|
Biodiversity Data Journal :
Research Article
|
|
Corresponding author: Patricia Esther Corro-Chang (estherpatricia04@gmail.com)
Academic editor: Bong-Kyu Byun
Received: 05 Dec 2016 | Accepted: 14 Feb 2017 | Published: 02 Mar 2017
© 2017 Patricia Corro-Chang
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Corro-Chang P (2017) Behavioural notes and attraction on Lepidoptera around the Gehry's Biodiversity Museum (Causeway, Calzada de Amador, Panamá, República de Panamá). Biodiversity Data Journal 5: e11410. https://doi.org/10.3897/BDJ.5.e11410
|
|
Abstract
This work represents a study of the diversity of Lepidoptera on the Gehry's Biodiversity Museum (MBG), in an attempt to generate a list of the diversity of butterflies and moths visiting the coastal ecosystem of the Causeway, Amador. Collection of insects was performed manually, over a period of ten months (June 16, 2014 to March 18, 2015) and photographic records of the behavior of other species preying on Lepidoptera were also included. A total of 326 specimens, representing 13 families were collected. These included 6 butterfly and 8 moth families and 52 genera, and 60 species. This study represents a contribution to the knowledge of species that frequent the area, and encourages the conservation and development of the Calzada de Amador as an important touristic site in the city of Panama.
Keywords
Richness of species, abundance, butterflies, moths, Causeway.
Introduction
Materials and Methods
Study Area
This study was carried out in the botanical garden and near-by areas of the Gehry's Biodiversity Museum MBG (
Sampling and Preservation
Specimens were collected with entomological nets, killed by a soft pressure over the thorax, and stored in wax paper envelopes. All the biological material collected was stored at 0°C, in hermetic plastic boxes until it was processed in lab facilities at Programa Centroamericano de Maestría en Entomología (PCMENT), Universidad de Panamá. Each envelope contained the collection data label, including the names of the collectors and the dates. Observation and collection were done daily, and continuously during June of 2014 and March of 2005; between 08:00 to 13:00 hours near to the main entrance of the museum in a 50 m long transect that includes bushes and shrubs that attract butterflies, including Lantana camara, during sunny days without heavy rains or strong winds. Specimens were set with #1 and #2 entomological pins. Processing and spreading techniques for Lepidoptera samples were done following
Identification
Determinations were made by comparisons with the material previously deposited at the Programa Centroamericano de Maestría en Entomología (Universidad de Panamá) already identified by specialists, and by review of
Results and Discussion
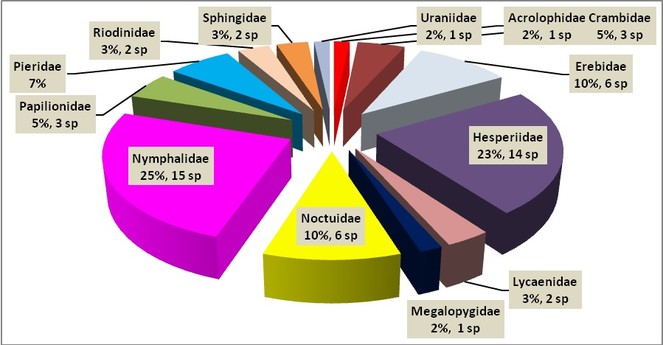
A total of 326 specimens, representing 13 families (6 butterfly and 8 moth families) distributed in 52 genera and 60 species were collected at the MBG (Table
|
Family |
Subfamily |
Tribe |
Species |
Common name (English)* |
|
Acrolophidae |
Acrolophus panamae Busck, 1914 |
Panama Grass Tube-worm Moth |
||
|
Crambidae |
Pyraustinae |
Herpetogramma phaeopteralis (Guenée, 1854) |
Sod Webworm Moth |
|
|
Crambidae |
Pyraustinae |
Spilomelini |
Palpita flegia (Cramer, 1777) |
|
|
Crambidae |
Pyraustinae |
Spilomelini |
Samea ecclesialis Guenée, 1854 |
|
|
Erebidae |
Arctiinae |
Calonotos metallicus Druce, 1884 |
||
|
Erebidae |
Arctiinae |
Arctiini |
Halysidota sp. |
|
|
Erebidae |
Arctiinae |
Arctiini |
Horama plumipes (Drury, 1773) |
Wasp moth |
|
Erebidae |
Arctiinae |
Arctiini |
Munona iridescens Schaus, 1894 |
|
|
Erebidae |
Spilosoma congrua (Walker, 1855) |
Agreeable Tiger Moth |
||
|
Erebidae |
Uranophora leucotelus (Butler, 1876) |
|||
|
Hesperiidae |
Hesperiinae |
Anthoptini |
Anthoptus epictetus (Fabricius, 1793) |
Trailside Skipper |
|
Hesperiidae |
Pyrginae |
Eudamini |
Astraptes fulgerator (Walch, 1775) |
Two-barred Flasher |
|
Hesperiidae |
Pyrginae |
Eudamini |
Astraptes talus (Cramer, 1777) |
Green Flasher |
|
Hesperiidae |
Pyrginae |
Pyrgini |
Bolla cupreiceps (Mabille, 1891) |
Copper-headed Sootywing |
|
Hesperiidae |
Hesperiinae |
Calpodini |
Carystoides lebbaeus (Hewitson, 1876) |
Lebbaeus Rubyeye |
|
Hesperiidae |
Pyrginae |
Pyrgini |
Nisoniades panama Evans, 1953 |
Panamanian Tufted- Skipper |
|
Hesperiidae |
Hesperiinae |
Moncini |
Parphorus nr. oeagrus (Godman, 1900) |
Tawny-washed Skipper |
|
Hesperiidae |
Pyrginae |
Pyrgini |
Pellicia arina Evans, 1953 |
Glazed Tufted- Skipper |
|
Hesperiidae |
Pyrginae |
Eudamini |
Phanus marshalli (W.F. Kirby, 1880) |
Common Ghost Skipper |
|
Hesperiidae |
Pyrginae |
Achlyodini |
Quadrus cerialis (Stoll, 1782) |
Common Blue- Skipper |
|
Hesperiidae |
Hesperiinae |
Calpodini |
Saliana esperi Evans, 1955 |
Saliana |
|
Hesperiidae |
Pyrginae |
Pyrgini |
Staphylus ascalaphus (Staudinger, 1876) |
Central American Sootywing |
|
Hesperiidae |
Pyrginae |
Eudamini |
Urbanus dorantes (Stoll, 1790) |
Dorantes Longtail |
|
Hesperiidae |
Pyrginae |
Eudamini |
Urbanus procne (Plötz, 1880) |
Brown Longtail |
|
Lycaenidae |
Theclinae |
Eumaeini |
Calycopis drusilla Field, 1967 |
Drusilla Groundstreak |
|
Lycaenidae |
Theclinae |
Eumaeini |
Magnastigma hirsuta (Prittwitz, 1865) |
Hirsuta Hairstreak |
|
Megalopygidae |
Megalopyginae |
Megalopyge lanata (Stoll, 1780) |
Mangrove Flannel Moth |
|
|
Noctuidae |
Catocalinae |
Anticarsia gemmatalis (Hübner, 1818) |
Velvetbean Caterpillar Moth |
|
|
Noctuidae |
Catocalinae |
Ascalapha odorata Linnaeus, 1758 |
Black Witch |
|
|
Erebidae |
Letis sp. |
Marbled Witch |
||
|
Noctuidae |
Plusiinae |
Noctuid sp. 1 |
||
|
Erebidae |
Catocalinae |
Noctuid sp. 2 |
||
|
Noctuidae |
Amphipyrinae |
Spodoptera sp. |
||
|
Nymphalidae |
Heliconiinae |
Heliconiini |
Agraulis vanillae (Linnaeus, 1758) |
Passion butterfly |
|
Nymphalidae |
Nymphalinae |
Kallimini |
Anartia fatima (Fabricius, 1793) |
Banded Peacok |
|
Nymphalidae |
Nymphalinae |
Kallimini |
Anartia jatrophae (Linnaeus, 1763) |
White Peacok |
|
Nymphalidae |
Nymphalinae |
Phyciodini |
Anthanassa frissia tulcis (H.W.Bates, 1864) |
Pale-banded Crescent |
|
Nymphalidae |
Brassolinae |
Brassolini |
Brassolis isthmia Bates, 1864 |
Small-spoted Owlet |
|
Nymphalidae |
Morphinae |
Brassolini |
Caligo atreus (Kollar, 1850) |
Atreus/ Banded Giant Owl |
|
Nymphalidae |
Nymphalinae |
Coeini |
Colobura dirce (Linnaeus, 1758) |
Dirce/Small Beauty |
|
Nymphalidae |
Danainae |
Danaini |
Danaus gillipus thersippus (H.W.Bates, 1863) |
Queen |
|
Nymphalidae |
Heliconiinae |
Heliconiini |
Dryadula phaetusa (Linnaeus, 1758) |
Banded Orange |
|
Nymphalidae |
Heliconiinae |
Heliconiini |
Dryas iulia (Fabricius, 1775) |
Julia |
|
Nymphalidae |
Heliconiinae |
Argynnini |
Euptoieta hegesia (Cramer, 1779) |
Mexican Fritillary |
|
Nymphalidae |
Biblidinae |
Biblidini |
Hamadryas laudamia (Cramer, 1777) |
Starry Night |
|
Nymphalidae |
Nymphalinae |
Kallimini |
Junonia evarete (Cramer, 1779) |
Tropical Buckeye |
|
Nymphalidae |
Brassolinae |
Brassolini |
Opsiphanes cassina C. Felder & R. Felder, 1862 |
Split-banded Owlet |
|
Nymphalidae |
Nymphalinae |
Kallimini |
Siproeta stelenes (Linnaeus, 1758) |
Malachite |
|
Papilionidae |
Papilioninae |
Troidini |
Battus polydamas (Linnaeus, 1758) |
Polydamas Swallowtail |
|
Papilionidae |
Papilioninae |
Papilionini |
Heraclides rumiko (Shiraiwa & Grishin, 2014) |
Western-giant Swallowtail |
|
Papilionidae |
Papilioninae |
Troidini |
Parides anchisiades farfan K.S. Brown, 1994 |
Anchisiades Cattleheart |
|
Pieridae |
Coliadinae |
Coliadini |
Eurema arbela gratiosa (Doubleday, 1847) |
Disjunct Yellow |
|
Pieridae |
Coliadinae |
Coliadini |
Eurema daira eugenia (Wallengren, 1860) |
Barred Yellow/ Barred sulphur |
|
Pieridae |
Coliadinae |
Coliadini |
Phoebis argante (Fabricius, 1775) |
Apricot Sulphur |
|
Pieridae |
Coliadinae |
Coliadini |
Phoebis sennae (Linnaeus, 1758) |
Cloudless Sulphur |
|
Riodinidae |
Theclinae |
Eumaeini |
Eumaeus godartii (Boisduval, 1870) |
White-tipped Cycadian |
|
Riodinidae |
Riodininae |
Riodinini |
Melanis pixe sanguinea (Stichel, 1910) |
Red-bordered Pixie |
|
Sphingidae |
Macroglossinae |
Dilophonotini |
Isognathus scyron (Cramer, 1780) |
|
|
Sphingidae |
Sphinginae |
Sphingini |
Manduca rustica rustica (Fabricius, 1775) |
Rustic Sphinx |
|
Uraniidae |
Uraniinae |
Urania fulgens (Walker, 1854) |
Urania Swallowtail |
The confection of the present checklist (Table
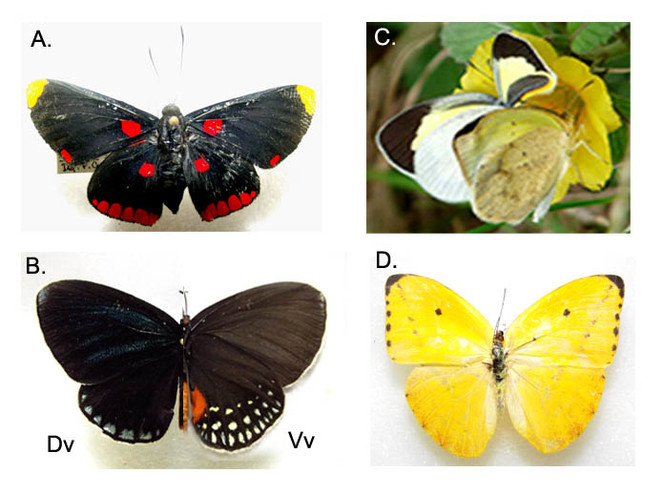
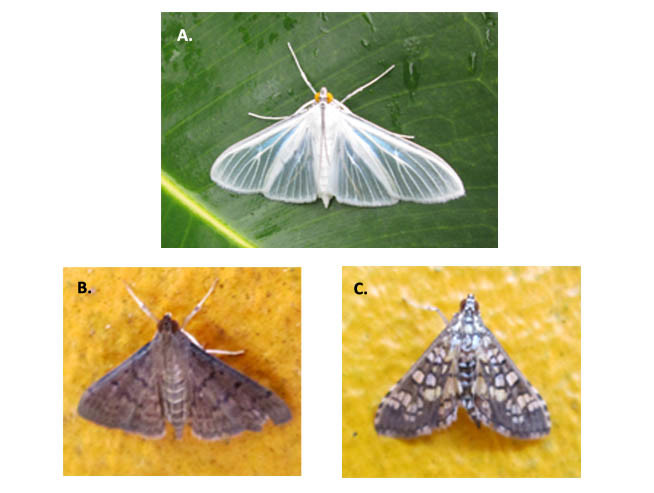
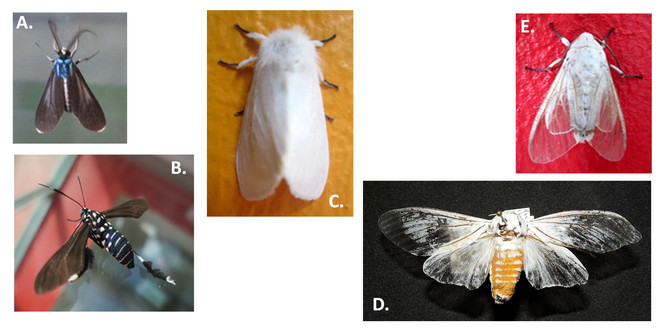
Some notes related to behavior and interaction patterns were registered over the colored surfaces of the building (Suppl. materials
Conclusion
The coastal ecosystem of Amador has a rich Lepidoptera fauna, besides the strong human intervention in this area; species exploit the various sources that the MBG offers. The addition of colors, a botanical garden and shapes on this construction serve as attractants for day active species and variety of interactions. This work represents a preliminary contribution to our knowledge of the species of Lepidoptera active in the Calzada de Amador, and will answer many of the questions asked by visitors interested in the fauna of this important tourist point of Panamá, thus promoting its conservation.
Acknowledgements
The author would like to express her sincere appreciation to Programa Centroamericano de Maestría en Entomología, Universidad de Panamá (Panamá) for supporting with lab facilities and collections in order to do the determinations of the sampled material. Thanks are also extended to Annette Aiello, Smithsonian Tropical Research Institute (Panamá) for confirming the identification of some specimens and corrections on the manuscript; to the personnel at the MBG, Amador for their kind assistance in collecting specimens and facilities. Marina Leccese, Smithsonian Institution, Punta Culebra Facilities (Panamá), for sharing literature about Fort Amador history.
References
- Ahlén I, Bach L, Baagøe HJ, Pettersson J (2007) Bats and offshore wind turbines studied in southern Scandinavia.Swedish Environmental Protection Agency,37pp.
- New genera and species of Microlepidoptera from Panama.Proceedings of the United States National Museum47(2043):1‑67. https://doi.org/10.5479/si.00963801.47-2043.1
- The Butterflies of Costa Rica and their Natural History.Princeton University Press,327pp.
- Report on the Lepidoptera of the Smithsonian Biological Survey of the Panama Canal Zone.Proceedings of the United States National Museum47(2050):139‑350. https://doi.org/10.5479/si.00963801.47-2050.139
- Butterflies of Central America: A Photographic Checklist of Common species. Vol.1: Papilionidae, Hesperiidae & Nymphalidae.I.RiCalé Publishing,Texas, USA,304pp.
- Butterflies of Central America: A Photographic Checklist of Common species. Vol.3: Hesperiidae, The Skippers.III.RiCalé Publishing,Texas, USA,288pp.
- Butterflies of Central America: A Photographic Checklist of Common species. Vol. 2: Lycaenidae & Riodinidae: The Hairstreaks and Metalmarks.II.RiCalé Publishing,Texas, USA,235pp.
- Atlas of Neotropical Lepidoptera, Checklist: Part 2 Hyblaeoidea, Pyraloidea, Tortricoidea. Association for Tropical Lepidoptera.2.Scientific Publishers,Gainesville, Florida,243pp.
- Atlas of Neotropical Lepidoptera, Checklist: Part 4B Drepanoidea-Bombycoidea-Sphingoidea. Association for Tropical Lepidoptera.IV.Scientific Publishers,Gainesville, Florida,87pp.
- A Study of Bird and Bat Collision Fatalities at the Montaineer Wind Energy Center, Tucker County, West Virginia: Annual Report for 2003.FPL Energy and Mountaineer Wind Energy Center Technical Review Committee.39pp.
- Korytkowski C (2013) Orden Lepidoptera. Manual de Sistemática de Insectos.Programa Centroamericano de Maestría en Entomología, Universidad de Panama,Panama,49pp. [InSpanish].
- of Neotropical Lepidoptera, Checklist: 4A Hesperioidea, Papilionoidea.Scientific Publishers,Gainesville, Florida,439pp.
- Insect attraction to wind turbines: does colour play a role?European Journal of Wildlife Research57(2):323‑331. https://doi.org/10.1007/s10344-010-0432-7
- Social Benefits Of Ecotourism: The Monarch Butterfly Reserve In Mexico.Enlightening Tourism. A Pathmaking Journal3(2):105‑124. URL: http://www.uhu.es/publicaciones/ojs/index.php/et/article/view/1733
- Piorkowski MD (2006) Breeding bird habitat use and turbine collisions of birds and bats located at a wind farm in Oklahoma mixed-grass prairie.MSc. Thesis, Oklahoma State University,Oklahoma,112pp. URL: http://www.batsandwind.org/pdf/Piorkowski_2006.pdf.
- Smith N (1983) Urania fulgens (Calipato Verde, Green Urania). In: Janzen DH (Ed.) Costa Rican Natural History.University of Chicago Press,Chicago,816pp.
- Borror and DeLong's lntroduction to the Study of lnsects.7.Thomson Brooks Cole,USA,864pp.