|
Biodiversity Data Journal :
Taxonomic Paper
|
|
Corresponding author:
Academic editor: Pavel Stoev
Received: 18 Oct 2015 | Accepted: 31 Mar 2016 | Published: 04 Apr 2016
© 2016 Wison Costa, Pedro Amorim, José Leonardo Mattos
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Costa WJEM, Amorim PF, Mattos JLO (2016) A new species of inseminating seasonal killifish of the Cynopoecilus melanotaeniacomplex from southern Brazil (Cyprinodontiformes: Rivulidae). Biodiversity Data Journal 4: e6888. https://doi.org/10.3897/BDJ.4.e6888
|
|
Abstract
Background
The Cynopoecilus melanotaenia complex is a morphologically homogeneous killifish group, endemic from an area encompassing southern Brazil and northeastern Uruguay. It presently comprises four valid species: C. melanotaenia, the type species of the genus, and C. fulgens, C. intimus, and C. nigrovittatus.
New information
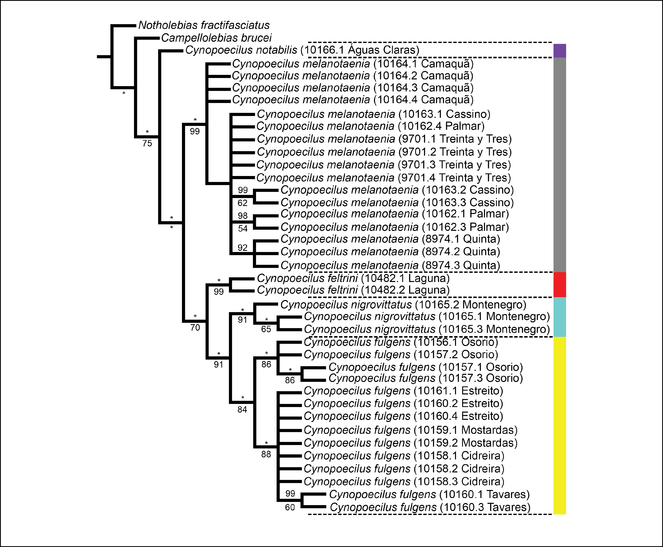
Cynopoecilus feltrini, n. sp., from the lower Tubarão river basin, southern Brazil, is distinguished from all congeners of the C. melanotaenia complex by having frontal E-scales medially overlapped, branchiostegal region orangish red in males and dorsum with few dark brown spots above opercular region. A phylogenetic tree derived from the analysis of a fragment of the mitochondrial gene cytochrome c oxidase subunit I (681 bp) indicates that C. feltrini is a member of the clade that includes all species of the C. melanotaenia complex except C. melanotaenia, as well as that C. feltrini is the sister group of a clade comprising C. fulgens and C. nigrovittatus.
Keywords
Aplocheiloid killifishes, Internal fertilization, Molecular phylogeny, Neotropical region, Systematics
Introduction
Killifishes of the tribe Cynopoecilini (Order Cyprinodontiformes, Family Rivulidae) comprise a well corroborated clade endemic of South America, between eastern Brazil and north-eastern Uruguay (
Cynopoecilus is diagnosed, among other characters, by a morphological apparatus on the male anal fin related to insemination that is unique among killifishes (
During the 90 years following its first description by
More recently, collecting trips provided large new collections making possible a detailed revision of the C. melanotaenia complex, which is presently in progress. Among the new findings is a new species herein first described after evidence from morphology and mitochondrial DNA.
Materials and methods
Material is deposited in the ichthyological collection of the Institute of Biology, Universidade Federal do Rio de Janeiro, Rio de Janeiro (UFRJ). Specimens were euthanized just after collection in a buffered solution of ethyl-3-amino-benzoat-methansulfonat (MS-222) at a concentration of 250 mg/l, for a period of 10 minutes or more, until completely ceasing opercular movements. Specimens fixed in formalin just after euthanasia for a period of 10 days, and then transferred to 70 % ethanol; specimens used in the molecular analysis were fixed in 98 % ethanol just after euthanasia and later preserved in the same fixative. List of specimens and respective GenBank accession numbers appear in Table
List of species, localities and respective catalogue numbers and GenBank accession numbers.
| Species | Catalog number | Locality | GenBank (COX1) |
| Notholebias fractifasciatus | |||
| UFRJ 8802.1 | Inoã | KT590062 | |
| (22°55'21"S, 42°55'42"W) | |||
| Campellolebias brucei | |||
| UFRJ 8383 | Florianópolis | KT590065 | |
| (27°40'59"S, 48°33'38"W) | |||
| Cynopoecilus melanotaenia | |||
| UFRJ 8974.1 | Quinta | KT590066 | |
| (32°04'13"S, 52°15'49"W) | |||
| UFRJ 8974.2 | Quinta | KT823646 | |
| (32°04'13"S, 52°15'49"W) | |||
| UFRJ 8974.3 | Quinta | KT823647 | |
| (32°04'13"S, 52°15'49"W) | |||
| UFRJ 9701.1 | Treinta y Tres | KT823648 | |
| (32°45'57"S, 53°44'09"W) | |||
| UFRJ 9701.2 | Treinta y Tres | KT823649 | |
| (32°45'57"S, 53°44'09"W) | |||
| UFRJ 9701.3 | Treinta y Tres | KT823650 | |
| (32°45'57"S, 53°44'09"W) | |||
| UFRJ 9701.4 | Treinta y Tres | KT823651 | |
| (32°45'57"S, 53°44'09"W) | |||
| UFRJ 10162.1 | Palmar | KT823665 | |
| (32°44'40"S, 52°38'41"W) | |||
| UFRJ 10162.3 | Palmar | KT823666 | |
| (32°44'40"S, 52°38'41"W) | |||
| UFRJ 10162.4 | Palmar | KT823667 | |
| (32°44'40"S, 52°38'41"W) | |||
| UFRJ 10163.1 | Cassino | KT823668 | |
| (32°06'00"S, 52°09'55"W) | |||
| UFRJ 10163.2 | Cassino | KT823669 | |
| (32°06'00"S, 52°09'55"W) | |||
| UFRJ 10163.3 | Cassino | KT823670 | |
| (32°06'00"S, 52°09'55"W) | |||
| UFRJ 10164.1 | Camaquã | KT823671 | |
| (31°04'41"S, 52°02'18"W) | |||
| UFRJ 10164.2 | Camaquã | KT823672 | |
| (31°04'41"S, 52°02'18"W) | |||
| UFRJ 10164.3 | Camaquã | KT823673 | |
| (31°04'41"S, 52°02'18"W) | |||
| UFRJ 10164.4 | Camaquã | KT823674 | |
| (31°04'41"S, 52°02'18"W) | |||
| Cynopoecilus fulgens | |||
| UFRJ 10156.1 | Osório | KT823652 | |
| (29°57'34"S, 50°13'53"W) | |||
| UFRJ 10157.1 | Osório | KT823653 | |
| (29°59'20"S, 50°11'32"W) | |||
| UFRJ 10157.2 | Osório | KT823654 | |
| (29°59'20"S, 50°11'32"W) | |||
| UFRJ 10157.3 | Osório | KT823655 | |
| (29°59'20"S, 50°11'32"W) | |||
| UFRJ 10158.1 | Cidreira | KT823656 | |
| (30°09'09"S, 50°14'25"W) | |||
| UFRJ 10158 2 | Cidreira | KT823657 | |
| (30°09'09"S, 50°14'25"W) | |||
| UFRJ 10158.3 | Cidreira | KT823658 | |
| (30°09'09"S, 50°14'25"W) | |||
| UFRJ 10159.1 | Mostardas | KT823659 | |
| (30°50'59"S, 50°41'21"W) | |||
| UFRJ 10159.2 | Mostardas | KT823660 | |
| (30°50'59"S, 50°41'21"W) | |||
| UFRJ 10160.1 | Estreito | KT590069 | |
| (31°15'52"S, 51°43'31"W) | |||
| UFRJ 10160.2 | Estreito | KT823661 | |
| (31°15'52"S, 51°43'31"W) | |||
| UFRJ 10160.3 | Estreito | KT823662 | |
| (31°15'52"S, 51°43'31"W) | |||
| UFRJ 10160.4 | Estreito | KT823663 | |
| (31°15'52"S, 51°43'31"W) | |||
| UFRJ 10161.1 | Estreito | KT823664 | |
| (31°49'18"S, 51°43'31"W) | |||
| Cynopoecilus nigrovittatus | |||
| UFRJ 10165.1 | Montenegro | KT590067 | |
| (29°40'12"S, 51°25'32"W) | |||
| UFRJ 10165.2 | Montenegro | KT823675 | |
| (29°40'12"S, 51°25'32"W) | |||
| UFRJ 10165.3 | Montenegro | KT823676 | |
| (29°40'12"S, 51°25'32"W) | |||
| Cynopoecilus notabilis | |||
| UFRJ 10166 | Aguas Claras | KT590068 | |
| (30°05'48"S, 50°51'06"W) | |||
| Cynopoecilus feltrini | |||
| UFRJ 10482.1 | Laguna | KT823677 | |
| (28°30'42"S, 48°47'59"W) | |||
| UFRJ 10482.2 | Laguna | KT823678 | |
| (28°30'42"S, 48°47'59"W) |
Data on colour patterns were based numerous photographs of both sides of five live males and three live females, taken in aquaria between 1 and 78 hours after collection. Morphometric and meristic data were taken following
Total genomic DNA was extracted from muscle tissue of the right side of the caudal peduncle using the DNeasy Blood & Tissue Kit (Qiagen), according to the manufacturer instructions. To amplify the fragment of the DNA were used the primers L4299, H2198 (
Species were diagnosed using two criteria: unique combination of morphological character states (diagnosability criterion; e.g.,
Taxon treatments
Cynopoecilus feltrini , sp. n.
-
country: Brazil; stateProvince:Santa Catarina; county:Laguna; locality:temporary pool near the confluence of Tubarão river and the Santo Antônio lagoon; verbatimElevation:5 m; verbatimLatitude:28°30'26"S; verbatimLongitude:48°48'01"W; verbatimCoordinateSystem:degrees minutes seconds; verbatimSRS:Córrego Alegre; year:2015; month:6; day:10; habitat:Temporary pool; fieldNotes:collectors = C. Feltrin et al.; institutionCode:UFRJ; collectionCode:10662; basisOfRecord:PreservedSpecimen; dynamicProperties:sex=male, SL= 45.6 mm
-
institutionID: UFRJ; collectionID:10597; basisOfRecord:Preserved Specimen; dynamicProperties:11 males, 18.2–48.0 mm SL, 11 females, 23.8–35.1 mm SL. Collected with holotype.
-
institutionCode: UFRJ; collectionCode:10598; basisOfRecord:Preserved Specimen; dynamicProperties:3 males, 32.3–35.9 mm SL, 3 females, 22.5–34.1 mm SL. Collected with holotype.
-
institutionCode: UFRJ; collectionCode:10482; basisOfRecord:Preserved Specimen; dynamicProperties:3 males, 20.3–32.2 mm SL, 2 females, 24.7–25.2 mm SL. Collected with holotype.
-
year: 2015; month:6; day:4; institutionCode:UFRJ; collectionCode:10620; basisOfRecord:Cleared and Stained; dynamicProperties:5 males, 27.9–46.9 mm SL, 2 females, 25.6–32.7 mm SL. Same locality of holotype.
Description
Morphometric data appear in Table
|
holotype |
paratypes |
||
|
male |
males (9) |
females (5) |
|
|
Standard length (mm) |
45.6 |
32.3–48.0 |
26.5–35.3 |
|
Percent of standard length |
|||
|
Body depth |
28.2 |
27.4–32.0 |
27.2–32.3 |
|
Caudal peduncle depth |
13.8 |
12.8–15.0 |
12.1–14.1 |
|
Pre-dorsal length |
54.3 |
53.4–57.4 |
59.6–62.8 |
|
Pre-pelvic length |
48.6 |
45.4–49.6 |
51.6–53.9 |
|
Length of dorsal-fin base |
28.8 |
26.0–30.4 |
24.5–26.4 |
|
Length of anal-fin base |
27.1 |
25.4–27.9 |
20.0–22.4 |
|
Caudal-fin length |
34.1 |
30.9–35.2 |
30.8–33.1 |
|
Pectoral-fin length |
19.1 |
20.0–22.5 |
19.4–21.5 |
|
Pelvic-fin length |
6.5 |
5.9–7.3 |
5.5–7.0 |
|
Head length |
27.9 |
27.6–30.2 |
27.2–29.8 |
|
Percent of head length |
|||
|
Head depth |
71.1 |
71.2–76.2 |
76.1–79.6 |
|
Head width |
68.8 |
65.1–72.9 |
71.0–79.1 |
|
Snout length |
14.3 |
12.7–14.7 |
12.7–14.1 |
|
Lower jaw length |
18.7 |
16.9–19.4 |
16.5–18.5 |
|
Eye diameter |
31.4 |
31.5–37.3 |
33.4–39.1 |
Eye positioned on dorsal portion of head side. Snout short, blunt. Premaxilla and dentary teeth conical, small, numerous, irregularly arranged, except for external series with longer fang-like teeth, slightly more robust in males. Vomerine teeth absent. Dermosphenotic absent. Frontal squamation usually E-patterned, sometimes D-patterned; E-scales often overlapping medially Fig.
Dorsal and anal fins pointed in males, rounded in females; caudal fin rounded in both sexes; often short filamentous ray on tip of dorsal and anal fins, and minute posterior filamentous extension on middle of caudal fin in males. No scales on dorsal and anal fins, scales extending on about 30 % of caudal fin. Four to six neuromasts on caudal-fin base. Pectoral fin rounded, posterior margin reaching vertical between anus and urogenital opening in males, shorter, not reaching pelvic-fin base in females. No scales on pectoral-fin base. Pelvic-fin small, tip reaching between anus and urogenital opening in males, not reaching anus in females; pelvic-fin bases medially in close proximity. Dorsal-fin origin in vertical just anterior to anal-fin origin. Dorsal-fin origin between neural spines of vertebrae 12 and 13; anal-fin origin between pleural ribs of vertebrae 10 and 12 in males, between pleural ribs of vertebrae 12 and 13 in females. Hypurals forming single plate. Ventral process of posttemporal absent. Dorsal-fin rays 18–19; anal-fin rays 25–27; caudal-fin rays 29–32; pectoral-fin rays 13–15; pelvic-fin rays 5–7. No contact organ on fins.
Colouration. Males: Side of body pale brown to light yellowish brown, lighter on ventral portion; broad dark reddish brown to black stripe between posterior orbital margin and caudal-fin base, other similar narrower stripe between pectoral-fin base and posterior portion of anal-fin base; longitudinal rows of greenish blue to greenish golden spots, consisting of one spot per scale, on head side and flank, sometimes interrupted or rudimentary. Dorsum pale brown, with few dark brown spots above opercular region. Ventral portion of head and venter white. Lower jaw dark reddish brown. Few dark reddish brown on suborbital region. Branchiostegal membrane orangish red. Iris yellow, with dark reddish brown bar on middle, anterior and posterior portion with greenish golden iridescence. Unpaired fins pale grey; small dark grey spots on basal region of dorsal fin; light blue iridescence on margins of caudal and anal fins. Pectoral fin hyaline. Pelvic fin pale grey.
Females: Colour pattern similar to that described for males, but iridescent colour paler, median stripe often forming row of dark brown or black blotches, and faint grey dots present on basal portion of anal fin.
Diagnosis
Distinguished from all other species of the Cynopoecilus melanotaenia complex by having frontal E-scales medially overlapped (vs. separated by interspace), branchiostegal region orangish red in males (vs. hyaline to pinkish hyaline), dorsum with few dark brown spots above opercular region (vs. dark brown spots over most region between snout and dorsal-fin origin).
Etymology
Named after Caio Feltrin, in recognition of his dedication in inventorying the fish fauna of southern Brazil.
Distribution
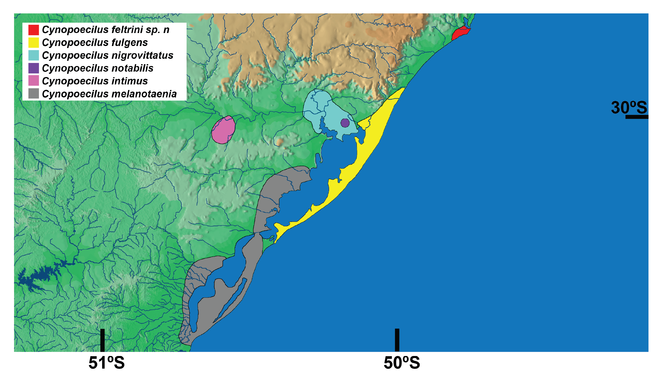
Known only from the type locality area, in temporary pools in the floodplains of the Tubarão river, Santa Catarina state, southern Brazil, corresponding to the northern-most record for the genus Cynopoecilus (Fig.
Taxon discussion
Cynopoecilus feltrini is easily identifiable by some morphological characters. Among them, the frontal squamation pattern consisting of E-scales medially overlapped is unique among cynopoeciline killifishes, which have been diagnosed by the E-scales medially separated by an interspace (
Cynopoecilus feltrini is presently known only from the lower Tubarão river basin, which is the northern-most record of the genus. The phylogenetic tree supports C. feltrini as a member of the clade that includes all species of the C. melanotaenia complex except C. melanotaenia, which is the taxon endemic of an area corresponding to the southern-most region of the genus distribution (Figs
Maximum parsimony tree obtained from the COXI sequences. Numbers below the nodes are referring to the value of Bootstrap test and over the nodes are the posterior probability of the Bayesian inference analysis, asterisks mean value of 100. Sequences used to generate the trees are listed in Table
Notholebias fractifasciatus
-
country: Brazil; municipality:Inoã; verbatimLatitude:22°55'21"S; verbatimLongitude:42°55'42"W; datasetID:8802; institutionCode:UFRJ
Notes
This taxon was included as terminal in phylogenetic analysis of this study. Genbank access code to the sequences in Table
Campellolebias brucei
-
country: Brazil; stateProvince:Santa Catarina; county:Florianópolis; verbatimLatitude:27°40'59"S; verbatimLongitude:48°33'38"W; verbatimSRS:Córrego Alegre; datasetID:8383; institutionCode:UFRJ; basisOfRecord:PreservedSpecimen
Notes
This taxon was included as terminal in phylogenetic analysis of this study. Genbank access code to the sequences in Table
Cynopoecilus melanotaenia
-
country: Brazil; stateProvince:Rio Grande do Sul; county:Quinta; verbatimLatitude:32°04'13"S; verbatimLongitude:52°15'49"W; datasetID:8974; institutionCode:UFRJ
-
country: Uruguay; stateProvince:Treinta y Tres; verbatimLatitude:32°45'57"S; verbatimLongitude:53°44'09"W; datasetID:9701; institutionCode:UFRJ
-
country: Brazil; stateProvince:Rio Grande do Sul; municipality:Palmar; verbatimLatitude:32°44'40"S; verbatimLongitude:52°38'41"W; datasetID:10162; institutionCode:UFRJ
-
country: Brazil; stateProvince:Rio Grande do Sul; county:Cassino; verbatimLatitude:32°06'00"S; verbatimLongitude:52°09'55"W; datasetID:10163; institutionCode:UFRJ
-
country: Brazil; stateProvince:Rio Grande do Sul; county:Camaquã; verbatimLatitude:31°04'41"S; verbatimLongitude:52°02'18"W; datasetID:10164; institutionCode:UFRJ
Notes
This taxon was included as terminal in phylogenetic analysis of this study. Genbank access code to the sequences in Table
Cynopoecilus fulgens
-
country: Brazil; stateProvince:Rio Grande do Sul; county:Osório; verbatimLatitude:29°57'34"S; verbatimLongitude:50°13'53"W; datasetID:10156; institutionCode:UFRJ
-
country: Brazil; stateProvince:Rio Grande do Sul; county:Cidreira; verbatimCoordinates:30°09'09"S, 50°14'25"W; datasetID:10158; institutionCode:UFRJ
-
country: Brazil; stateProvince:Rio Grande do Sul; county:Mostardas; verbatimCoordinates:30°50'59"S, 50°41'21"W; datasetID:10159; institutionCode:UFRJ
-
country: Estreito; stateProvince:Rio Grande do Sul; county:Estreito; verbatimCoordinates:31°15'52"S, 51°43'31"W; datasetID:10160; institutionCode:UFRJ
Notes
This taxon was included as terminal in phylogenetic analysis of this study. Genbank access code to the sequences in Table
Cynopoecilus nigrovittatus
-
country: Brazil; stateProvince:Rio Grande do Sul; county:Montenegro; verbatimCoordinates:29°40'12"S, 51°25'32"W; datasetID:10165; institutionCode:UFRJ
Notes
This taxon was included as terminal in phylogenetic analysis of this study. Genbank access code to the sequences in Table
Cynopoecilus notabilis
-
country: Brazil; stateProvince:Rio Grande do Sul; county:Águas Claras; verbatimCoordinates:30°05'48"S, 50°51'06"W; datasetID:10166; institutionCode:UFRJ
Notes
This taxon was included as terminal in phylogenetic analysis of this study. Genbank access code to the sequences in Table
Acknowledgements
Special thanks are due to C. Feltrin for calling our attention to the occurrence of Cynopoecilus near Tubarão and help during field collection, and to A. Katz for providing photographs of living specimens used in this study and help in the field. We are also grateful to F. Llanos and F. Pereira for help in field studies, and to M. Cheffe and T. Litz for providing specimens from Quinta, Brazil and Treinta y Tres, Uruguay, respectively, used in the molecular analysis. This study was funded by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico - Ministério de Ciência e Tecnologia) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). Collections were made with licenses provided by ICMBio (Instituto Chico Mendes de Conservação da Biodiversidade).
References
- Multiple sequence alignment with the Clustal series of programs.Nucleic Acids Research31(13):3497‑3500. [InEnglish]. https://doi.org/10.1093/nar/gkg500
- Classificação e distribuição da família Rivulidae (Cyprinodontiformes, Aplocheiloidei).Revista Brasileira de Biologia50:83‑89.
- Phylogeny and classification of Rivulidae revisited: origin and evolution of annualism and miniaturization in rivulid fishes.Journal of Comparative Biology3:33.
- The neotropical annual fish genus Cynolebias (Cyprinodontiformes: Rivulidae): phylogenetic relationships, taxonomic revision and biogeography.Ichthyological Exploration of Freshwaters12:333‑383.
- The annual fish genus Cynopoecilus (Cyprinodontiformes: Rivulidae): taxonomic revision, with descriptions of four new species.Ichthyological Exploration of Freshwaters13:11‑24.
- Monophyly and taxonomy of the Neotropical seasonal killifish genus Leptolebias (Teleostei: Aplocheiloidei: Rivulidae), with the description of a new genus.Zoological Journal of the Linnean Society153(1):147‑160. https://doi.org/10.1111/j.1096-3642.2008.00380.x
- A new genus of miniature cynolebiasine from the Atlantic Forest and alternative biogeographical explanations for seasonal killifish distribution patterns in South America (Cyprinodontiformes: Rivulidae).Vertebrate Zoology64:23‑33.
- A new annual killifish species of the Hypsolebias flavicaudatus complex from the São Francisco River basin, Brazilian Caatinga (Cyprinodontiformes: Rivulidae).Vertebrate Zoology61:99.
- Systématique et distribution du genre néotropical Campellolebias (Cyprinodontiformes, Rivulidae), avec description de deux nouvelles espèces.Revue Française d'aquariologie et d'herpetologie15:65.
- Sistemática e distribuição do complexo de espécies Cynolebias minimus (Cyprinodontiformes, Rivulidae), com a descrição de duas espécies novas.Revista Brasileira de Zoologia5(4):557‑570. https://doi.org/10.1590/s0101-81751988000400004
- Revision of the Neotropical Annual Fish Genus Cynopoecilus (Cyprinodontiformes: Rivulidae).Copeia1995(2):456. https://doi.org/10.2307/1446910
- Historical biogeography of cynolebiasine annual killifishes inferred from dispersal-vicariance analysis.Journal of Biogeography37:1995‑2004. https://doi.org/10.1111/j.1365-2699.2010.02339.x
- jModelTest 2: more models, new heuristics and parallel computing.Nature Methods9(8):772‑772. https://doi.org/10.1038/nmeth.2109
- Populations, Genetic Variation, and the Delimitation of Phylogenetic Species.Systematic Biology41(4):421‑435. https://doi.org/10.1093/sysbio/41.4.421
- Description of a new species and phylogenetic analysis of the subtribe Cynopoecilina, including continuous characters without discretization (Cyprinodontiformes: Rivulidae).Zoological Journal of the Linnean Society172(4):846‑866. https://doi.org/10.1111/zoj.12190
- DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates.Molecular Marine Biology and Biotechnology3(5):294.
- TNT, a free program for phylogenetic analysis.Cladistics24(5):774‑786. https://doi.org/10.1111/j.1096-0031.2008.00217.x
- The frontal scalation pattern in some groups of toothcarps (Pisces, Cyprinodontiformes).Bulletin of Aquatic Biology1:23.
- Annual fishes.Aquarium Journal23:125‑141.
- A phylogenetic and biogeographic analysis of cyprinodontiform fishes (Teleostei, Atherinomorpha).Bulletin of the American Museum of Natural History168:335‑557.
- Tracer.1.5. URL: http://beast.bio.ed.ac.uk/Trace
- LXXXII.— Sexual differences in the Pœciliid fishes of the genus Cynolebias.Journal of Natural History Series 810(60):641‑642. https://doi.org/10.1080/00222931208693288
- MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space.Systematic Biology61(3):539‑542. https://doi.org/10.1093/sysbio/sys029
- Ichthyologische Beiträge 5.Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Classe74:49.
- MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0.Molecular Biology and Evolution30(12):2725‑2729. https://doi.org/10.1093/molbev/mst197
- Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study.Cybium9:107.
- Especies del genero Cynolebias Steindachner, 1876 en el Uruguay.Boletín de la Sociedad Zoológica del Uruguay1:24.
- Campellolebias brucei n. gen. n. sp., cyprinodontido con especializacion de la papila gentital y de los primeros radios de la aleta anal.Comunicaciones zoológicas del museo de historia natural de montevideo10:1‑17.
- Delimiting species using DNA and morphological variation and discordant species limits in spiny lizards (Sceloporus).Systematic Biology51:69. https://doi.org/10.1080/106351502753475880