1841). This common species utilizes a wide range of stream sizes (Fig.
Agnetina flavescens (Walsh, 1862). This Agnetina is also common, occupying similar stream sizes (Fig.
Attaneuria ruralis (Hagen, 1861). The four Ohio records for this species predate 1926, because of this we consider it extirpated from the state (
Eccoptura xanthenes (Newman, 1838). This species inhabits small, usually ravine associated streams (Fig.
Neoperla catharae Stark & Baumann, 1978. This species occurs mainly in medium sized streams and rivers (Fig.
Neoperla coosa Smith & Stark, 1998. Small streams to medium rivers support this species (Fig.
Neoperla gaufini Stark & Baumann, 1978. This is a rare find in Ohio since only four unique locations, all in the southwestern region of the state, are known (Fig.
Neoperla mainensis Banks, 1948. Records exist for the Bass Islands of Lake Erie, the Olentangy River near Columbus, and the Clear Fork of the Mohican River near Loudonville (Figs
Neoperla occipitalis (Pictet, 1841). This uncommon species occurs in large streams and medium rivers (Fig.
Neoperla robisoni Poulton & Stewart, 1986. This species inhabits large streams and medium rivers (Fig.
Neoperla stewarti Stark & Baumann, 1978. This common species occupies small streams to medium rivers (Fig.
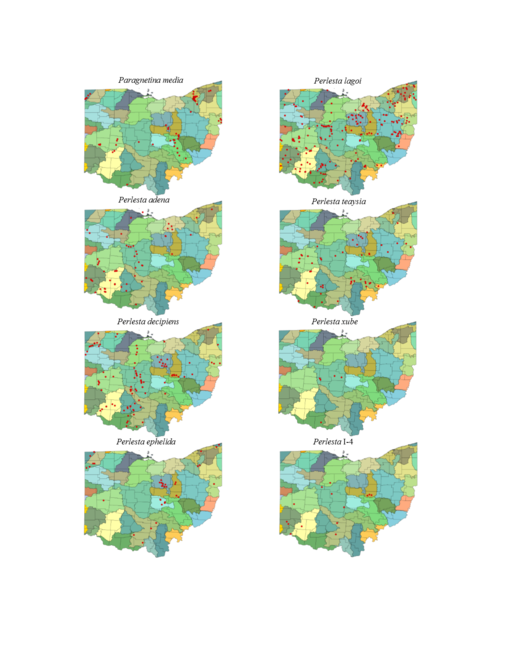
Paragnetina media (Walker, 1852). This a common species in Ohio. It inhabits a wide range of stream sizes (Fig.
Perlesta adena Stark, 1989. This common species inhabits a wide range of stream sizes (Fig.
Perlesta decipiens (Walsh, 1862). This is also a common species and exhibits nearly the same stream size usage (Fig.
Perlesta ephelida Grubbs & DeWalt, 2012. This species inhabits a large range of stream sizes (Fig.
Perlesta lagoi Stark, 1989. The distribution of this species is statewide (Fig.
Perlesta teaysia Kirchner & Kondratieff, 1997. This species utilizes mainly small streams to small rivers in Ohio (Fig.
Perlesta xube Stark & Rhodes, 1997. This rare species utilizes large streams to small rivers (Fig.
Perlesta I-4. This undescribed species inhabits large streams and small rivers (Fig.
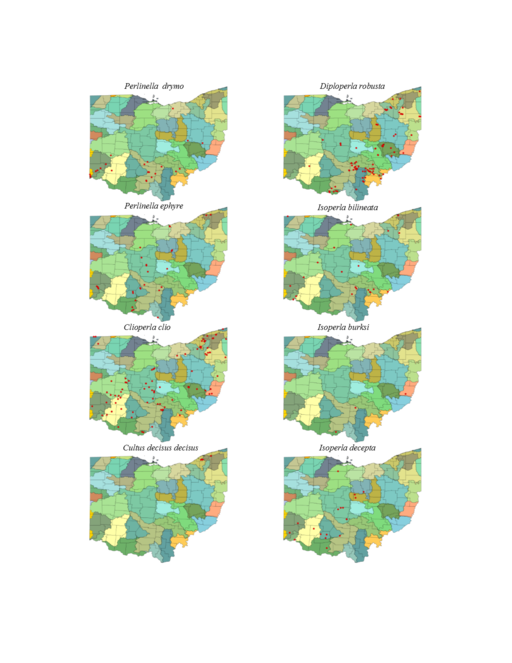
Perlinella drymo (Newman, 1839). This species occurs in the largest streams and rivers (Fig.
Perlinella ephyre (Newman, 1839). Large streams and rivers support this species (Fig.
Perlodidae. Spring Stones
Clioperla clio (Newman, 1839). This common species most often inhabits small to medium sized streams (Fig.
Cultus decisus (Walker, 1852). This species inhabits four small streams (Fig.
Diploperla robusta Stark & Gaufin, 1974. This is a small stream species (Fig.
Isoperla bilineata (Say, 1823). This species occurs mainly in larger streams and rivers (Fig.
Isoperla burksi Frison, 1942. Larvae of this rare Ohio species occur in small streams (Fig.
Isoperla decepta Frison, 1935. This species occurs mainly in small to mid-order streams (Fig.
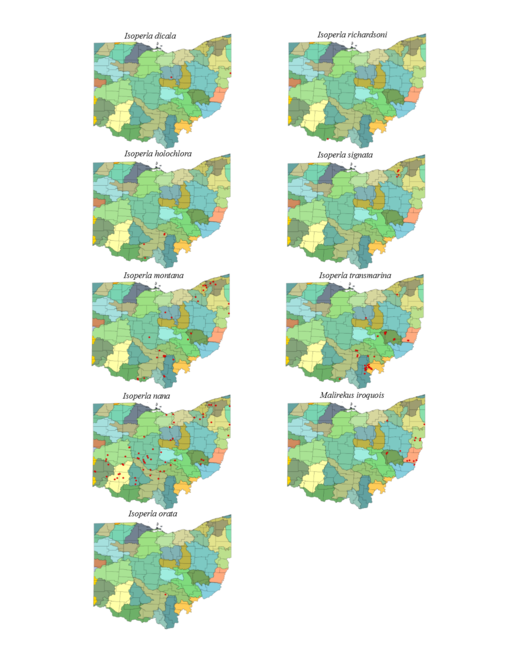
Isoperla dicala Frison, 1942. This species is rare in Ohio (Fig.
Isoperla holochlora Klapálek, 1923. This species too is rare, being known from only four small streams (Fig.
Isoperla montana (Banks, 1898). This common species inhabits mainly small streams (Fig.
Isoperla nana (Walsh, 1862). This common species inhabits small streams to medium sized rivers (Fig.
Isoperla orata Frison, 1942. This is the rarest species in Ohio, being known from only one locality in Crane Hollow Preserve in Hocking County (Fig.
Isoperla richardsoni Frison, 1935. This is a new state record, confirmed from a single adult female from the Ohio River in Adams County (Figs
Isoperla signata (Banks, 1902). This rare species inhabits only four small streams (Fig.
Isoperla transmarina (Newman, 1838). This common species inhabits mainly small streams (Fig.
Malirekus iroquois Stark & Szczytko, 1988. Larvae inhabit only small streams (Fig.
Discussion
Our present study added 3717 records to the data set of
Most portions of the state were satisfactorily sampled (Fig.
The use of museum specimens and agency data was exceedingly valuable for this project. Less than 600 records (7.7%) were added as new specimens to this project by RED and SAG since 2005. Existing data were sufficient to characterize the assemblage to a relatively fine scale. This was perhaps an extraordinary situation with coauthors having started this project decades ago (BJA, RWB, SMC) or providing a continuous source of agency data (MJB) with high confidence identifications. Our experience should give others confidence that they too could obtain enough material to characterize a region given the presence of regional museums and trusted agency data.
Little stonefly data were present in GBIF and iDigBio, other than what was already provided by the INHS. Regional collections had not digitized their material in time for our use. We agree that with time and diligent work by plecopterologists, GBIF will become an important source of stonefly data in the future. To this end, we support the mission of GBIF and iDigBio by providing our data in Darwin Core Archive format from the INHS portal and through an archived data set (
Some state water quality agencies support robust biological monitoring programs where well trained aquatic macroinvertebrate taxonomists are employed and where specimens are vouchered to ensure replicability of the science conducted. Unfortunately, there are many governments that either do not have the resources or believe this level of work is unnecessary to achieve their goals. These organizations only meet the objectives of determining attainment of use designations and adherence to permit regulations. Alternatively, because the OEPA hires qualified taxonomists and vouchers specimens, they also meet the additional objectives of providing data for biodiversity analyses and conservation status assessment. The OEPA has earned a lofty reputation because they set the standard for the water quality monitoring community.
Conclusions
This work culminates at least 91 years (
Acknowledgements
We are grateful to the Ohio Department of Natural Resources, Division of Wildlife, Wildlife Diversity and Endangerment Species Program who funded the Ohio Biological Survey to conduct extensive survey work from 1993-2000 that resulted in a large collection of stoneflies. Other agencies, corporations, and individuals who were integral to this survey work were acknowledged in Armitage et al. (2011). We also acknowledge partial funding from the National Science Foundation (DEB 09–18805 ARRA, DBI CSBR 14-58285) that supported digitization of these records. We extend warm thanks to colleagues & collection managers for loaning specimens, these include: Ian Smith (CNC), Gregory Dahlem (CNHM), Joe Keiper (formerly CLEV), Daniel Summers (formerly FMNH), Gary Parsons (MSUC), Bob Glotzhober (OHSC), Norm Johnson (OSUC), Arwin Provonsha (PERC), Fred Kirchner (RFKC), Antonia Guidotti (ROME), Jay McPherson (SIUC), and Mark O’Brien (UMMZ). Chris Bedel of Edge of Appalachia Preserve kindly provided housing during work on the preserve. Heather Stehle of Crane Hollow Nature Preserve provided funding for the identification and digitization of the Gary A. Coovert collection of stoneflies. Joe Moosbrugger of Crane Hollow Preserve was instrumental in sharing field notes and answering questions about them. Undergraduates Elise Snyder and Alex Nelson transcribed the field notes and digitized specimens for the Crane Hollow work. We cannot list the names of all who participated in the collection of stoneflies from 1880 until 2016—there are over 275 unique names. Without them this project would not have been possible.
Funding program
USA National Science Foundation, Division of Environmental Biology, through American Recovery and Reinvestment Act of 2009 funds.
USA National Science Foundation, Division of Biological Infrastructure, Collections in Support of Biological Research funds.
Author contributions
DeWalt conceived of the project, collected and identified specimens, digitized data, conducted analyses, wrote text.
Grubbs collected and identified specimens, conducted analyses, wrote text.
Armitage was instrumental in initial conception of the project in the 1990s, collected specimens, conducted analyses, produced maps, wrote text.
Baumann was instrumental in initial conception of project in 1990s, collected many specimens, wrote text.
Clark was instrumental in initial conception of project in 1990s, collected many specimens, wrote text.
Bolton headed statewide sampling effort through Ohio Environmental Projection Agency, collected and identified many specimens, and digitized material.
References
- Acme Mapper 2.1. http://mapper.acme.com. Accessed on: 2016-6-20.
- List of Ohio Counties. https://en.wikipedia.org/wiki/List_of_counties_in_Ohio. Accessed on: 2016-7-22.
- Atlas of Ohio Aquatic Insects, Volume I. Trichoptera.Ohio Biological Survey Miscellaneous Contribution13:1‑88.
- What is Alloperla imbecilla (Say)? Designation of a neotype, and a new Alloperla from eastern North America (Plecoptera: Chloroperlidae).Proceedings of the Biological Society of Washington87:257‑264. URL: http://www.biodiversitylibrary.org/page/34672952
- Plecoptera of the Ohio River: community composition and species phenologies of nymphs collected near Cincinnati, Ohio.Ohio Journal of Science87:41‑45. URL: https://kb.osu.edu/dspace/bitstream/1811/23183/1/V087N1_041.pdf
- Species loss of stoneflies (Plecoptera) in the Czech Republic during the 20th century.Freshwater Biology57(12):2550‑2567. https://doi.org/10.1111/fwb.12027
- New state records of aquatic insects for Ohio, U.S.A. (Ephemeroptera, Plecoptera, Trichoptera, Coleoptera).Entomological News121(1):75‑90. https://doi.org/10.3157/021.121.0115
- Using Maxent to model the historic distributions of stonefly species in Illinois streams: The effects of regularization and threshold selections.Ecological Modelling259:30‑39. https://doi.org/10.1016/j.ecolmodel.2013.03.012
- The external morphology of Acroneuria evoluta Klapálek (Perlidae, Plecoptera).Ohio Journal of Science34:121‑128. URL: https://kb.osu.edu/dspace/bitstream/1811/2682/1/V34N02_121.pdf
- Updates to the stonefly fauna of Illinois and Indiana.Illiesia7:31‑50. URL: http://illiesia.speciesfile.org/papers/Illiesia07-03.pdf
- Ephemeroptera, Plecoptera, and Trichoptera on Isle Royale National Park, USA, compared to mainland species pool and size distribution.ZooKeys532:137‑158. https://doi.org/10.3897/zookeys.532.6478
- Life histories of stoneflies (Plecoptera) in the Rio Conejos of southern Colorado.The Great Basin Naturalist.55:1‑18. https://doi.org/10.5962/bhl.part.22804
- Just how imperiled are aquatic insects? a case study of stoneflies (Plecoptera) in Illinois.Annals of the Entomological Society of America98:941‑950. https://doi.org/10.1603/0013-8746(2005)098[0941:jhiaai]2.0.co;2
- Plecoptera Species File Online. Version 5.0/5.0. http://Plecoptera.SpeciesFile.org. Accessed on: 2016-8-31.
- Ohio Plecoptera Atlas. v1.0. Biodiversity Data Journal. Dataset/Occurrence.http://ipt.pensoft.net/resource?r=ohioplecopteraatlas&v=1.0. Accessed on: 2016-11-14.
- Ohio USA stoneflies (Insecta, Plecoptera): species richness estimation, distribution of functional niche traits, drainage affiliations, and relationships to other states.ZooKeys178:1‑26. https://doi.org/10.3897/zookeys.178.2616
- Emergence patterns and an assessment of collecting methods for adult stoneflies (Plecoptera) in an Ozark foothills stream.Canadian Journal of Zoology63:2962‑2968.
- Comparing the Ephemeroptera and Plecoptera specimen databases at the Illinois Natural History Survey and using them to document changes in the Illinois fauna.Annals of the Entomological Society of America95:35‑40.
- Stoneflies (Plecoptera) in Gray's Run in northeastern Ohio.Ohio Journal of Science87:67‑72. URL: https://kb.osu.edu/dspace/bitstream/1811/23197/1/V087N3_067.pdf
- Studies of North American Plecoptera, with special reference to the fauna of Illinois.Illinois Natural History Survey Bulletin22:235‑355. URL: http://www.biodiversitylibrary.org/page/14780275
- Nymphs of the stonefly genus Taeniopteryx (Plecoptera: Taeniopterygidae) of North America.Journal of the Kansas Entomological Society53:237‑259. URL: http://www.jstor.org/stable/25084028
- An annotated checklist of the stoneflies of Ohio (Plecoptera).Ohio Journal of Science56:321‑324. URL: http://https://kb.osu.edu/dspace/bitstream/1811/4394/1/V56N06_321.pdf
- Soyedina alexandria and S. calcarea (Plecoptera: Nemouridae), new stonefly species from the eastern Nearctic region and notes on the life cycle of S. calcarea.Illiesia2:39‑49. URL: http://illiesia.speciesfile.org/papers/Illiesia02-06.pdf
- Leuctra schusteri, a new stonefly species (Plecoptera: Leuctridae) of the L. tenuis (Pictet) group from the southeastern USA.Illiesia11:147‑166. URL: http://illiesia.speciesfile.org/papers/Illiesia11-12.pdf
- Taxonomic and distributional notes on Perlesta teaysia, P. golconda, and P. shawnee (Plecoptera: Perlidae).Illiesia4:143‑149. URL: http://illiesia.speciesfile.org/papers/Illiesia04-14.pdf
- Perlesta ephelida, a new Nearctic stonefly species (Plecoptera, Perlidae).ZooKeys194:1‑15. https://doi.org/10.3897/zookeys.194.2972
- Notes on Perlesta (Plecoptera: Perlidae) from Eastern North America.Aquatic Insects23:119‑122. URL: http://www.tandfonline.com/doi/abs/10.1076/aqin.23.2.119.4915
- Michigan Plecoptera (Stoneflies): distribution patterns and an updated species list.Illiesia8:162‑173. URL: http://illiesia.speciesfile.org/illiesia/papers/Illiesia08-18.pdf
- Distribution patterns of Ohio stoneflies, with an emphasis on rare and uncommon species.Journal of Insect Science13:72. URL: http://www.insectscience.org/13.72
- A review of the Nearctic genus Prostoia (Ricker) (Plecoptera, Nemouridae), with the description of a new species and a surprising range extension for P. hallasi Kondratieff & Kirchner.ZooKeys401:11‑30. https://doi.org/10.3897/zookeys.401.7299
- A review of the Nearctic genus Zealeuctra Ricker (Plecoptera, Leuctridae), with the description of a new species from the Cumberland Plateau region of eastern North America.ZooKeys344:17‑47. https://doi.org/10.3897/zookeys.344.5912
- Geology of the Hocking Hills State Park Region.Ohio Department of Natural Resources, Division of Geological Survey, Guidebook4:1‑23.
- The Teays River, GeoFacts No. 10, Ohio Department of Natural Resources, Division of Geological Survey. https://geosurvey.ohiodnr.gov/portals/geosurvey/PDFs/GeoFacts/geof10.pdf. Accessed on: 2016-9-26.
- Wet Collections Digitization Workshop. https://www.idigbio.org/wiki/images/8/83/Digitization_Workflows_wet_collections.pdf. Accessed on: 2016-8-01.
- Documentation-Data Ingestion. https://www.idigbio.org/wiki/images/8/83/Digitization_Workflows_wet_collections.pdf. Accessed on: 2016-8-01.
- The Black Swamp: a study in historical geography.Annals of the Association of American Geographers45(1):1‑35. https://doi.org/10.1111/j.1467-8306.1955.tb01481.x
- Teays-age drainage effects on present distributional patterns of Ohio biota.Ohio Biological Survey Informative Circular11:1‑14.
- Kondratieff BC (2004) Perlodidae. Perlodinae (the springflies). pp. 149-190. In: Stark BP, Armitage BJ (Eds) Stoneflies (Plecoptera) of Eastern North America. Volume II. Chloroperlidae, Perlidae, Perlodidae (Perlodinae).Ohio Biological Survey Bulletin New Series 14(4),1-192pp.
- A Reclarification of the males of Alloperla concolor and A. neglecta Species.Entomological News104:73‑78. URL: http://www.biodiversitylibrary.org/page/2672847#page/85/mode/1up
- Kondratieff BC, Kirchner RF (2009) A new species in the Sweltsa onkos complex (Plecoptera: Chloroperlidae). pp. 295-300. In: Roble SM, Mitchell JC (Eds) A Lifetime of Contributions to Myriapodology and the Natural History of Virginia: A Festschrift in Honor of Richard L. Hoffman’s 80th Birthday.Virginia Museum of Natural History Special Publication 16,1-458pp.
- A Review of Perlinella Banks (Plecoptera: Perlidae).Annals of the Entomological Society of America81(1):19‑27. https://doi.org/10.1093/aesa/81.1.19
- Mehrhoff LJ (1997) Museums, research collections, and the biodiversity challenge, pp. 447-465. In: Reaka-Kukla ML, Wilson DE, Wilson EO (Eds) Biodiversity II.Joseph Henry Press,Washington, DC.
- A monograph of the Plecoptera or stoneflies of America north of Mexico.The Thomas Say Foundation 11,1-397pp. https://doi.org/10.5962/bhl.title.6826
- Nelson CH (2000) Pteronarcyidae (The Salmonflies). pp. 29-39. In: Stark BP, Armitage BP (Eds) Stoneflies (Plecoptera) of Eastern North America, Volume I, Pteronarcyidae, Peltoperlidae, and Taeniopterygidae.Ohio Biological Survey Bulletin New Series 14(1),1-99pp.
- Refugia and postglacial expansion of Acroneuria frisoni Stark & Brown (Plecoptera:Perlidae) in North America.Freshwater Science33(1):232‑249. https://doi.org/10.1086/675306
- Systematic studies in Plecoptera.Indiana University Publications Science Series18:1‑200.
- North American species of Taeniopteryx (Plecoptera, Insecta).Journal of the Fisheries Research Board of Canada25:1423‑1439.
- The genus Zealeuctra and its position in the family Leuctridae (Plecoptera, Insecta).Canadian Journal of Zoology47:1113‑1127. URL: http://www.nrcresearchpress.com/doi/pdf/10.1139/z69-176
- An annotated list of stoneflies (Plecoptera) from Penitentiary Glen, Lake County, Ohio.The Great Lakes Entomologist12:225. URL: http://michentsoc.org/gle-pdfs/vol12no4.pdf
- The aquatic insect community in Penitentiary Glen, a Portage Escarpment stream in northeastern Ohio.Ohio Journal of Science84:113‑119. URL: https://kb.osu.edu/dspace/bitstream/1811/23006/1/V084N3_113
- New species of winter stoneflies, genus Allocapnia (Plecoptera, Capniidae).Transactions of the Illinois State Academy of Sciences57:88‑93.
- The Classification, Evolution, and Dispersal of the Winter Stonefly Genus Allocapnia.Illinois Biological Monographs45:1‑166. URL: http://www.biodiversitylibrary.org/bibliography/50280#/summary
- Variations in the winter stonefly Allocapnia granulata as indicators of Pleistocene faunal movements.Annals of the Entomological Society of America60:447‑458. URL: http://aesa.oxfordjournals.org/content/60/2/447.full.pdf
- Postglacial colonization of Canada by its subboreal winter stoneflies of the genus Allocapnia.Canadian Entomologist99:703‑712. URL: https://www-cambridge-org.proxy2.library.illinois.edu/core/journals/canadian-entomologist/article/postglacial-colonization-of-canada-by-its-subboreal-winter-stoneflies-of-the-genus-allocapnia1/340A6F4A331D168CAEE0C60A28F1715A
- On natural history collections, digitized and not: a response to Ferro and Flick.ZooKeys618:145‑158. https://doi.org/10.3897/zookeys.618.9986
- The Nearctic species of Agnetina (Plecoptera: Perlidae).Journal of the Kansas Entomological Society59:437‑445.
- Perlesta placida (Hagen), an eastern Nearctic species complex (Plecoptera: Perlidae).Entomologica Scandinavica20:263‑286. URL: http://booksandjournals.brillonline.com/content/journals/10.1163/187631289x00339
- Stark BP (2000) Peltoperlidae (the roachflies). pp. 41-54. In: Stark BP, Armitage BJ (Eds) Stoneflies (Plecoptera) of Eastern North America, Volume I, Pteronarcyidae, Peltoperlidae, and Taeniopterygidae.Ohio Biological Survey Bulletin New Series 14(1),1-99pp.
- Stark BP (2004) Perlidae (the stones). pp. 61-148. In: Stark BP, Armitage BJ (Eds) Stoneflies (Plecoptera) of Eastern North America. Volume II. Chloroperlidae, Perlidae, Perlodidae (Perlodinae).Ohio Biological Survey Bulletin New Series 14(4),1-192pp.
- New species of Nearctic Neoperla (Plecoptera: Perlidae), with notes on the genus.Great Basin Naturalist38:97‑114. URL: https://ojs.lib.byu.edu/spc/index.php/wnan/issue/view/2200
- The winter stonefly genus Paracapnia (Plecoptera: Capniidae).Monographs of the Western North American Naturalist2:96‑108. URL: http://www.bioone.org/doi/pdf/10.3398/1545-0228-2.1.96
- The Genus Diploperla (Plecoptera: Perlodidae).Journal of the Kansas Entomological Society47:433‑436. URL: http://www.jstor.org/stable/25082676
- The Nearctic species of Acroneuria (Plecoptera: Perlidae).Journal of the Kansas Entomological Society49:221‑253. URL: http://www.jstor.org/stable/25082817
- Larvae of eight eastern Nearctic Alloperla Species (Plecoptera: Chloroperlidae).Illiesia6:267‑276. URL: http://illiesia.speciesfile.org/papers/Illiesia06-20.pdf
- Epiproct and dorsal process structure in the Allocapnia forbesi Frison, A. pygmaea (Burmeister), and A. rickeri Frison species groups (Plecoptera: Capniidae), and inclusion of A. minima (Newport) in a new species group.Illiesia8:45‑77. URL: http://illiesia.speciesfile.org/papers/Illiesia08-05.pdf
- Egg morphology and phylogeny in Pteronarcyidae (Plecoptera).Annals of the Entomological Society of America75(0):519‑529. https://doi.org/10.1093/aesa/75.5.519
- The Cultus decisus complex of eastern North America (Plecoptera: Perlodidae).Proceedings of the Entomological Society of Washington90:91‑96. URL: http://www.biodiversitylibrary.org/part/54364
- Stewart KW (2000) Taeniopterygidae (the willowflies). pp. 55-88. In: Stark B, Armitage B (Eds) Stoneflies (Plecoptera) of Eastern North America, Volume I, Pteronarcyidae, Peltoperlidae, and Taeniopterygidae.14.Ohio Biological Survey Bulletin New Series,1-99pp.
- Nymphs of North American Stonefly Genera (Plecoptera).Caddis Press,Columbus, Ohio,510pp.
- Surdick R (2004) Chloroperlidae (the sallflies). pp. 1-60. In: Stark B, Armitage B (Eds) Stoneflies (Plecoptera) of Eastern North America. Volume II. Chloroperlidae, Perlidae, Perlodidae (Perlodinae).Ohio Biological Survey Bulletin New Series,1-192pp.
- A Review of the Eastern Nearctic Isoperlinae (Plecoptera: Perlodidae) with the description of twenty-two new species.Monographs of Illiesia1:1‑289. URL: http://illiesia.speciesfile.org/papers/Monographiae-of-Illiesia.pdf
- Isoperla bilineata: designation of a neotype and allotype, and further descriptions of egg and nymph.Annals of the Entomological Society of America71:212‑217. https://doi.org/10.1093/aesa/71.2.212
- Reevaluation of the Genus Clioperla.Annals of the Entomological Society of America74:563‑569. https://doi.org/10.1093/aesa/74.6.563
- Tkac MA (1979) The Plecoptera of Northeastern Ohio. PhD Dissertation.Kent State University,Kent, Ohio,229pp.
- Annotated list of stoneflies (Plecoptera) from Stebbins Gulch in northeastern Ohio.The Great Lakes Entomologist11:139‑142. URL: http://michentsoc.org/gle-pdfs/vol11no3.pdf
- The Teays River.The Ohio Journal of Science46:297‑307. URL: https://kb.osu.edu/dspace/bitstream/1811/3568/1/V46N06_297.pdf
- A list of the stoneflies, Plecoptera, known to occur in southeastern Ohio.Ohio Journal of Science47:134‑136. URL: https://kb.osu.edu/dspace/bitstream/1811/5760/1/V47N03_134.pdf
- The effect of dispersal ability in winter and summer stoneflies on their genetic differentiation.Ecological Entomology32(4):399‑404. https://doi.org/10.1111/j.1365-2311.2007.00881.x
- Colonization of Lake Erie tributaries by Allocapnia recta (Capniidae).Illiesia11:41‑50. URL: http://illiesia.speciesfile.org/papers/Illiesia11-05.pdf
- Description of male Ostrocerca Ricker (Plecoptera: Nemouridae) using the scanning electron microscope.Proceedings of the Entomological Society of Washington91:257‑268. URL: http://www.biodiversitylibrary.org/page/16134999#page/267/mode/1up
- Notes on the genus Perlinella and a generic synonymy in North American Perlidae (Plecoptera).The Florida Entomologist54:315. https://doi.org/10.2307/3493593
Supplementary material
All known references were tracked beginning in 1925 with the first work to mention stoneflies from Ohio. Names used and reconciliation of names presented. A total of 53 references, denoted as microcitations (full references in text), are presented within this pdf document.