|
Biodiversity Data Journal :
Taxonomic paper
|
Report on the occurrence of synanthropic derived form of Chrysomya megacephala (Diptera: Calliphoridae) from Royapuram fishing harbour, Chennai, Tamil Nadu, India
|
Corresponding author:
Academic editor: Pierfilippo Cerretti
Received: 13 May 2014 | Accepted: 25 Jun 2014 | Published: 26 Jun 2014
© 2014 Paulchamy Ramaraj, Chellappa Selvakumar, Arumugam Ganesh, Sundaram Janarthanan
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Ramaraj P, Selvakumar C, Ganesh A, Janarthanan S (2014) Report on the occurrence of synanthropic derived form of Chrysomya megacephala (Diptera: Calliphoridae) from Royapuram fishing harbour, Chennai, Tamil Nadu, India. Biodiversity Data Journal 2: e1111. https://doi.org/10.3897/BDJ.2.e1111
|
|
Abstract
The occurrence of dipteran fly, Chrysomya megacephala (Fabricius, 1794) is reported for the first time from Royapuram fishing harbour (Chennai), Tamil Nadu, South East India. The fully grown third instar larvae of C. megacephala were collected from decaying fishes near Royapuram fishing harbour. This site is found to be the regular breeding site for C. megacephala. Larvae were reared under laboratory condition and freshly emerged adult flies from pupae were collected and identified by morphological features and molecular tools. Molecular identification through generation of DNA barcoding using mitochondrial COI gene of C. megacephala is appended.
Keywords
Blowfly, Molecular identification, DNA barcoding, India
Introduction
Calliphoridae is a cosmopolitan group of calyptrate flies comprising nearly 1500 recognized species worldwide (
Chrysomya megacephala (Fabricius, 1794) is commonly found in cadavers in many parts of the world (
Presently, three forms of C. megacephala are recognized, namely, the normal form (nf), the synanthropic derived form (sdf) and the recently reported feral derived form (fdf) (
However, the occurrence of the synanthropic derived form of C. megacephala has not been documented in the State of Tamil Nadu, India. In this context, the present study reports for the first time the synanthropic derived form of C. megacephala from Royapuram fishing harbour, Chennai, Tamil Nadu, South East India and provides key characters based on morphological features and molecular analysis.
Materials and methods
Collection, rearing and morphological identification
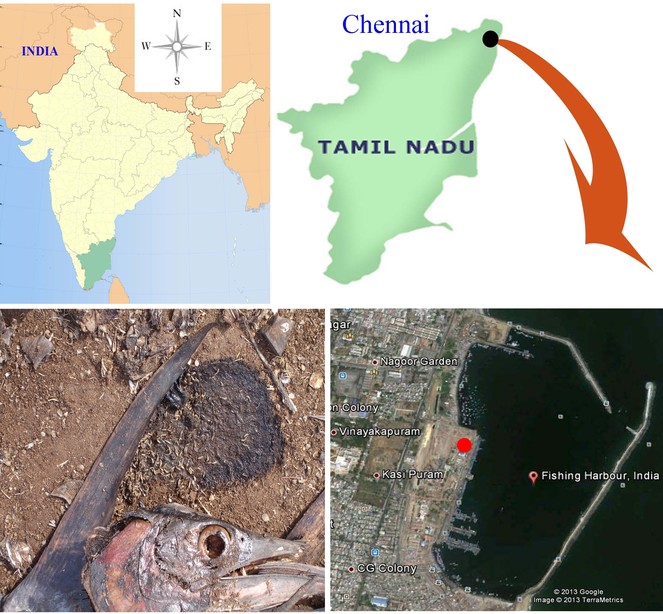
A colony of C. megacephala was established from numerous second and third instar larvae collected from decaying fishes of Royapuram fishing harbour in North Chennai, Tamil Nadu, South India (Fig.
Morphological description
The procedure adopted to identify the morphological features and terminology used in the description were based on the previous reports of
Molecular identification
Genomic DNA was extracted from a single morphologically identified adult male and female species of C. megacephala (sdf) after removing the gut region as per the standard phenol/chloroform extraction protocol (
Taxon treatment
Chrysomya megacephala
- GenBank AB910389
- GenBank AB910390
- Barcode of Life SPLID033-14
- Barcode of Life SPLID013-13
-
taxonomicStatus: accepted; kingdom:Animalia; phylum:Arthropoda; class:Insecta; order:Diptera; family:Calliforidae; taxonRank:species; vernacularName:Oriental latrine fly; genus:Chrysomya; specificEpithet:megacephala; country:India; stateProvince:Tamil Nadu; municipality:Chennai; verbatimLocality:Royapuram fishing horbour; verbatimElevation:2 m; verbatimLatitude:13°07'44.73 N; verbatimLongitude:80°17'52.70 E; samplingProtocol:Hand picking; eventDate:2013-12-22; individualCount:25; sex:12 male, 13 female; catalogNumber:I/D 28: 3 male and I/D 29: 3 female; recordedBy:Dr. S. Janarthanan; disposition:Zoological Survey of India, Southern Regional Centre, Chennai, Tamil Nadu, India; identifiedBy:P. Ramaraj & C. Selvakumar; dateIdentified:29 Jan 2014; identificationReferences:Senior-White et al., 1940; identificationRemarks:Eye facets of upper two-thirds greately enlarged and sharply demarcated from small facets of lower third; institutionCode:University of Madras, Chennai
Diagnosis
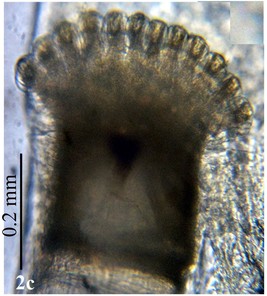
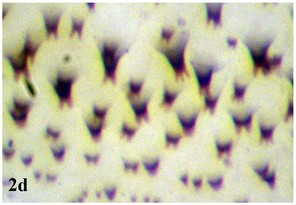
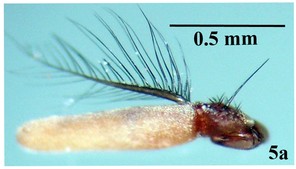
Larva: Fully mature third instar muscoid-shaped (11 mm length), composed of 12 segments with pointed anterior and blunt posterior end (Fig.
Larger in size than larvae; cylindrical in shape (Fig.
Male (Fig.
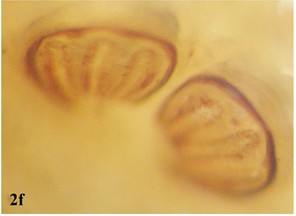
Head: Eye facets of upper two-thirds greatly enlarged and sharply demarked from small facets of lower third (Fig.
Female: (Fig.
Head: Eyes separated by one-quarter total width of head; facets uniformly small; frons clearly separated; widest part of frons more than width of ocellar triangle; frontal reddish to black, with small hairs on the upper part; frontal bristles short and weak; parafrontalia slightly narrower than width of frons, covered with golden tomentum, but appearing black towards vertex in certain lights; parafacilia yellowish brown with silvery pollen and white hairs (Fig.
The 651 bp mitochondrial cytochrome c oxidase subunit I (COI) sequences of C. megacephala (sdf) were 100% identical with available NCBI database sequences of C. megacephala using BLAST analysis. These mtCOI gene sequences of C. megacephala were submitted to NCBI database (Accession No. AB910389-male; AB910390-female) and DNA barcodes were generated for both the sexes based on their COI sequences using Barcode of Life Database (BOLD system; Process ID SPLID013-13 and SPLID033-14).
Distribution
South India – Tamil Nadu (Chennai); Kerala (Calicut); North India - West Bengal and rapidly throughout the continent.
Ecology
Life cycle: Second and third instar larvae of C. megacephala (sdf) were collected from decaying fishes of Royapuram fishing harbour of North Chennai, Tamil Nadu, South India. Adults emerged in the rearing chamber 7-9 d after pupation. Mating began 2 d after emergence and oviposition occurred at 3-4 d of age and laid egg mass on pieces of spoiled chicken in a squat cup. Each egg mass contained 200-300 eggs. Eggs held at 28°C hatched on 1 d. Second instars emerged 2 d after hatching, becoming 3rd instar 2 d later, and then pupation occurred 4-6 d.
Discussion
This study reports for the first time the occurrence of C. megacephala (sdf) in Tamil Nadu, India and provides, therefore, key identification characters of this blowfly based on distinct morphological features of larvae, pupae and adult males and females as well as molecular barcode analysis in the adults of both sexes. Chrysomya megacephala also known as the oriental latrine fly (
Acknowledgements
The authors thank the financial assistance received from UGC-SAP. C. Selvakumar thanks University Grants Commission, NewDelhi for the award of Dr. D. S. Kothari Post Doctoral Fellowship [No.F.4-2/2006(BSR) /13-670/2012 (BSR)].
Author contributions
All authors are equally contributed
References
- An updated checklist of blowflies (Diptera: Calliphoridae) from India.Halteres3:34‑37.
- Finding of feral derived form (fdf) of Chrysomya megacephala (Fabricius) from India with an evolutionary novelity (Diptera, Calliphoridae).Japan Journal of Systematic Entomology15:411‑413.
- First record of Chrysomya megacephala (Fabricius, 1794) (Diptera, Calliphoridae) from Portugal.Graellsia65(1):75‑77. https://doi.org/10.3989/graellsia.2009.v65.i1.139
- Seasonal patterns of arthropods occurring on sheltered and unsheltered pig carcasses in Buenos Aires Province (Argentina).Forensic Science International126:63‑70. https://doi.org/10.1016/S0379-0738(02)00037-3
- Key to the adults of the most common forensic species of Diptera in South America.Revista Brasileira de Entomologia52:390‑406. https://doi.org/10.1590/S0085-56262008000300012
- Diptera and Coleoptera of potential forensic importance in south eastern Brazil: relative abundance and seasonality.Medical and Veterinary Entomology11:8‑12. https://doi.org/10.1111/j.1365-2915.1997.tb00284.x
- Biology of Chrysomya megacephala (Diptera: Calliphoridae) and reduction of losses caused to the salted-dried fish industry in south-east Asia.Bulletin of Entomological Research81:33‑41. https://doi.org/10.1017/S0007485300053219
- DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates.Molecular Marine Biology and Biotechnology3:294‑299.
- Problems in estimation of postmortem interval resulting from wrapping of the corpse: a case study from Hawaii.Journal of Agricultural Entomology9:237‑243.
- Forensic Entomology in the Hawaiian Islands - Three case studies.American Journal of Forensic Medicine and Pathology8:45‑50. https://doi.org/10.1097/00000433-198703000-00011
- Estimation of postmortem interval by arthropod succession - Three case studies from the Hawaiian Islands.American Journal of Forensic Medicine and Pathology9:220‑225. https://doi.org/10.1097/00000433-198809000-00009
- Flies and Disease - Ecology, Classification and Biotic Associations.Princeton University Press, Princeton, New JerseyVol: 1:856 pp.
- Forensically important Calliphoridae (Diptera) associated with pig carrion in rural north-central Florida.Journal of Medical Entomology44:509‑515. https://doi.org/10.1603/0022-2585(2007)44[509:FICDAW]2.0.CO;2
- Geographical expansion of the range of Chrysomya blowflies.Transactions of the Royal Society of Tropical Medicine and Hygiene75:130‑131. https://doi.org/10.1016/0035-9203(81)90040-7
- Old World blowflies in the New World.Parisitology Today2:77‑79. https://doi.org/10.1016/0169-4758(86)90162-6
- Quantifying the potential pathogens transmission of the blowflies (Diptera: Calliphoridae).Memorias do Instituto Oswaldo Cruz98:213‑216. https://doi.org/10.1590/S0074-02762003000200008
- Rapid isolation of high molecular weight DNA.Nucleic Acids Research8:4321‑4325. https://doi.org/10.1093/nar/8.19.4321
- Checklist of Calliphoridae (Diptera) of India.Record of Zoological Survey of India, Occ. Paper231:1‑47.
- Seasonal occurrence of muscid, calliphorid and sarcophagid flies in Silguri, West Bengal, with a note on the identity of Musca domestica L.Oriental Insects9:351‑374. https://doi.org/10.1080/00305316.1975.10434505
- The Fauna of British India, including the remainder of the oriental region, Diptera, Family Calliphoridae.Taylor and Francis, LondonVol. VI:288 pp.
- A check list of blow flies (Diptera: Calliphoridae) from North-west of India.Uttar Pradesh Journal of Zoology24:63‑71.
- Two New species of Melinda Robineau-Desvoidy (Diptera:Calliphoridae) from India, with a key to the Indian species of this genus.Journal of Bombay Natural History Society104:55‑57.
- Notes on calliphorid flies (Diptera: Calliphoridae) from Sunderbans Biosphere Reserve and their impact on man and animals.Journal of Bombay Natural History Society101:415‑420.
- Morphology of the puparia of the housefly, Musca domestica (Diptera: Muscidae) and blowfly, Chrysomya megacephala (Diptera: Calliphoridae).Parasitology Research96:166‑170. https://doi.org/10.1007/s00436-005-1343-5
- A Manual of Forensic Entomology.The Trustees of the British Museum (Natural History), London.,205pp.
- Morphology and developmental rate of blowflies Chrysomya megacephala and Chrysomya rufifacies in Thailand: application in forensic entomology.Parasitology Research102:1207‑1216. https://doi.org/10.1007/s00436-008-0895-6
- Forensic entomology cases in Thailand: a review of cases from 2000 to 2006.Parasitology Research101(5):1417‑1423. https://doi.org/10.1007/s00436-007-0659-8
- Arthropod succession on exposed rabbit carrion in Alexandria, Egypt.Journal of Medical Entomology33:566‑580.
- The seasonal abundance of blowflies infesting drying fish in south-west India.Journal of Applied Ecology38:339‑348.
- Wang JF, Li ZG, Chen YC, Chen QS, Yin XH (2008) The succession and development of insects on pig carcasses and their significances in estimating PMI in south China. Forensic Science International 179: 11-18.
- Chrysomya megacephala (Diptera: Calliphoridae) has reached the continental United States: review of its biology, pest status, and spread around the world.Journal of Medical Entomology28:471‑473.
- Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae) development, rate, variation and the implications for forensic entomology.Japanese Journal of Sanitary Zoology45:303‑309.
- Myiasis in man and animals in the Old World: a textbook for physicians, veterinarians and zoologists.Butterworth,London, UK,267pp.