|
Biodiversity Data Journal :
Taxonomic Paper
|
|
Corresponding author:
Academic editor: Luis Cayuela
Received: 29 Jan 2016 | Accepted: 28 Mar 2016 | Published: 05 Apr 2016
© 2016 Nathan Howell, Alexander Krings, Richard Braham
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Howell N, Krings A, Braham R (2016) Guide to the littoral zone vascular flora of Carolina bay lakes (U.S.A.). Biodiversity Data Journal 4: e7964. https://doi.org/10.3897/BDJ.4.e7964
|
|
Abstract
Background
Carolina bays are elliptic, directionally aligned basins of disputed origin that occur on the Atlantic Coastal Plain from the Delmarva Peninsula to southern Georgia. In southeastern North Carolina, several large, natural, lacustrine systems (i.e., Carolina bay lakes) exist within the geomorphological features known as Carolina bays. Within the current distribution of Carolina bays, Bladen and Columbus counties (North Carolina) contain the only known examples of Carolina bay lakes. The Carolina bay lakes can be split into two major divisions, the “Bladen Lakes Group” which is characterized as being relatively unproductive (dystrophic – oligotrophic), and Lake Waccamaw, which stands alone in Columbus County and is known for its high productivity and species richness. Although there have been several studies conducted on these unique lentic systems, none have documented the flora comprehensively.
New information
Over the 2013−2014 growing seasons, the littoral zone flora of Carolina bay lakes was surveyed and vouchered. Literature reviews and herbarium crawls complemented this fieldwork to produce an inventory of the vascular plant species. This survey detected 205 taxa (species/subspecies and varieties) in 136 genera and 80 vascular plant families. Thirty-one species (15.2%) are of conservation concern. Lake Waccamaw exhibited the highest species richness with 145 catalogued taxa and 26 species of conservation concern. Across all sites, the Cyperaceae (25 spp.), Poaceae (21 spp.), Asteraceae (13 spp.), Ericaceae (8 spp.), Juncaceae (8 spp.), and Lentibulariaceae (6 spp.) were the six most species-rich vascular plant families encountered. A guide to the littoral zone flora of Carolina bay lakes is presented herein, including dichotomous keys, species accounts (including abundance, habitat, phenology, and exsiccatae), as well as images of living species and vouchered specimens.
Keywords
North American southeastern Coastal Plain lakes, floristics, aquatic, emersed vegetation
Introduction
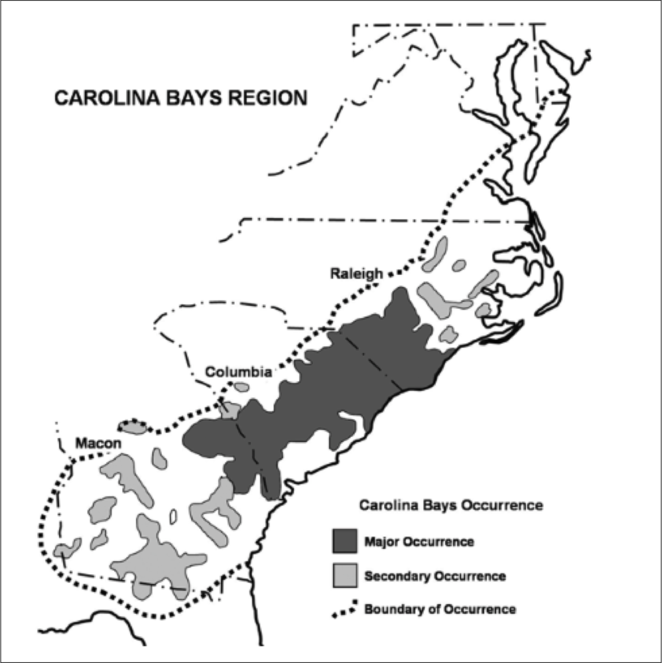
Carolina bays are shallow elliptical depressions of disputed origin aligned in a northwest-southeast direction on the Atlantic Coastal Plain of the eastern United States from the Delmarva Peninsula to southern Georgia (
Although there have been several studies conducted on these unique lentic (freshwater) systems (
A narrow time frame exists to study the few remaining natural freshwater systems not affected by severe degradation.
Background
Lake Ecosystems and Abiotic Factors
Catchment Area
Lakes (also referred to as lentic systems along with ponds) exhibit physical and chemical characteristics unique to the soils, vegetation, and land use activities present on immediately surrounding lands; thus, no two lakes are exactly the same (
Water Color
The observed color of natural lake waters is caused by the selective absorption of wavelengths as light penetrates through the water column (
Trophic status
Trophic status refers to the rate at which organic matter is supplied by or transported into a lake. Humic substances are the most common component in allochthonous organic matter; consequently, wetlands that receive the bulk of their organic matter from allochthonous sources (e.g., Carolina bay lakes, bogs, pocosins) are heavily “tea-stained” and are commonly referred to in the southeastern United States as “black water” lakes, streams, rivers, ponds. Lakes receiving the majority of their organic matter from allochthonous sources have been given the term dystrophic. Dystrophic lakes have low productivity and are often acidic due to large quantities of allochthonous humic input.
Phosphorous is limiting in freshwater systems and is therefore a useful determinant for production. Phosphorous concentrations are easier to quantify than carbon content and production, and, as a result, trophic status is often classified based on phosphorous content (
pH
The unit commonly used to measure acidity is pH. It is technically defined as the reciprocal of the activity of free hydrogen ions (H+;
Photosynthesis and respiration are also known to affect the pH of waters by influencing the amount of carbon dioxide (CO2) in the water column. When CO2 is taken up and stored by aquatic macrophytes, phytoplankton, and algae during photosynthesis, free hydrogen ions (H+) are neutralized or taken up by carbonates, bicarbonates, and hydroxides, causing a reduction in H+ and thus a higher pH. Respiration adds CO2 into the system, thus releasing free H+ into the water column and lowering the pH (
Alkalinity
Alkalinity refers to a lake's ability to neutralize strong inorganic acids (i.e., it is a measure of how sensitive a lake is to acidification). It is now used synonymously with acid neutralizing capacity (ANC;
Carolina Bays, Bay Lakes, and Pocosins
Carolina Bays
The core concentration of Carolina bays occurs in southeastern North Carolina and northeastern South Carolina (
Core distribution of Carolina bays. Carolina bays are known to occur from the Delmarva Peninsula south to southern Georgia. Although many historical texts frequently cite the distribution range of Carolina bays as occurring from New Jersey south to Florida, the more narrow range from the Delmarva Peninsula to southern Georgia is more accurate. Conversations with state agencies and personnel from all states included in the broader range of Carolina bays confirm their “apparent absence” in southern New Jersey and northern Florida. The core distribution of Carolina bays is located in northeast South Carolina and southeast North Carolina (darker gray). The bays in this region would be considered “classic” Carolina bays (i.e., matching all of the well-known and consistent geomorphological criteria in the literature), whose geomorphology is described well by
The term bay is used to describe these landscape features not because they commonly contain hydric soils or are inundated with water, but because of the presence of three species of bay tree typically found within and around their elliptical boundaries (i.e., Magnolia virginiana L. [sweetbay; Magnoliaceae], Persea palustris (Raf.) Sarg. [swamp bay; Lauraceae], and Gordonia lasianthus (L.) J. Ellis [loblollybay; Theaceae]. Traditionally, the term “bay” tree has been used when speaking of the laurel trees within the Lauraceae family. While Persea palustris may be properly referred to as a “bay” tree, Gordonia lasianthus and Magnolia virginiana may not (sensu stricto), hence their common names being one word (i.e., loblollybay and sweetbay). Gordonia lasianthus and Magnolia virginiana bear a noticeable morphological resemblence to the laurels of the Lauraceae; thus, they are generally referred to as “bay” trees (sensu lato). North of Virginia, these mysterious landscape features are referred to as Delmarva potholes, bays, or basins (Tiner and Burke 1995, Lide 1997, Sharitz 2003, Tiner 2003). The inability to agree upon a clear-cut definition and universal name for these unique geological features has caused some discrepancy among estimates of bay numbers (Lide 1997).
Collectively, Carolina bays and pocosins represent the largest total acreage of palustrine wetlands in the Carolinas (
Geographic location, soil depth, soil type, surrounding land use, varying hydrology, and fire regimes interact to create vastly different vegetative and wetland assemblages within Carolina bays.
Carolina bays can be divided into two classes based on soil substrate: clay-based bays and peat-based bays. The vast majority of Carolina bay literature has referenced peat-based bays, frequently using terms such as “pocosin” or “evergreen shrub bog” to describe the vegetation growing over deep organic soils. However, there are about 27 bays (as of 1982) located in the Carolinas that contain clay subsoil not overlain with sand or peat (
Clay-based bays are species-rich communities, often supporting rare taxa within their boundaries (
Bladen County, North Carolina, is well-known for its many Carolina bays.
Carolina bays should not be confused with pocosins; they are two distinct physiographic features that just so happen to coexist with one another on the Atlantic Coastal Plain. These two landscape features differ from one another and using the terms synonymously is a common mistake among both laymen and professionals (
Historically, the Atlantic and Gulf Coastal Plains supported a heterogeneous landscape of longleaf pine savannas, xeric sandhills, upland mixed-pine hardwoods, pocosins, Carolina bays, bottomland hardwood forests, natural lakes, and black and brown-water river systems (
Carolina bays are valuable components of our national and state natural heritage (
Carolina Bay Lakes
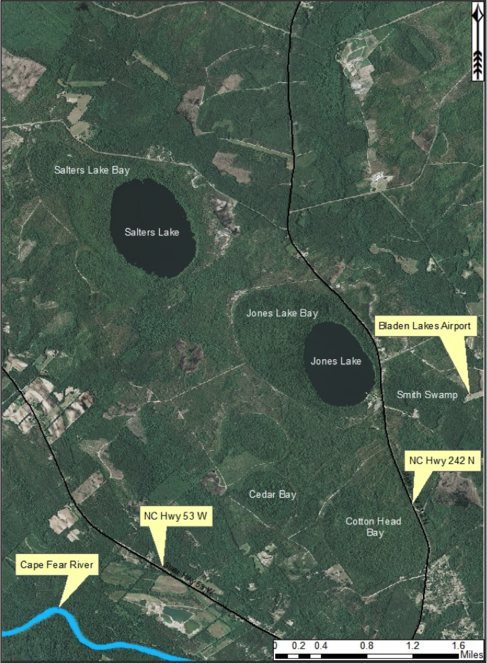
Several Carolina bays in southeastern North Carolina contain large (i.e., > 50 hectares) natural lakes within their elliptic boundaries (
Position of Carolina bay lakes within Carolina bays. Carolina bay lakes are located in the southeasternmost portions of Carolina bays. The northern portions of the bays (i.e., the portion not inundated by lake waters) support shrub-bog plants over organic soils. Here, Salters (top left) and Jones (middle right) Lakes exemplify the typical bay lake position within Carolina bays. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (
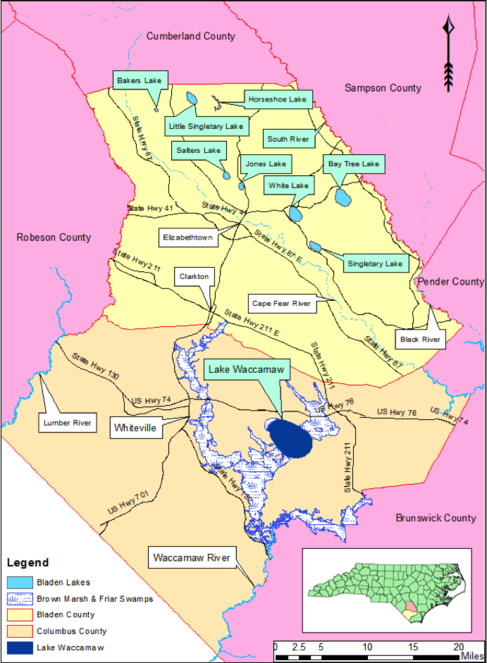
Nine Carolina bay lakes (i.e., Bakers Lake, Bay Tree Lake, Horseshoe Lake, Jones Lake, Lake Waccamaw, Little Singletary Lake, Salters Lake, Singletary Lake and White Lake) are known to exist within the known distribution of Carolina bays. All nine lakes occur in Bladen and Columbus counties, North Carolina (
Geographic location of all nine Carolina bay lakes (green text boxes). Bladen County (light yellow) supports eight of the nine Carolina bay lakes known to exist; all eight lakes occur within the Cape Fear River Valley between the Cape Fear River and South River. Bay Tree Lake is the largest Carolina bay lake in Bladen County; the smallest is Bakers Lake. Lake Waccamaw is the largest Carolina bay and bay lake in North Carolina and is the only bay lake known to exist in Columbus County (tan). Baseline vector data obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map Produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (
Although some Carolina bays may contain shallow marshes or ponds (
Carolina bays are considered to be geographically isolated wetlands with their primary water source coming directly from precipitation (
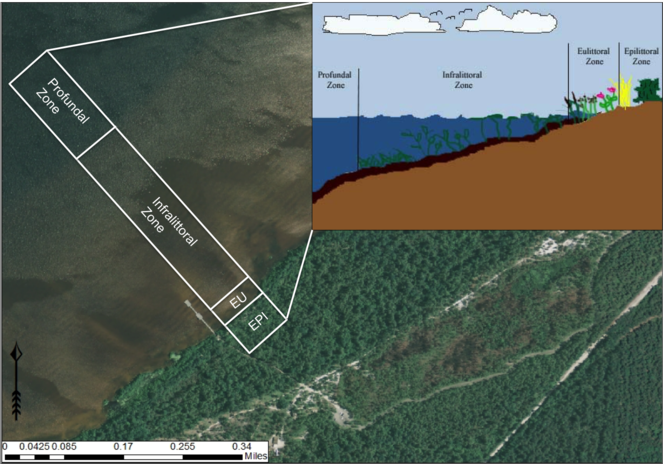
Lacustrine Zonation (derived from
Lakes, including Carolina bay lakes, can be divided into distinct transitional zones, moving from the shoreline to the center of the lake (Fig.
Lacustrine zonation. EPI = epilittoral zone, EU = eulittoral zone. Aerial imagery obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map Produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (
(1) Epilittoral zone: The zone that lies entirely above the lake surface and is not influenced by the spray of surf. This zone can be thought of as the terrestrial or upland zone; the highest water levels never reach it and it is not affected by lakeshore dynamics or hydrology.
(2) Supralittoral zone: The zone that lies entirely above the lake surface and is influenced by the spray of the surf.
(3) Eulittoral zone: The zone encompassing the entire region of the shoreline from the highest and lowest seasonal water levels. This zone experiences natural disturbances such as water level fluctuations and wave dynamics.
(4) Infralittoral zone: This zone is subdivided into three zones in relation to the occurrence and distribution of the major classes of aquatic macrophytes: upper infralittoral zone where emergent rooted macrophytes persist; middle infralittoral zone where floating-leaved rooted macrophytes occur; and lower infralittoral zone where submersed-rooted, adnate, or free-floating macrophytes occur. The eulittoral and infralittoral zones collectively constitute the littoral zone.
(5) Littoriprofundal zone: The zone occupied by photosynthetic algae and bacteria, often associated with the metalimnion (i.e., the stratum between the epilimnion and hypolimnion representing a marked thermal change; also synonymous with thermocline) of stratified lakes.
(6) Profundal zone: The zone that consists of the remainder of the vegetation free sediments.
The Littoral Zone
The littoral zone of lakes (i.e., the eulittoral and infralittoral zones) is an important transition zone between adjacent uplands and the deeper pelagic area of the lake. This zone contains vascular macrophytes (i.e., aquatic vascular plants large enough to see with the naked eye) that have evolved from their terrestrial ancestors to cope with the physical and physiological demands of persisting in an aquatic environment (
Aquatic Macrophytes (derived from
Aquatic macrophytes may be divided into four classes. Moving from the shoreline out to deeper water, these classes are as follows [taxa vouchered or reported from Carolina bay lakes are indicated by c]:
(1) Emergent macrophytes: Species rooted in saturated and inundated soils with a water depth up to 1.5 meters; root systems remain in anoxic soil conditions while leaves and reproductive organs stay above the water surface. These plants are often rhizomatous, stoloniferous, or cormous with the potential to reproduce asexually. Heterophyllous (i.e., when a plant exhibits vegetative polymorphism, having morphologically different submersed and aerial organs) species may also be emergent. Examples of genera that may be grouped in this category include Carex L.c, Cephalanthus L.c, Cladium P. Brownec, Juncus L.c, Panicum L.c, Pontederia L.c, Rhynchospora Vahlc, Scirpus L.c, and Typha L.
(2) Floating-leaved macrophytes: Species rooted in the substratum with floating leaves attached to long flexible petioles or on short petioles attached to an ascending stem.
Submersed leaves precede the floating leaves in heterophyllous species. Reproductive organs remain atop or above the water surface. Examples of genera grouped into this category include Brasenia Schreb.c, Nelumbo Adans.c, Nuphar Sm.c, Nymphaea L.c, Nymphoides Ség.c, and Potamogeton Lc.
(3) Submersed macrophytes: Species that remain completely submersed in the water column, but are rooted to the substratum. Leaf morphology is highly variable in this group, from finely dissected to very broad, and reproductive organs may be emersed, floating, or submersed. Examples of genera included in this group are Ceratophyllum L., Isoetes L., and Myriophyllum Lc.
(4) Freely floating macrophytes: Species that remain unattached to the substratum and are completely dependent upon the nutrients in the water column for survival. Reproductive organs may be floating or aerial. Examples of genera include Azolla Lam., Eichhornia Kunth, Hydrocharis L., Limnobium Rich., Trapa L., and Utricularia Lc.
Factors affecting Aquatic Macrophyte Richness in Lakes
Latitude
It is well known that generally the number of species occuring at the equator greatly exceeds that of the temperate and northern latitudes (
pH and Alkalinity
Peat-based Carolina bays are known to have acidic (< 7 pH), nutrient poor, organic soils (
Water Color
Waters with increased levels of humic substances are typically, dystrophic, acidic, and tea-stained. Tea-stained waters are not as transparent as lakes with low humic substances, thus humic lakes have a shallow euphotic zone and a narrow littoral zone, reducing the abundance and depth at which aquatic macrophytes may grow (
Hydrography
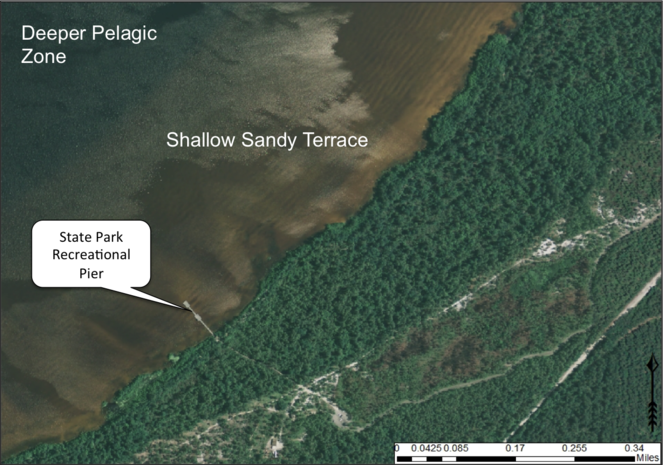
Broad, shallow, sandy terrace along Lake Waccamaw’s southern shoreline. The gentle relief of this terrace creates a wide littoral zone. Wide littoral zones are more floristically diverse and contain more available area for the establishment of aquatic macrophytes. Alternatively, narrow littoral zones do not have much area for the establishment of aquatic macrophytes and are species-poor. Aerial imagery obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (
Lake Size
As a general rule, species richness usually increases with increasing area (
Water Level Variation, Disturbance, and Soil Fertility
Shorelines exposed to frequent disturbances typically have silt and clay stripped from them; and consequently, contain few nutrients. Sheltered shorelines receive clay and silt deposits and therefore contain a higher nutrient content. Foreshores will have a distinct vegetative community characterized as having low biomass and rare species, while backshores (bays or backwater areas sheltered from disturbance) will support a higher biomass community composed of a few clonal dominants (
Study Sites
Bakers Lake
Bakers Lake (30.35 hectares; 75 acres) is a small, privately owned, Carolina bay lake, located in northwestern Bladen County between Little Singletary Lake and the Cape Fear River north of Thoroughfare Bay, ca. 1.5−2 miles east of the intersection of SR 1318 (Old River Road) and SR 1320 (Middle Road;
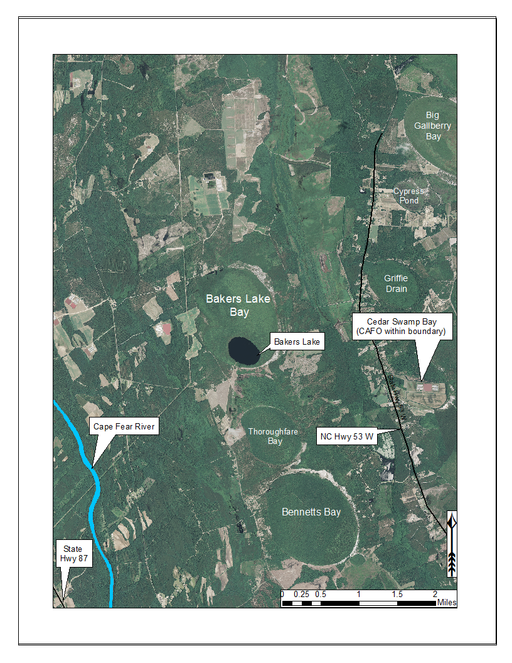
Bakers Lake and surrounding lands. Bakers Lake is located in northern Bladen County and is surrounded by a mix of agriculture and forestland. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (
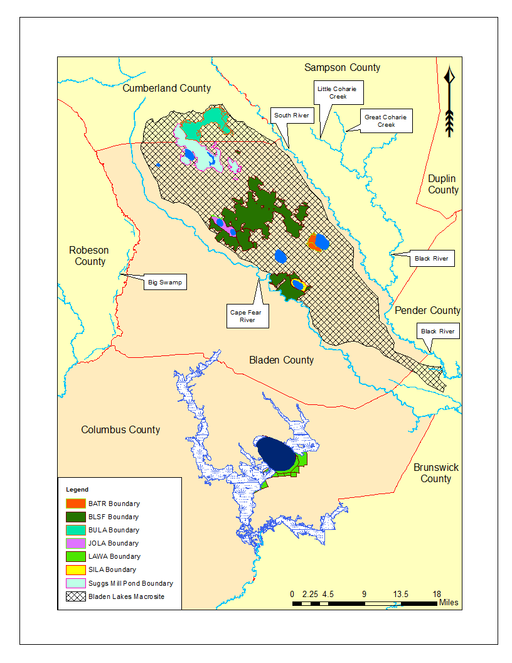
Bladen Lakes Macrosite (vector). The Bladen Lakes Macrosite (hatched pattern) is a large area encompassing parts of southern Cumberland County, eastern Bladen County, and northwest Pender County. Historically, macrosites were established by the North Carolina Natural Heritage Program (NCNHP) in efforts to identify large, intact, natural areas that withheld numerous other smaller natural areas within their boundaries. The NCNHP no longer uses macrosites as viable natural area boundaries, but it is useful to show the extent of the Bladen Lakes Macrosite boundary. When moving from north to south, the lands are as follows: Bushy Lake State Natural Area (teal green), Suggs Mill Pond Gameland (light mint green), Bladen Lakes State Forest (forest green), Jones Lake State Park (pink), Bay Tree Lake State Park (orange), and Singletary Lake State Park (yellow). Lake Waccamaw State Park (neon green) can be seen farther south along with Friar and Brown Marsh Swamps in Columbus County. Baseline vector data obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (
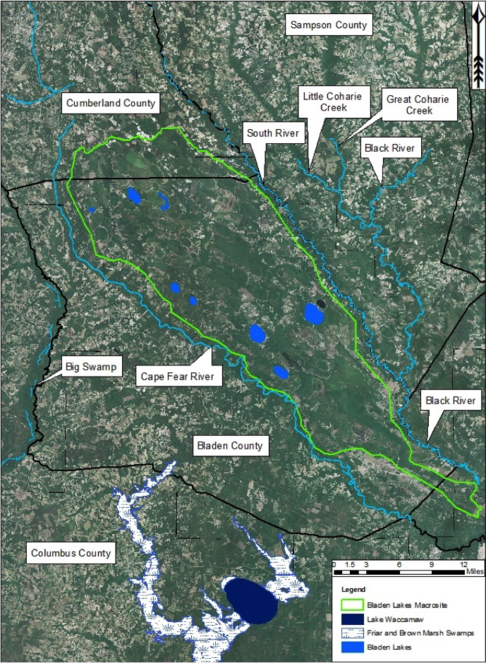
Bladen Lakes Macrosite (ortho). The North Carolina Natural Heritage Program no longer uses macrosites as viable natural area boundaries, but here it is useful to show the extent of the Bladen Lakes Macrosite boundary. Note the large areas of fragmented land surrounding the macrosite and the relatively unfragmented land within the boundaries of the macrosite. This large tract of land contains one of the largest remaining portions of intact unaltered Carolina bay complexes known to exist. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (
Dr. Clemuel Johnson and wife Nancy Johnson, of Elizabethtown, have owned Bakers Lake and surrounding lands (451.40 hectares; 1,155.45 acres) since 1980. Prior to the Johnson’s ownership, Agnes Holden Williams owned the lake and surrounding lands. Ms. Williams’ father acquired the land from an unknown seller during the early 20th century. This seller was able to successfully purchase the lake before 1929, when North Carolina legislation mandated that all lakes greater than 50 acres in size be made property of the state.
Bakers Lake forms the headwaters of Phillips Creek, which drains southward into the Cape Fear River. Bakers Lake Natural Area (i.e., Bakers Lake bay and immediate surrounding lands) is known to support five natural community types (i.e., Pond Pine Woodland – Typic Subtype (S3,G3), Peatland Atlantic White Cedar Forest (S1,G2), Low Pocosin – Gallberry/Fetterbush Subtype (S2,G2), Sand Barren – Typic Subtype (S2,G2), and Natural Lake Shoreline – Cypress Subtype (S2,G3;
Anthropogenic disturbances (i.e., silvicultural practices, dam installation in the outflow channel, agricultural fields, confined animal feeding operations (CAFOs), fire supression, and rural residential development) have either been documented on site or on adjacent properties (
The water quality of Baker’s Lake has not been formally tested by state agencies, but appears high in humic substances (N. Howell, pers. obs.) and the chemistry is likely similar to that of the other Bladen lakes. The lake is here considered dystrophic and relatively unproductive.
Bay Tree Lake
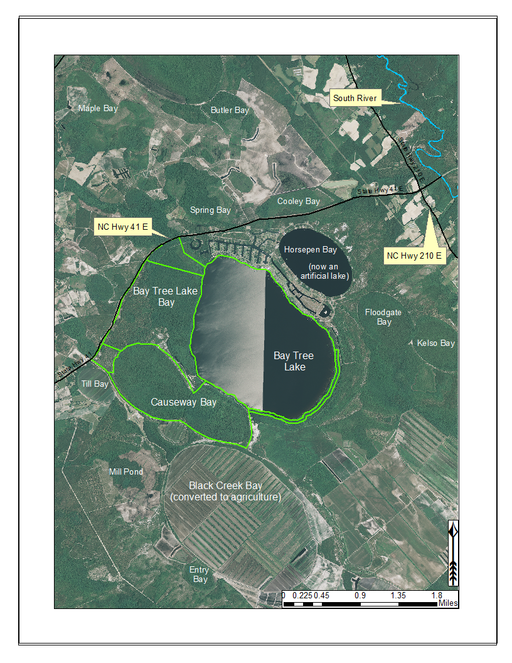
Bay Tree Lake (formerly Black Lake; 588.81 hectares; 1,455 acres) is a large, state-owned Carolina bay lake, located in east-central Bladen County along NC Hwy 41 east of White Lake and west of NC Hwy 210. Bay Tree Lake is part of Bay Tree Lake State Park, a 1,006.85 hectare (2,488 acre) park that includes Bay Tree Lake bay and large parcels of land lying to the north and west of Bay Tree Lake (Fig.
Bay Tree Lake State Park (highlighted in green) and surrounding lands. Lands surrounding Bay Tree Lake State Park to the south are privately owned and have been partially converted to agriculture. Black Creek Bay and several others in the vicinity have been cleared of their original vegetation and converted to agriculture (primarily blueberry farms in this area). Historically, Horsepen Bay was a peat-filled Carolina bay. During the development of the residential community seen along the northeast shoreline (Bay Tree Resorts), it was turned into a body of open water. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (
The North Carolina General Assembly passed legislation in 1911 confirming the status of Bay Tree Lake as a state-owned public trust resource (
In January 1965, a private land development group had the option to purchase 5,665.59 hectares (14,000 acres) of land surrounding Bay Tree Lake with the intent of creating an inland resort community (
The purpose of the drainage project was to release tannic, tea-colored, waters from the lake and divert all incoming tannic waters from a northerly adjacent swamp to below the outflow channel. Drainage of the lake was completed in the winter of 1966. The lake remained dry for 5 years while developers removed debris and peat deposits and imported large quantities of white sand, which would later be distributed around the entirety of the lakeshore. In 1970, the lakes outflow channel was plugged and the lake began to refill (
Bay Tree Lake State Park contains five natural community types (Mesic Pine Savanna – Coastal Plain Subtype [S2,G2G3]; Sand Barren – Typic Subtype [S2,G2]; Small Depression Drawdown Meadow – Typic Subtype [S2S3,G2?]; Small Depression Pocosin – Blueberry Subtype [S2,G3?]; and Xeric Sandhill Scrub – Typic Subtype [S3S4,G3?]. A Natural Lake Shoreline community was not assigned to Bay Tree Lake by the NCNHP due to the shoreline’s disturbance history. The present authors agree with this determination and have chosen not to assign a natural lake shoreline community to this site. However, it is worth noting that the shoreline flora of Bay Tree Lake differs only slightly from the other Bladen Lakes.
Bay Tree Lake forms the headwaters of Lake Creek, a small blackwater creek that drains southeast to the South River (the boundary between Bladen and Sampson counties). Much of the land surounding Bay Tree Lake State Park has been cleared for agriculture (particularly blueberry farms) and has limited the landscape connectivity between it and other intact natural areas. Several bay complexes occur in the immediate vicinity of Bay Tree Lake including Beagle Bay, Black Creek Bay, Causeway Bay, Cooley Bay, Horsepen Bay (now an artificially created lake/pond), Floodgate Bay, Kelso Bay, and Spring Bay. A residential resort community is located along the north and east shorelines of the lake. The boundaries of this community have continued to extend around the east and southeast shorelines. Residential development, agricultural expansion, severe offroad vehicle use, and fire supression are the primary threats to biological diversity within and around Bay Tree Lake State Park (N. Howell pers. obs.). Available water quality parameters for Bay Tree Lake are provided in Table
Water Quality Data for Bay Tree Lake (Bladen County, North Carolina).
|
Frey (1949) |
Weiss & Kuenzler (1976) |
DWQ 2009 |
|
|
Trophic Status |
− |
− |
Dystrophic |
|
Watershed Area (km2) |
− |
− |
10.36 |
|
Surface Area (ha) |
− |
573.84 |
− |
|
Max Width (km) |
− |
1.77 |
− |
|
Max Length (km) |
− |
3.05 |
− |
|
Max Depth (m) |
− |
1.83 |
− |
|
Mean Depth (m) |
− |
− |
0.9 |
|
Secchi Depth (m) |
0.55 |
0.3−0.4 |
1.4−1.8 |
|
Min Temp. (°C) |
− |
13.3 |
23.2 |
|
Max Temp. (°C) |
− |
30.5 |
30 |
|
Dissolved Oxygen (mg/L) |
6.4 |
7.1−10.9 |
6.8−8 |
|
Alkalinity (meq/L) |
− |
0.159−0.231 |
− |
|
pH (s.u.) |
4.4 |
6.3−7.1 |
4.1−4.5 |
|
Total N (mg/L) |
− |
0.48−1.568 |
− |
|
Total P (mg/L) |
− |
0.13−0.238 |
− |
|
Chlorophyll-A (μg/L) |
− |
− |
2−6 |
Horseshoe Lake
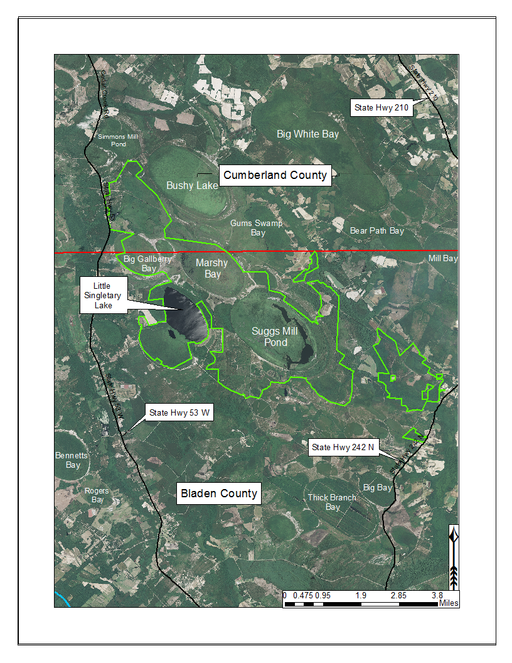
Horseshoe Lake (also known as Suggs Mill Pond; 109 hectares; ca. 270 acres) is an irregularly shaped Carolina bay lake located in northern Bladen County south of Bushy Lake State Natural Area, east of Little Singletary Lake, north of SR 1325 (Gum Springs Rd), and west of SR 1002 (Old Fayetteville Rd). Horseshoe Lake is one of two Carolina bay lakes within Suggs Mill Pond Game Land (4469.34 hectares; 11,044 acres; Fig.
Suggs Mill Pond Game Land (outlined in green) and surrounding lands. Lands north of the red dividing line occur in Cumberland County while lands south of the red line occur in Bladen County. Suggs Mill Pond Game Land contains two large bay lakes within its boundary. Little Singletary Lake is located along the western boundary of the property and Horseshoe Lake (aka Suggs Mill Pond) is located in the center of the property. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (
The state first gained rights to the property in 1994 when a 62-acre (25 ha) parcel was donated to the NCWRC from Canal Woods Industries. Thereafter, much of the remaining property was purchased from Canal Woods. The fact that Horseshoe Lake and Little Singletary lake were not owned by the state of North Carolina until the mid-1990s suggests that these lakes were involved in a similar ownership situation as Bakers Lake (i.e., these lakes must have been privately owned prior to 1929 when legislation mandated that all lakes greater than 50 acres (20.2 ha) in size be released to the state of North Carolina). Suggs Mill Pond Game Land is one of four North Carolina game lands enrolled in the Cooperative Upland habitat Restoration and Enhancement program (CURE), where management for early successional habitat is the top priority (
The largest bay on site contains a horsehoe-shaped artificial impoundment (Horseshoe Lake). Horseshoe Lake forms the headwaters of Ellis Creek, which drains southwest to the Cape Fear River. Although an old milldam currently maintains Horseshoe Lake, it is thought that a smaller body of open water may have been present prior to the dam’s installation in the late 19th or early 20th centuries. Horseshoe Lake was formed subsequent to the dam installation, as water levels began to rise into the peat-filled Carolina bay. Today, it is best described as a semi-permanent impoundment; however, the presence of floating bogs within the lake makes it unique from other semi-permanent impoundments in North Carolina. Parts of the lake support patches of the rare floating bog community (the largest extent known from the state), which is dominated by sedges, orchids, carnivorous plants, and ericaceous shrubs. Other portions comprise the Coastal Plain Semipermanent Impoundment community, which is characterized by open water, dominated by floating-leaved macrophytes, and a sparse overstory of Taxodium ascendens Brongn.
The floating bog community type is quite unique. Manifestations of this community type occur just above the water surface and range in size from ca. 10 × 10 m to a few hectares in size (N. Howell, pers. obs.). Some bogs may contain well-developed herbaceous vegetation in addition to small (e.g., < 3 m tall) trees of Chamaecyparis thyoides, Nyssa biflora, and Taxodium ascendens, while others contain a strictly herbaceous component. Exposed portions of peat can be seen around the peripheries of some bogs; here, Drosera intermedia Hayne, Eleocharis baldwinii (Torr.) Chapm. /E. vivipara Link, Pogonia ophioglossoides (L.) Ker Gawl, Utricularia striata Leconte ex Torr., Utricularia purpurea Walter, and other small-statured herbaceous plants can be seen colonizing the apparently young peat formations. Isolated floating bogs (i.e., bogs surrounded by open water and separated from adjacent bogs and upland habitats) of varying size show a consistent zonation pattern. Small statured herbaceous taxa colonize the outer periphery and are slowly replaced by larger herbaceous taxa (Andropogon glaucopsis, Dulichium arundinaceum (L.) Britton, Hypericum virginicum L., Rhexia nashii Small, Rhynchospora alba (L.) Vahl, Rhynchospora inundata (Oakes) Fernald, Xyris fimbriata Elliott, and Xyris smalliana Nash) and woody species (Acer rubrum L., Chamaecyparis thyiodes, Decodon verticillatus (L.) Elliott, Nyssa biflora, and Taxodium ascendens) when moving toward the center. Thus, a dome-shaped appearance is typically seen.
Few examples of floating bogs or mats of vegetation are known to science. The floating peat mats of New Hampshire are most similar to those of Horseshoe Lake. These peat mats possess the same general structure and abiotic conditions as those of Horseshoe Lake and are known to contain several overlapping taxa, inculding Drosera intermedia, Dulichium arundinaceum, Eleocharis R. Br. spp., Hypericum virginicum, Nymphaea odorata W.T. Aiton, Rhynchospora alba, and Utricularia spp. (
A separate but similar case of floating vegetation mats, forming as a result of dam installation, has been observed at Goose Creek Reservoir in South Carolina (
The floating “sudd” vegetation of the upper Nile River is also somewhat similar, forming large floating mats of marsh vegetation both along the margins and within the river.
Eleven natural community types exist within Suggs Mill Pond Game Land, but the low and high pocosin communities are dominant, comprising 48% (2,119.74 hectares; 5,238 acres) of the site (
Jones Lake
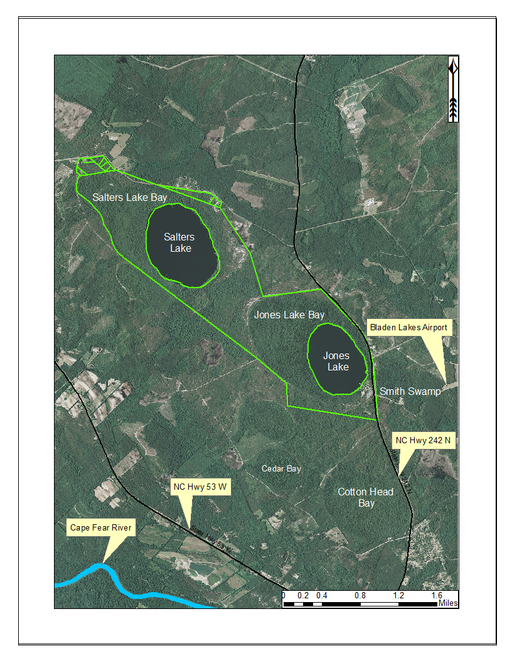
Jones Lake (91.05 hectares; 225 acres) is one of two dystrophic Carolina bay lakes located within Jones Lake State Park (893.54 hectares; 2,208 acres; Fig.
Jones Lake State Park (outlined in green) and surrounding lands. Jones Lake State Park is located between state highways 53 and 242, north of the Cape Fear River. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (
Eleven natural community types have been described from Jones Lake State Park (i.e., Bay Forest, Coastal Plain Small Stream Swamp, High Pocosin, Low Pocosin, Natural Lake Shoreline, Peatland Atlantic White Cedar Forest, Pine/Scrub Oak Sandhill Mixed Oak Variant, Pond Pine Woodland, Wet Pine Flatwoods Wet Spodosol Variant, Xeric Sandhill Scrub Coastal Plain Variant, Xeric Sandhill Scrub Sandbarren Variant;
Water Quality Data for Jones Lake (Bladen County, North Carolina).
|
Frey (1949) |
Weiss & Kuenzler (1976) |
DWQ 2009 |
|
|
Trophic Status |
− |
− |
Dystrophic |
|
Watershed Area (km2) |
− |
− |
5.18 |
|
Surface Area (ha) |
− |
90.65 |
− |
|
Max Width (km) |
− |
0.48 |
− |
|
Max Length (km) |
− |
0.80 |
− |
|
Max Depth (m) |
− |
2.13 |
− |
|
Mean Depth (m) |
− |
− |
0.9 |
|
Secchi Depth (m) |
0.73 |
0.3−1.22 |
1.3−2.4 |
|
Min Temp. (°C) |
− |
14 |
21.9 |
|
Max Temp. (°C) |
− |
30.5 |
29.6 |
|
Dissolved Oxygen (mg/L) |
5.7 |
6.7−10.6 |
6.2−7.5 |
|
Alkalinity (meq/L) |
− |
0−0.002 |
− |
|
pH (s.u.) |
4.34 |
3.1−4.8 |
3.6−4.2 |
|
Total N (mg/L) |
− |
0.32−0.73 |
− |
|
Total P (mg/L) |
− |
0.013−0.025 |
− |
|
Chlorophyll-A (μg/L) |
− |
− |
1−11 |
Lake Waccamaw
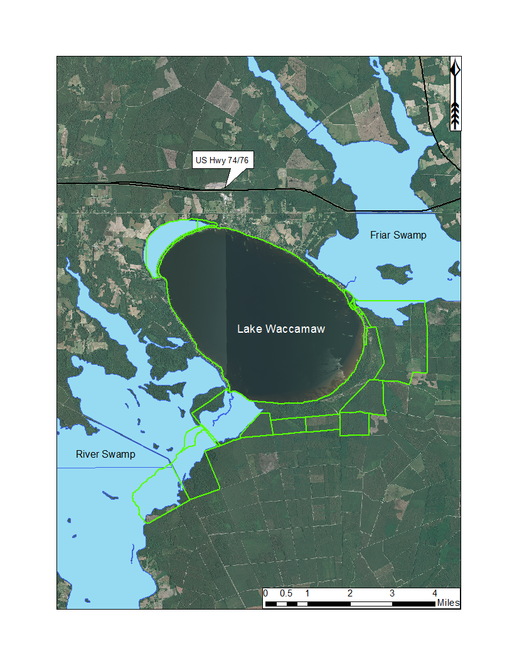
Lake Waccamaw is located south of the township of Lake Waccamaw, between Friar Swamp to the northeast, and the Waccamaw River to the south. It is the only Carolina bay lake located in Columbus County and is the largest Carolina bay and bay lake (3,617.48 hectares; 8,939 acres) in North Carolina (
Lake Waccamaw State Park (outlined in green) and surrounding lands. Lake Waccamaw State Park is a large state park encompassing Lake Waccamaw and adjacent swampland and uplands. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (
Prior to European civilization in the Southeast, the Waccamaw-Sioux Native American peoples, one of five Native American tribes known to inhabit the Cape Fear Region, inhabited the lands surrounding the lake (
This bay lake differs from the Bladen lakes in its larger size, neutral pH, mesotrophic status, and presence of alluvial hydrologic inputs (Big Creek). Tea-stained waters from Friar Swamp are delivered into northeast Lake Waccamaw via Big Creek, the largest of several creeks draining into the lake from Friar Swamp. Lake Waccamaw forms the headwaters of the Waccamaw River, a species-rich river system known to support several rare plant (e.g., Fimbristylis perpusilla R.M. Harper ex Small & Britton, Ilex amelanchier M.A. Curtis ex Chapm., Lipocarpha micrantha (Vahl) G.C. Tucker, Oldenlandia boscii (DC.) Chapm., Rhynchospora decurrens Chapm., and Sabatia kennedyana Fernald) and animal taxa (Alligator mississipiensis [American Alligator], Elliptio folliculata [Pod Lance], Etheostoma perlongum [Waccamaw Darter], Lampsilis ochracea [Tidewater Mucket], Menidia extensa [Waccamaw Silverside], Noturus spp. 2 [Broadtail Madtom], and Procambarus leptodactylus [Pee Dee Lotic Crayfish;
Much of the land surrounding Lake Waccamaw has been converted to agriculture (north of the lake) and loblolly pine plantations (south of the lake). A small portion of Lake Waccamaw’s bay is still present on the northern end.
The Coastal Plain Marl Outcrop occurs along a roughly 394 m (1,000 ft.) stretch of northern shoreline and is characterized by having vertical and overhanging low cliffs in the supralittoral zone of the lake. Portions of these cliffs are submerged in the upper eulittoral zone, but local residents privately own terrestrial portions. This marl community is known for supporting the only naturally occuring population of Venus hair fern (Adiantum capillus-veneris L.) in the state.
Shoreline residential development extends along the northern shores of the lake from the lake outlet (southwest corner of lake) to just south of Big Creek. These shorelines support the globally rare Natural Lake Shoreline Marsh (Lake Waccamaw Pondlily Subtype) community. Undeveloped shorelines (i.e., Natural Lake Shoreline Swamp – Lake Waccamaw Subtype) occur from just south of Big Creek to the lake’s outlet. Historically, Lake Waccamaw experienced wide-ranging water level fluctuations determined by precipitation. In 1925, a poorly constructed dam was built at the lakes outlet in an effort to stabilize lake levels for increased recreational use. Before construction began, lake levels were so low that vehicles could be driven to the construction site on the dried lake bed (
The physical and hydrographic nature of Lake Waccamaw’s shoreline also differs from the other bay lakes. Lake Waccamaw’s shoreline is sandy around its entire periphery (
A broad, sandy, terrace (lacking in Bladen lakes) is also present along the southeast shoreline of Lake Waccamaw (Fig.
The buffering effect of subsurface and surficial limestone on the naturally acidic waters of Lake Waccamaw result in an unusually diverse fauna. Lake Waccamaw contains the largest number of endemic animal species (i.e., endemic to this lake and nowhere else in the world; 10 taxa) of any site in North Carolina (
Water Quality Data for Lake Waccamaw (Columbus County, North Carolina).
|
Frey (1949) |
Weiss & Kuenzler (1976) |
DWQ 2012 |
|
|
Trophic Status |
− |
− |
Mesotrophic |
|
Watershed Area (km2) |
− |
− |
181.29 |
|
Surface Area (ha) |
− |
3617.08 |
− |
|
Max Width (km) |
− |
5.47 |
− |
|
Max Length (km) |
− |
8.36 |
− |
|
Max Depth (m) |
− |
3.35 |
− |
|
Mean Depth (m) |
− |
− |
1.5 |
|
Secchi Depth (m) |
1.34 |
0.61−2.38 |
1.1−1.9 |
|
Min Temp. (°C) |
− |
14 |
23.5 |
|
Max Temp. (°C) |
− |
31.5 |
29.9 |
|
Dissolved Oxygen (mg/L) |
5.2 |
7.8−11 |
6.9−8.1 |
|
Alkalinity (meq/L) |
− |
0.14−0.24 |
− |
|
pH (s.u.) |
6.95 |
6.8−7.5 |
7.0−8.5 |
|
Total N (mg/L) |
− |
0.297−1.56 |
− |
|
Total P (mg/L) |
− |
0.017 − .055 |
− |
|
Chlorophyll-A (μg/L) |
− |
− |
2.8−8 |
Little Singletary Lake
Little Singletary Lake (626 acres; 253.33 hectares) is located in the western half of Suggs Mill Pond Game Land (Fig.
Lands abutting the southern shoreline are privately owned and were once subject to residential development. Remnants of bulkheads and recreational piers can still be seen today along the southeast shoreline. The North Carolina Wildlife Resources Commission gained property rights to all remaining lands surrounding Little Singletary Lake before residential development could ensue. On June 20, 2011, a lightning caused wildfire (Simmons Road Fire) started just west of Little Singletary Lake and by August 18th, had burned over 2,023 hectares (5,000 acres) of Carolina bay and pocosin habitat, much of which surrounded Little Singletary Lake. During growing seasons of extreme drought, water levels have been known to recede low enough to reveal a clean sandy lake bottom 90−275 m (100−300 yds) out into the lake (G. Lewis, pers. comm.). Native American projectile points have been found on this lake bottom during drought years (G. Lewis, pers. comm.).
The water quality of Little Singletary Lake has not been documented by state agencies. The water appears high in humic substances and is likely similar to the other Bladen lakes (i.e., dystrophic, acidic, shallow, nutrient poor).
Salters Lake
Salters Lake (127.47 hectares; 315 acres) is the larger of the two Carolina bay lakes located in Jones Lake State Park (Fig.
Salters Lake is similar to Jones Lake in many respects, but quite possibly could be the most “pristine” of all Carolina bay lakes. Salters Lake has no shoreline development, appreciable recreational activities (e.g., outboard motor use), immediate surrounding agricultural (crop or animal production) land use, water level control structures, or historical manipulation of any kind. Natural communities and landscape features for Salters Lake are the same as those for Jones Lake (above). Available water quality parameters for Salters Lake are provided in Table
Water Quality Data for Salters Lake (Bladen County, North Carolina).
|
Frey (1949) |
Weiss & Kuenzler (1976) |
DWQ 2009 |
|
|
Trophic Status |
− |
− |
Dystrophic |
|
Watershed Area (km2) |
− |
− |
7.77 |
|
Surface Area (ha) |
− |
127.47 |
− |
|
Max Width (km) |
− |
0.80 |
− |
|
Max Length (km) |
− |
1.12 |
− |
|
Max Depth (m) |
− |
1.82 |
− |
|
Mean Depth (m) |
− |
− |
2.13 |
|
Secchi Depth (m) |
0.55 |
0.6−0.91 |
− |
|
Min Temp. (°C) |
− |
15 |
21.7 |
|
Max Temp. (°C) |
− |
25.4 |
31.2 |
|
Dissolved Oxygen (mg/L) |
6 |
7.9−10.1 |
6.5 – 8.1 |
|
Alkalinity (meq/L) |
− |
0.0019 |
− |
|
pH (s.u.) |
4.49 |
4.1−4.8 |
3.6 – 4.1 |
|
Total N (mg/L) |
− |
0.293−0.374 |
− |
|
Total P (mg/L) |
− |
0.015−0.016 |
− |
|
Chlorophyll-A (μg/L) |
− |
− |
4.7 – 26 |
Singletary Lake
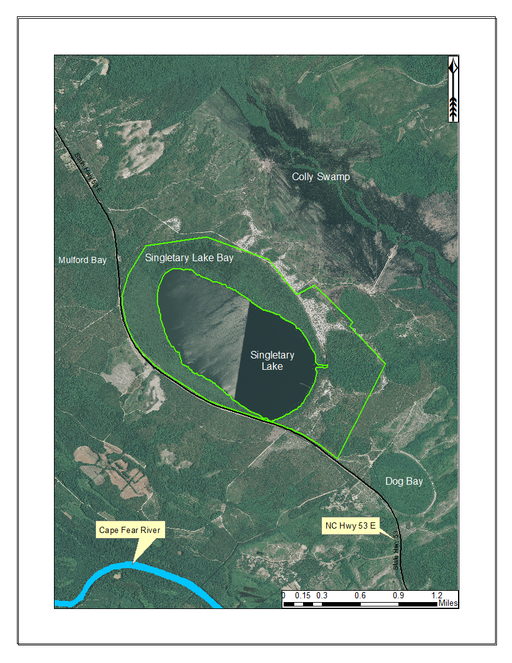
Singletary Lake (233.09 hectares; 576 acres) is located within Singletary Lake State Park (494.12 hectares; 1,221 acres; Fig.
Singletary Lake State Park (outlined in green) and surrounding lands. Singletary Lake State Park is primarily comprised of lands immediately surrounding Singletary Lake. In addition to the lands surrounding Singletary Lake, White Lake is also managed by Singletary Lake State Park. Singletary Lake State Park is located north of the Cape Fear River and State Hwy 53 and southeast of White Lake. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (
Singletary Lake is similar to the other Bladen lakes in that it is dystrophic, acidic, and nutrient poor. It contains high quality examples of the Natural Lake Shoreline Swamp (Cypress Subtype) and Natural Lake Shoreline Marsh (Typic Subtype) communities.
Water Quality Data for Singletary Lake (Bladen County, North Carolina).
|
Frey (1949) |
Weiss & Kuenzler (1976) |
DWQ 2009 |
|
|
Trophic Status |
− |
− |
Dystrophic |
|
Watershed Area (km2) |
− |
− |
5.18 |
|
Surface Area (ha) |
− |
231.48 |
− |
|
Max Width (km) |
− |
0.64 |
− |
|
Max Length (km) |
− |
2.09 |
− |
|
Max Depth (m) |
− |
2.74 |
− |
|
Mean Depth (m) |
− |
− |
2.13 |
|
Secchi Depth (m) |
0.76 |
0.48−1.21 |
0.6−1 |
|
Min Temp. (°C) |
− |
13.8 |
24.8 |
|
Max Temp. (°C) |
− |
31 |
30.6 |
|
Dissolved Oxygen (mg/L) |
6.6 |
7.3−11.2 |
6−7.8 |
|
Alkalinity (meq/L) |
− |
0.0019 |
− |
|
pH (s.u.) |
4.5 |
3.2−4.6 |
3.9−4.2 |
|
Total N (mg/L) |
− |
0.255−0.515 |
− |
|
Total P (mg/L) |
− |
0.018−0.075 |
− |
|
Chlorophyll-A (μg/L) |
− |
− |
4.8−44 |
White Lake
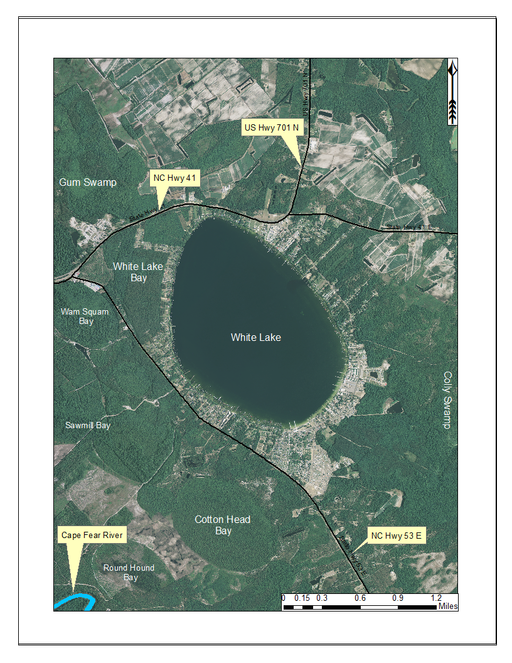
Although not included in the sampling aspect of this study, White Lake is unique and deserves a brief summary. White Lake (432.20 hectares; 1,068 acres) is a large Carolina bay lake located in east-central Bladen County about 6 miles east of Elizabethtown, just east of the intersection of NC Hwy 53 and U.S. Hwy 701 (Fig.
White Lake and surrounding lands. Like the majority of Carolina bay lakes, White Lake is a state-owned lake. All but a very small portion of White Lake’s shoreline has been altered. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (
White Lake’s remarkable water clarity is attributed to the presence of artesian springs on the lake bottom (
Water Quality Data for White Lake (Bladen County, North Carolina).
|
Frey (1949) |
Weiss & Kuenzler (1976) |
DWQ 2009 |
|
|
Trophic Status |
− |
− |
Oligotrophic |
|
Watershed Area (mi2) |
− |
− |
− |
|
Surface Area (ha) |
− |
432.2 |
− |
|
Max Width (km) |
− |
1.61 |
− |
|
Max Length (km) |
− |
2.57 |
− |
|
Max Depth (m) |
− |
3.35 |
− |
|
Mean Depth (m) |
− |
− |
3.04 |
|
Secchi Depth (m) |
3.35 |
3.35 |
3.35 |
|
Min Temp. (°C) |
− |
15.1 |
22.3 |
|
Max Temp. (°C) |
− |
26.1 |
30.1 |
|
Dissolved Oxygen (mg/L) |
6.7 |
8.6−10.1 |
6.8−8.2 |
|
Alkalinity (meq/L) |
− |
0.0019−.0099 |
− |
|
pH (s.u.) |
4.92 |
4.6−4.8 |
4.6−5.2 |
|
Total N (mg/L) |
− |
0.123−0.211 |
− |
|
Total P (mg/L) |
− |
0.010−0.017 |
− |
|
Chlorophyll-A (μg/L) |
− |
− |
4.8−44 |
Climate
Bladen Lake Group (Bladen County, NC)
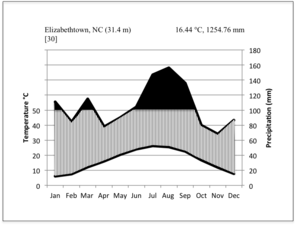
Climate data from the nearest weather station to the Bladen County bay lakes, ca. 1.6 kilometers away in Elizabethtown, North Carolina (Bladen County: 34.68° N, -78.58°W; 30.5 m elev.), show that during the thirty-year period between 1971-2000, the average annual temperature was 16.44 °C (61.6 °F) and mean annual precipitation 1,254.76 mm (49.4 in). Average daily maximum and minimum temperatures were 22.83 °C (73.1 °F) and 10.11 °C (50.2 °F;
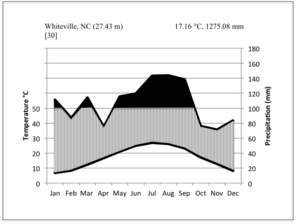
Walter climate diagrams for weather stations closest to the Bladen Lakes (Bladen County, NC; a) and Lake Waccamaw (Columbus County, NC; b), based on data from the
The lowest temperature recorded for Bladen County was -14.4 °C (6 °F) on January 17, 1977 (
Lake Waccamaw (Columbus County, NC)
Climate data from the nearest weather station to Lake Waccamaw, ca. 16 km away in Whiteville, North Carolina (Columbus County: 34.27287° N, -78.71499° W; 29.8 meters above sea level), show that for the 30-year period between 1971 and 2000, the average annual temperature was 17.16 °C (62.9 °F) and mean annual precipitation 1,275.08 mm (50.2 in). The average daily maximum and minimum temperatures over the same thirty-year period were 24.3 °C (75.8 °F) and 10 °C (50 °F;
The lowest temperature recorded for Columbus County was -15 °C (5 °F) on February 12, 1973 (
Plant Communities
Four plant community types and two subtypes can be distinguished within the littoral zone of Carolina bay lakes (
Plant community types occurring within the littoral zone of Carolina bay lakes. Community types follow
Species Richness |
Plant Community Types |
State Rank |
Global Rank |
Lowest Highest |
Natural Lake Shoreline Marsh (Lake Waccamaw Pondlily Subtype) |
S1 |
G1 |
|
Coastal Plain Semipermanent Impoundment |
S4 |
G4G5 |
|
|
floating Bog |
S1 |
G1? |
|
|
Natural Lake Shoreline Swamp (Cypress Subtype) |
S2 |
G3 |
|
|
Natural Lake Shoreline Marsh (Typic Subtype) |
S1 |
G1 |
|
|
Natural Lake Shoreline Swamp (Lake Waccamaw Subtype) |
S1 |
G1 |
|
|
S1 = Critically Imperiled, 1–5 occurrences in state; S2 = Imperiled, 6–20 occurrences in state; S4 = Apparently Secure, 101–1000 occurrences in state; G1 = Critically Imperiled, 1–5 occurrences in the world; G3 = Vulnerable, 21–100 occurrences in the world; G4 = Apparently Secure, 101–1000 occurrences in the world; G5 = Secure, 1001+ occurrences in the world. |
|||
Natural Lake Shoreline Swamp (Cypress Subtype; S2G3) [Taxodium distichum – T. ascendens / Panicum hemitomon Schult. Woodland (CES203.044)].
This natural community type covers Carolina bay lake shorelines with narrow littoral zones characterized by an absent to sparse herbaceous component and a nearly closed canopy of Chamaecyparis Spach, Nyssa L., or Taxodium Rich. in the upper eulittoral zone. If a cross-section of this littoral zone were to be drawn, the epilittoral vegetation would abruptly coincide with the littoral zone (i.e., a zone of emergent herbaceous vegetation is lacking where it typically would occur between the epilittoral and infralittoral zones). This “two-staged” zonation pattern typical of this community type is directly attributable to the steeper hydrography and narrow littoral zone. The Natural Lake Shoreline Swamp (Lake Waccamaw Subtype) and the Natural Lake Shoreline Marsh community types can be distinguished from the depauperate Natural Lake Shoreline Swamp (Cypress Subtype) community type by having a broader littoral zone, a well-developed zone of herbaceous emergent macrophytes, a sparse to open canopy of Nyssa, Taxodium, or other obligate wetland hardwoods, and the absence of Nuphar sagittifolia (Walter) Pursh. Examples of this community type are found at Bakers Lake, and the western, northern, and eastern shorelines of Jones, Salters, Little Singletary, and Singletary Lakes.
Natural Lake Shoreline Swamp (Lake Waccamaw Subtype; S1G1) [Taxodium distichum – T. ascendens / Panicum hemitomon – Sclerolepis uniflora (Walter) Britton, Sterns & Poggenb. Woodland (CEGL004465)].
This natural community type covers the southern shoreline of Lake Waccamaw located between Big Creek and the lake’s outlet on the southwest shore. This stretch of natural shoreline is characterized by gentle hydrography, which results in a broad littoral zone, and a species-rich flora dominated by emergent herbaceous macrophytes, many of which are rare. Emergent macrophytes typical of this community type include Cladium mariscoides (Muhl.) Torr., Eriocaulon aqutaicum (Hill) Druce, Panicum hemitomon, Sclerolepis uniflora, and Xyris smalliana, among others. This community type can be distinguished from the species-poor Natural Lake Shoreline Swamp (Cypress Subtype) community type by its broader littoral zone and species-rich herbaceous component (95 taxa). It can be distinguished from the Natural Lake Shoreline Marsh community types by the absence or only irregular presence of Nuphar sagittifolia and the unique assemblage of diverse herbaceous taxa (e.g., Bacopa caroliniana (Walter) B.L. Rob., Boltonia asteroides var. glastifolia, Cladium mariscoides, Ludwigia brevipes (B.H. Long ex Britton, A. Braun & Small) Eames, L. sphaerocarpa Elliott, and Sclerolepis uniflora).
Natural Lake Shoreline Marsh (Typic Subtype; S1G1) [Panicum hemitomon – Juncus spp. Coastal Plain Lakeshore Herbaceous Vegetation (CEGL004307)].
This natural community type covers the southern shorelines of the Bladen Lakes. The southern shorelines have a broader littoral zone than the remaining portions of the lakes. Consequently, they support a more diverse emergent herbaceous component. Herbs found in this community type include Eleocharis baldwinii, E. equisetoides (Elliott) Torr., E. vivipara, Juncus pelocarpus E. Mey., Panicum hemitomon, Panicum verrucosum Muhl., Rhexia nashii, Rhynchospora distans, Saccharum giganteum (Walter) Pers., Sacciolepis striata, Scirpus cyperinus (l.) Kunth, and Xyris smalliana. This community type is also characterized as having a sparse to open canopy of Nyssa and Taxodium. This community type can be distinguished from the Natural Lake Shoreline Marsh (Lake Waccamaw Pondlily Subtype) by the absence of Nuphar sagittifolia and from the Natural Lake Shoreline Swamp (Lake Waccamaw Subtype) by the occurence of < 30 herbaceous taxa, none of which include the unique and rare herbs found at Lake Waccamaw. Examples of this community type include the southern shorelines of Jones, Little Singletary, Salters, and Singletary Lakes.
Natural Lake Shoreline Marsh (Lake Waccamaw Pond-lily Subtype; S1G1) [Nuphar sagittifola – Eriocaulon aquaticum Lakeshore Herbaceous Vegetation (CEGL004297)].
This natural community type covers the western, northern, and eastern shorelines of Lake Waccamaw (i.e., where residential and commercial development is present). It is the only Natural Lake Shoreline community type dominated by Nuphar sagittifolia (a distinguishing feature) and Eriocaulon aquaticum. Nuphar sagittifolia is essentially absent from the Natural Lake Shoreline Swamp (Lake Waccamaw Subtype) community type save for small stands around the mouth of Big Creek and around the dam at the lakes outlet.
floating Bog [Rhynchospora alba Saturated Herbaceous Vegetation (CEGL004463)]
This natural community type covers the rare examples of vegetation occuring on floating peat mats in deep water of natural or artificial ponds and lakes. Horseshoe Lake is the only Carolina bay lake known to support floating bogs. The floating bogs of Horseshoe Lake are the largest in the state. These floating bogs are saturated and nutrient-poor, supporting taxa that characteristically inhabit such stressful conditions (e.g., Calopogon tuberosus (L.) Britton, Sterns & Poggenb., Drosera intermedia, Dulichium arundinaceum, Hypericum virginicum, Pogonia ophioglossoides, Rhynchospora alba, R. inundata, and Xyris fimbriata). This community type’s “floating” nature and the presence of the aforementioned plant taxa sets it apart from all others.
Coastal Plain Semipermanent Impoundment (Cypress-Gum Subtype; G4G5) [Taxodium distichum / Lemna minor L. Forest (CEGL002420)]
All portions of Horseshoe Lake not considered floating Bog fall into the Coastal Plain Semipermanent Impoundment community type. This community type is characterized by a sparse to absent canopy of Taxodium ascendens with sporadically occurring beds of floating-leaved and submersed aquatics (e.g., Brasenia schreberi J.F. Gmel, Cabomba caroliniana A. Gray, Nymphaea odorata ssp. odorata, and Utricularia spp.). This community type can be distinguished from all others by the sparse presence of Taxodium throughout the lake with floating-leaved and submersed aquatics occurring underneath.
Floristic Summary
Across All Sites
The littoral zone vascular flora of Carolina bay lakes, based on vouchered collections, reports, and personal observations, consists of 205 taxa (170 species, 4 subspecies, 30 varieties, 1 hybrid) in 136 genera and 80 vascular plant families (Table
Summary of vascular plant taxa collected or reported from Carolina bay lake littoral zones
|
Species and Subspecies/Varieties |
|||||
|
Group |
Families |
Genera |
Native |
Exotic |
Total |
|
Basal Angiosperms & Magnoliids |
4 |
6 |
6 |
0 |
6 |
|
Pteridophytes |
6 |
7 |
7 |
0 |
7 |
|
Gymnosperms |
2 |
3 |
5 |
0 |
5 |
|
Monocotyledons |
17 |
41 |
84 |
2 |
86 |
|
Eudicotyledons |
51 |
79 |
98 |
3 |
101 |
|
Total |
80 |
136 |
200 |
5 |
205 |
List of North Carolina Significantly Rare and Watch List taxa collected or reported from Carolina bay lake littoral zones. Status and rank designations follow
|
Taxon |
Vouchered ? |
State Status |
Fed. Status |
State Rank |
Global Rank |
|
|
Significantly Rare: |
||||||
|
1 |
Bacopa caroliniana (Walter) B.L. Rob. |
✓ |
T |
− |
S1 |
G4G5 |
|
2 |
Boltonia asteroides var. glastifolia (Hill) Fernald |
✓ |
SR−O |
− |
S2 |
G5TNR |
|
3 |
Cladium mariscoides (Muhl) Torr. |
✓ |
SR−O |
− |
S3 |
G5 |
|
4 |
Eleocharis vivipara Link |
✓ |
E |
− |
S1 |
G5 |
|
5 |
Epidendrum magnoliae Muhl. |
✓ |
T |
− |
S1S2 |
G4 |
|
6 |
Eriocaulon aquaticum (Hill) Druce |
✓ |
SC−V |
− |
S2 |
G5 |
|
7 |
Ludwigia brevipes (Long) Eames |
✓ |
SR−T |
FSC |
S1S2 |
G2G3 |
|
8 |
Ludwigia sphaerocarpa Elliott |
✓ |
E |
− |
S1 |
G5 |
|
9 |
Luziola fluitans var. fluitans |
✓ |
SR−P |
− |
S2 |
G4,G5 |
|
10 |
Lycopus angustifolius Elliott |
✓ |
SR−P |
− |
S1 |
G4?Q |
|
11 |
Rhexia aristosa Britton |
SC−V |
FSC |
S3 |
G3,G4 |
|
|
12 |
Rhynchospora alba (L.) Vahl |
✓ |
SR−P |
− |
S2 |
G5 |
|
13 |
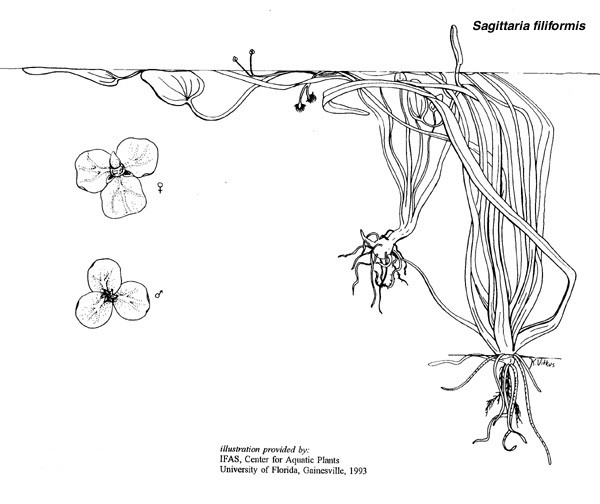
Sagittaria filiformis J.G. Sm. |
✓ |
SR−P |
− |
SH |
G4,G5 |
|
14 |
Sagittaria isoetiformis J.G. Sm. |
✓ |
T |
− |
S2 |
G4? |
|
15 |
Sagittaria weatherbiana Fernald |
✓ |
E |
FSC |
S2 |
G3G4 |
|
16 |
Sclerolepis uniflora (Walter) Britton, Sterns & Poggenb. |
✓ |
SR−T |
− |
S2 |
G4 |
|
17 |
Spiranthes laciniata (Small) Ames |
✓ |
SC−V |
− |
S2 |
G4,G5 |
|
18 |
Utricularia cornuta Michx. |
✓ |
T |
− |
S1S2 |
G5 |
|
19 |
Utricularia resupinata B.D. Greene ex Bigelow |
✓ |
E |
− |
S1 |
G4 |
|
Watch List: |
||||||
|
1 |
Dichanthelium dichotomum var. roanokense (Ashe) LeBlond |
✓ |
W1 |
− |
S2 |
G5T4? |
|
2 |
Dichanthelium erectifolium (Nash) Gould & C.A. Clark |
✓ |
W1 |
− |
S2 |
G4 |
|
3 |
Dryopteris ludoviciana (Kunze) Small |
✓ |
W1 |
− |
S2 |
G4 |
|
4 |
Eleocharis equisetoides (Elliott) Torr. |
✓ |
W1 |
− |
S3 |
G4 |
|
5 |
Habaneria repens Nutt. |
W1 |
− |
S2 |
G5 |
|
|
6 |
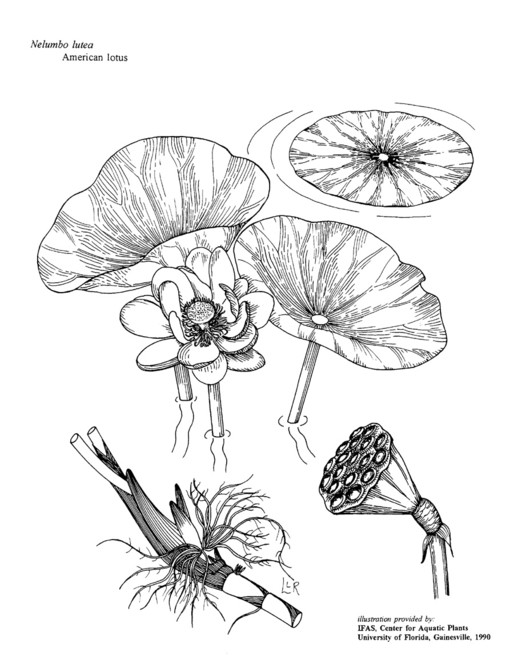
Nelumbo lutea Willd. |
✓ |
W7 |
− |
S2 |
G4 |
|
7 |
Nuphar sagittifolia (Walter) Pursh |
✓ |
W1 |
FSC |
S2 |
G5T2 |
|
8 |
Rhexia cubensis Griseb. |
✓ |
W1 |
− |
S3 |
G4G5 |
|
9 |
Rhynchospora inundata (Oakes) Fernald |
✓ |
W1 |
− |
S3 |
G4? |
|
10 |
Rhynchospora nitens (Vahl) A. Gray |
✓ |
W1 |
− |
S3 |
G4? |
|
11 |
Xyris iridifolia Chapm. |
W7 |
− |
S2 |
G4G5T4T |
|
|
12 |
Xyris smalliana Nash |
✓ |
W1 |
− |
S3 |
G5 |
|
STATE STATUS: E = Endangered; T = Threatened; SC-V = Special Concern-Vulnerable; SR = Significantly Rare: −T = Throughout; −P = Periphery of Range; −O = Other; W = Watchlist: W1 = rare but relatively secure; W7 = rare and poorly known. FEDERAL STATUS: FSC = Federal Species of Concern. STATE RANK: SH = historical (known only from historical populations in the state); S1 = Critically Imperiled, 1–5 populations in the state; S2 = Imperiled, 6–20 populations in the state; S3 = Vulnerable, 21–100 populations in the state. FEDERAL RANK: G2 = Imperiled, 6–20 populations in the world; G3 = Vulnerable, 21–100 populations in the world; G4 = Apparently Secure, 101–1000 populations in the world; G5 = Secure, 1001+ populations in the world; T# = Global rank of a subspecies or variety; NR = Not Ranked; Q = Questionable taxonomy; ? = Uncertain. |
||||||
Sørenson’s Similarity Index for Carolina bay lakes. Values in this table are represented as percentiles (i.e., when looking in the second column from the left under Bakers Lake, Bakers Lake is considered to be 16.4% similar to Bay Tree Lake, 23.5% similar to Horseshoe Lake, and 40.8% similar to Jones Lake). Based solely on littoral zone plant taxa, Jones Lake and Singletary Lake are 83.3% alike.
Bakers Lake |
Bay Tree Lake |
Horseshoe Lake |
Jones Lake |
Lake Waccamaw |
Little SingletaryLake |
Salters Lake |
Singletary Lake |
|
|
Bakers Lake |
100 |
16.4 |
23.5 |
40.8 |
12.4 |
39.3 |
41.0 |
41.5 |
|
Bay Tree Lake |
16.4 |
100 |
37.4 |
38.6 |
33.0 |
46.3 |
32.4 |
41.3 |
|
Horseshoe Lake |
23.5 |
37.4 |
100 |
38.6 |
26.7 |
42.2 |
24.7 |
48.3 |
|
Jones Lake |
40.8 |
38.6 |
38.5 |
100 |
20.5 |
42.5 |
55.6 |
83.3 |
|
Lake Waccamaw |
12.4 |
33.0 |
26.7 |
20.5 |
100 |
22.5 |
21.3 |
28.9 |
|
Little Singletary Lake |
39.3 |
46.3 |
42.2 |
42.5 |
22.5 |
100 |
29.5 |
56.0 |
|
Salters Lake |
41.0 |
32.4 |
24.7 |
55.5 |
21.3 |
29.5 |
100 |
51.7 |
|
Singletary Lake |
41.5 |
41.3 |
48.3 |
83.3 |
28.9 |
56.0 |
51.7 |
100 |
Among all taxa treated in this guide, the major vascular plant groups consisted of the following total taxa: Eudicotyledons (101 taxa; 86 species, 1 subspecies, 13 varieties, 1 hybrid), monocotyledons (86 taxa; 71 species, 1 subspecies, 14 varieties), pteridophytes (7 taxa; 6 species and 1 subspecies), gymnosperms (5 species), basal angiosperms (4 taxa; 3 species and 1 subspecies), and magnoliids (2 taxa; 1 species and 1 variety; Table
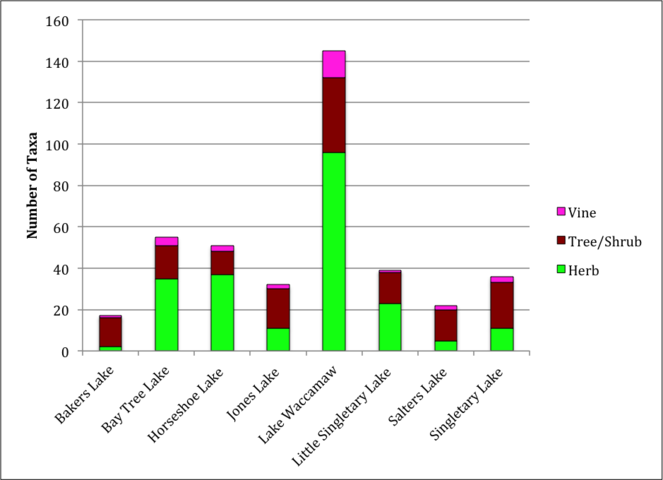
Distribution of plant habit across all Carolina bay lakes. Lakes dominated by herbs have broader littoral zones, which encourage the establishment of herbaceous emergent macrophytes. Lakes dominated by trees and shrubs have narrow littoral zones, which discourage the establishment of herbaceous emergent macrophytes.
Among all taxa treated in this guide, the most species-rich habit is herbs (140 taxa; 119 species, 2 subspecies, 18 varieties, 1 hybrid), followed by trees and shrubs (51 taxa; 42 species, 1 subspecies, 8 varieties), and vines (14 taxa, 12 species, 2 varieties; Fig.
Among the natural community types included in this work, the Natural Lake Shoreline Swamp (Lake Waccamaw Subtype) is the most species-rich (145 taxa) and the Natural Lake Shoreline Marsh (Lake Waccamaw Pondlily Subtype) is the least species-rich (< 10 taxa; Table
Individual Lakes
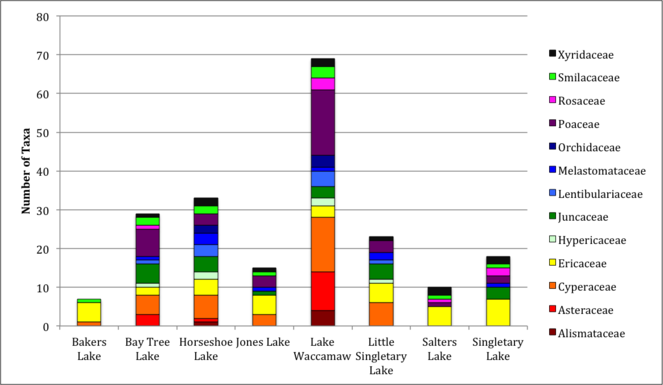
Among the lakes, the largest number of littoral zone taxa (i.e., species, subspecies, and varieties) occurred in Lake Waccamaw (145 taxa), followed by Bay Tree Lake (56 taxa) and Horseshoe Lake (52 taxa; Table
Number of taxa (species, subspecies, and varieties) by major taxonomic group across study sites. Sites are arranged from taxonomically richest to most depauperate. BALA = Bakers Lake; BATR = Bay Tree Lake; HOLA = Horseshoe Lake; JOLA = Jones Lake; LAWA = Lake Waccamaw; LISI = Little Singletary Lake; SALA = Salters Lake; SILA = Singletary Lake.
|
LAWA |
BATR |
HOLA |
LISI |
SILA |
JOLA |
SALA |
BALA |
|
|
Pteridophytes |
7 |
3 |
1 |
2 |
1 |
1 |
1 |
1 |
|
Gymnosperms |
2 |
3 |
3 |
3 |
5 |
4 |
2 |
1 |
|
Basal angiosperms |
3 |
-- |
3 |
-- |
1 |
-- |
-- |
-- |
|
Magnoliids |
2 |
-- |
-- |
1 |
2 |
2 |
2 |
2 |
|
Monocots |
60 |
23 |
21 |
17 |
9 |
10 |
5 |
3 |
|
Eudicots |
71 |
27 |
24 |
16 |
18 |
16 |
12 |
11 |
|
Total |
145 |
56 |
52 |
39 |
36 |
33 |
22 |
18 |
Bakers Lake
The littoral zone vascular flora of Bakers Lake is depauperate with respect to the other bay lakes (Table
The most species-rich habit class was trees and shrubs (14 taxa; 10 species, 4 varieties), followed by herbs (3 taxa), and vines (1 taxa; Fig.
Bay Tree Lake
The littoral zone vascular flora of Bay Tree Lake is comprised of 56 taxa (48 species, 2 subspecies, and 6 varieties), in 47 genera and 34 vascular plant families (Table
The richest eudicotyledon families are Asteraceae (3 taxa), followed by Ericaceae (2 taxa) and Aquifoliaceae (2 taxa;) . The richest monocotyledonous families are Poaceae (7 taxa; 6 species, 1 subspecies), Cyperaceae (5 taxa; 4 species, 1 variety), and Juncaceae (5 taxa). The richest monocotyledon genera are Juncus (5 taxa; 3 species, 1 subspecies, 1 variety) and Panicum (3 taxa).
The most species-rich habit class was herbs (35 taxa; 29 species, 2 subspecies, 4 varieties), followed by trees and shrubs (16 taxa; 15 species, 1 variety), and vines (4 species, 1 variety; Fig.
Horseshoe Lake
The littoral zone vascular flora of Horseshoe Lake is comprised of 52 taxa (45 species, 2 subspecies, and 5 varieties), in 41 genera and 29 vascular plant families (Table
The richest eudicotyledon families are Ericaceae (4 taxa), Lentibulariaceae (3 taxa) and Melastomataceae (3 taxa). The richest eudicotyledonous genera are Rhexia (3 taxa), Utricularia (3 taxa), followed by Hypericum (2 taxa). The richest monocotyledonous families are Cyperaceae (5 taxa), Juncaceae (4 taxa), Poaceae (3 taxa), followed by Orchidaceae (2 taxa), Smilacaceae (2 taxa) and Xyridaceae (2 taxa). The richest monocotyledonous genera are Juncus (4 taxa), followed by Rhynchospora (2 taxa), Smilax (2 taxa), and Xyris (2 taxa).
The most species-rich habit class was herbs (38 taxa; 31 species, 2 subspecies, 4 varieties), followed by trees and shrubs (11 taxa; 10 species, 1 variety), and vines (3 taxa; Fig.
Jones Lake
The littoral zone vascular flora of Jones Lake is comprised of 33 taxa (29 species, 1 subspecies, and 3 varieties), in 31 genera and 23 vascular plant families (Table
The richest eudicotyledonous family is Ericaceae (5 taxa). The richest eudicotyledonous genus is Lyonia (2 taxa; 1 species, 1 variety). The richest monocotyledonous families are Cyperaceae (3 taxa) and Poaceae (3 taxa). Monocotyledons are comprised of ten different genera.
The most species-rich habit class was trees and shrubs (20 taxa; 16 species, 1 subspecies, 3 varieties), followed by herbs (11 taxa), and vines (2 taxa; Fig.
Lake Waccamaw
The littoral zone vascular flora of Lake Waccamaw is comprised of 145 taxa (122 species, 3 subspecies, 19 varieties, 1 hybrid), in 111 genera and 72 vascular plant families (Table
The richest eudicotyledonous families are Asteraceae (10 taxa; 8 species, 1 variety, 1 hybrid), followed by Lentibulariaceae (4 taxa), Ericaceae (3 taxa), Rosaceae (3 taxa), and Salicaceae (3 taxa). The richest eudicotyledonous genera are Utricularia (4 taxa), Eupatorium L. (2 taxa), Hypericum (2 taxa), Ludwigia L. (2 taxa), Nyssa (2 taxa), and Salix L. (2 taxa). The richest monocotyledonous families are Poaceae (17 taxa; 13 species, 1 subspecies, 3 varieties), Cyperaceae (14 taxa; 11 species, 3 varieties), Alismataceae (4 taxa), Juncaceae (3 taxa), Orchidaceae (3 taxa), and Smilacaceae (3 taxa). The richest monocotyledonous genera are Dichanthelium (Hitchc. & Chase) Gould (6 taxa; 5 species and 1 variety), Rhynchospora (6 taxa; 5 species and 1 variety), Sagittaria L. (4 taxa), Juncus (3 taxa; 3 species, 1 subspecies, 1 variety) and Smilax L. (3 taxa).
The most species-rich habit class was herbs (96 taxa; 80 species, 3 subspecies, 13 varieties, 1 hybrid), followed by trees and shrubs (36 taxa; 32 species, 4 varieties), and vines (13 taxa; 11 species, 2 varieties; Fig.
Little Singletary Lake
The littoral zone flora of Littoral Singletary Lake is comprised of 39 taxa (35 species, 1 subspecies, 3 varieties), in 32 genera and 21 vascular plant families (Table
The richest eudicotyledonous genus is Rhexia (2 taxa). The richest monocotyledonous families are Cyperaceae (6 taxa; 5 species and 1 variety), Juncaceae (4 taxa; 2 species, 1 subspecies, 1 variety), and Poaceae (3 taxa). The richest monocotyledonous genera are Juncus (4 taxa), Eleocharis (3 taxa), and Panicum (2 taxa).
The most species-rich habit class was herbs (23 taxa; 20 species, 1 subspecies, 2 varieties), followed by trees and shrubs (15 taxa; 14 species and 1 variety), and vines (1 taxon; Fig.
Salters Lake
The littoral zone flora of Salters Lake is comprised of 22 taxa (16 species, 2 subspecies, 4 varieties), in 18 genera and 16 vascular plant families (Table
The richest eudicotyledon family is Ericaceae (5 taxa). The richest eudicotyledonous genera are Lyonia (2 taxa) and Vaccinium (2 taxa). The richest monocotyledonous family is Xyridaceae (2 taxa). The richest monocotyledon genus is Xyris (2 taxa).
The most species-rich habit class was trees and shrubs (15 taxa; 11 species, 1 subspecies, 3 varieties), herbs (5 taxa; 4 species and 1 subspecies), and vines (2 taxa; Fig.
Singletary Lake
The littoral zone vascular flora of Singletary Lake is comprised of 36 taxa (32 species, 1 subspecies, 3 varieties), in 30 genera and 22 vascular plant families (Table
The richest eudicotyledonous families are Ericaceae (7 taxa) and Rosaceae (2 taxa). The richest eudicotyledonous genus is Vaccinium (2 taxa). The richest monocotyledonous families are Juncaceae (3 taxa), Poaceae (2 taxa), and Xyridaceae (2 taxa). The richest monocotyledonous genera are Juncus (3 taxa) and Xyris (2 taxa).
The most species-rich habit class was trees and shrubs (22 taxa; 19 species and 3 varieties), herbs (11 taxa; 10 species and 1 subspecies), and vines (3 taxa; Fig.
White Lake
White Lake was not included in this study due to the severity of the lake’s shoreline development. A provisional checklist of plants known to occur within the littoral zone of White Lake (from historical vouchers, personal observation, and literature review) is provided in Suppl. material
Materials and methods
This work is restricted to the littoral zone vascular flora of unaltered Carolina bay lake shorelines. The littoral zone was defined as the zone of vegetation occurring between the maximum annual high water mark and the point at which submerged aquatic plants cease to persist (Fig.
During the 2013 and 2014 growing seasons, 36 total visits were made to the eight study sites meeting the criteria articulated above (i.e., Bakers Lake, Bay Tree Lake, Horseshoe Lake, Jones Lake, Lake Waccamaw, Little Singletary Lake, Salters Lake, Singletary Lake), resulting in 121 field hours and the identification of 204 taxa (species, subspecies, and varieties). A 10-foot aluminum boat with a transom-mounted trolling motor was used to transport equipment along Carolina bay lake shorelines. Where water was too shallow for the use of the trolling motor, we walked and pulled the boat by rope. GPS locations (NAD 83) were taken at numerous intervals and associated with all specimens collected within 30 m of each point. Digital photographs of plant habit and overall morphology were taken prior to collection using a Panasonic Lumix FZ−150. Plant specimens were pressed while in the field. Tissue samples were taken in the field and dessicated with blue indicating silica gel (purchased from Delta Enterprises Inc.) in ziploc bags. Voucher specimens and tissue samples were deposited respectively at the North Carolina State University Vascular Plant Herbarium (NCSC) and its DNA bank. The entirety of Carolina bay lake shorelines was surveyed, but it was quickly observed that all shorelines, save for the southernmost, were relatively depauperate. All taxa occurring along western, northern, and eastern shorelines could be found within the littoral zone of the southern shoreline, but the inverse did not hold true. The significantly gentler hydrography (see
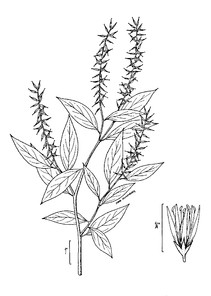
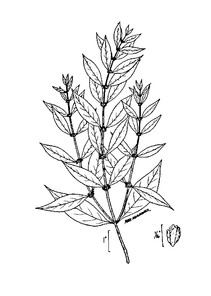
The flora is organized by the following major vascular plant groups: (1) pteridophytes, (2) gymnosperms, (3) monocots, and (4) basal angiosperms, magnoliids, and eudicotyledons. Dichotomomous keys are provided to each major group, as well as to families, genera, and species within each group. Notes are provided above some keys to aid in the identification process. Within each group, taxa are arranged alphabetically, by family, then genus, then species.
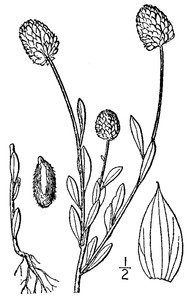
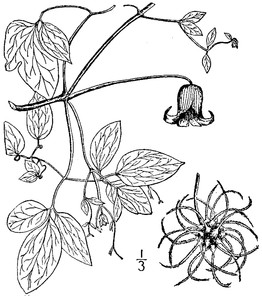
The following information is provided for each taxon account: taxon concept mapping, basionym, conservation status, habit, habitat, flowering and fruiting phenology, abundance, and presence/absence data for each site (Suppl. material
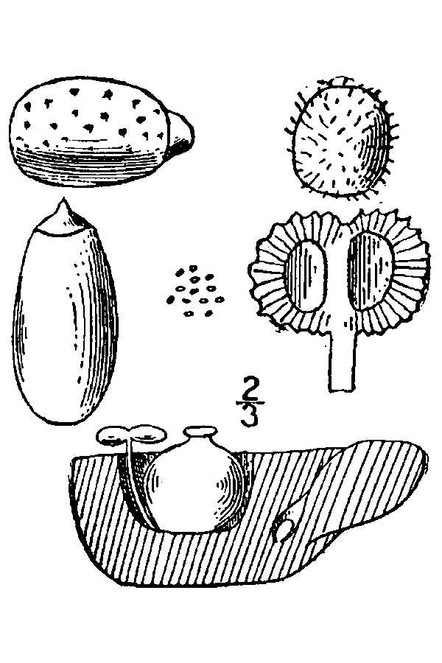
Abundance estimates following the recommendations of
Descriptions for estimating the abundance of taxa (adapted from
|
Density |
Description |
|
Abundant |
Dominant or co-dominant in one or more communities. |
|
Frequent |
Easily seen or found in one or more common communities but not dominant in any common community |
|
Occasional |
Widely scattered but not difficult to find |
|
Infrequent |
Difficult to find with few individuals or colonies but found in several locations |
|
Rare |
Very difficult to find and limited to one or very few locations or uncommon communities |
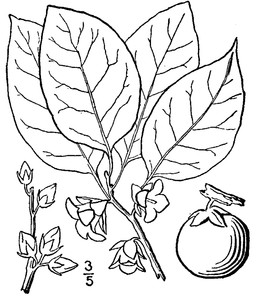
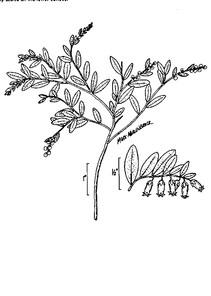
When available, digital photographs and line drawings were obtained from:
In addition, relevant historical vouchers are cited based on systematic searches of the three major herbaria−DUKE, NCSC, and NCU. Unfortunately, it is not uncommon to find historical specimens containing vague habitat or locality descriptions. For a taxon to be included in the present study, a clear label statement referencing Carolina bay lake shoreline habitat was required (e.g., “collected from peat-drained lake bed of Suggs Mill Pond”). Herbarium vouchers meeting this criterion were annotated (following taxon concepts accepted here) and their label information was subsequently entered into spreadsheets for organization. Label information for new collections resulting from this study was captured in a DarwinCore compliant spreadsheet for upload to the online portal of the Southeastern Regional Network of Expertise and Collections (www.sernecportal.org), which feeds into iDigBio and the Global Biodiversity Data Facility (GBIF).
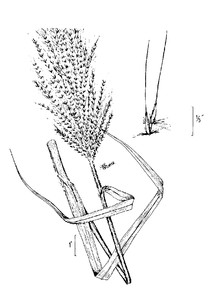
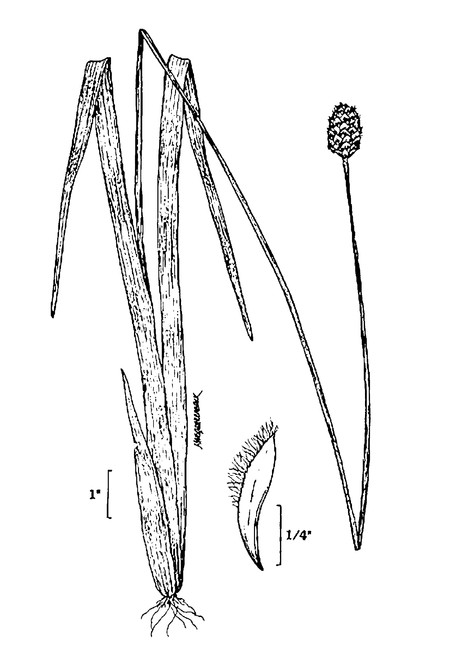
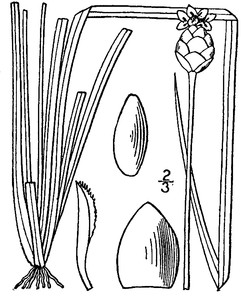
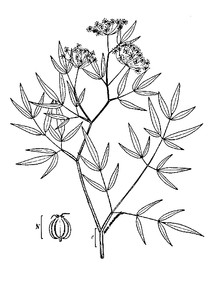
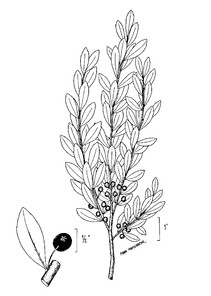
PTERIDOPHYTES
Families represented: 6
Blechnaceae
Anchistea virginica
Basionym: Blechnum virginicum L.
Taxon concept: [= Woodwardia virginica (L.) Sm. − RAB, FNA, Weakley]
Bakers Lake (Infrequent): Howell BALA−14 (NCSC!)
Bay Tree Lake (Occasional): Howell BATR−4, 24 (NCSC!)
Jones Lake (Rare): Howell JOLA−44 (NCSC!)
Lake Waccamaw (Infrequent): Howell LAWA−59 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−42 (NCSC!)
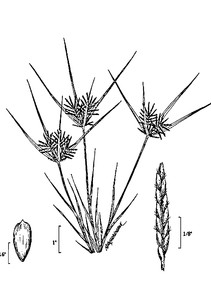
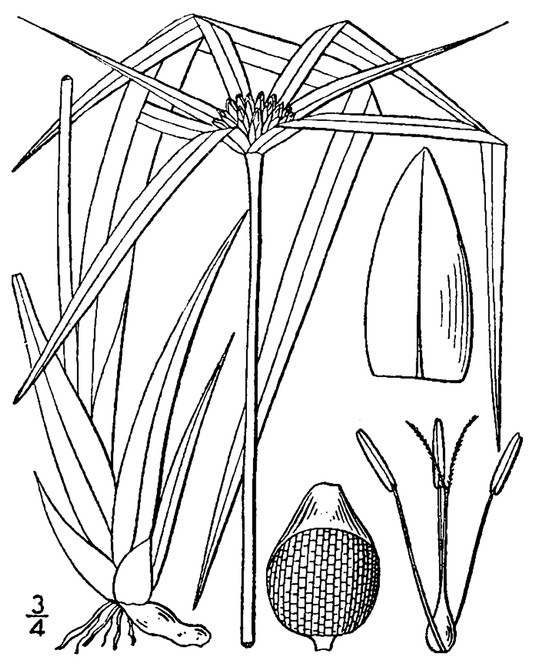
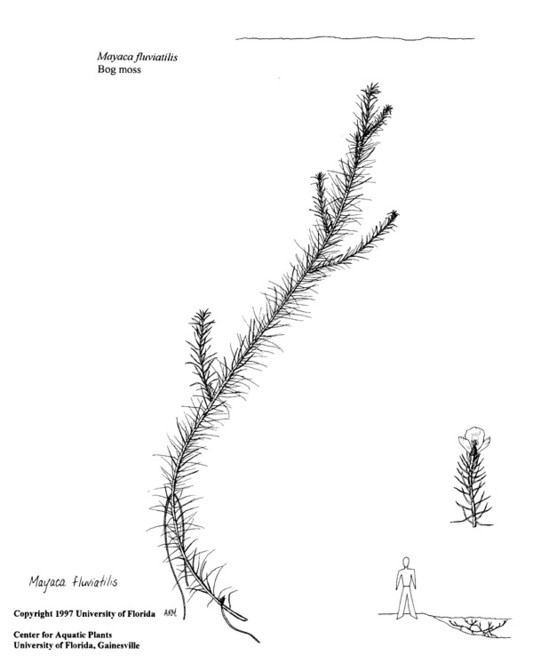
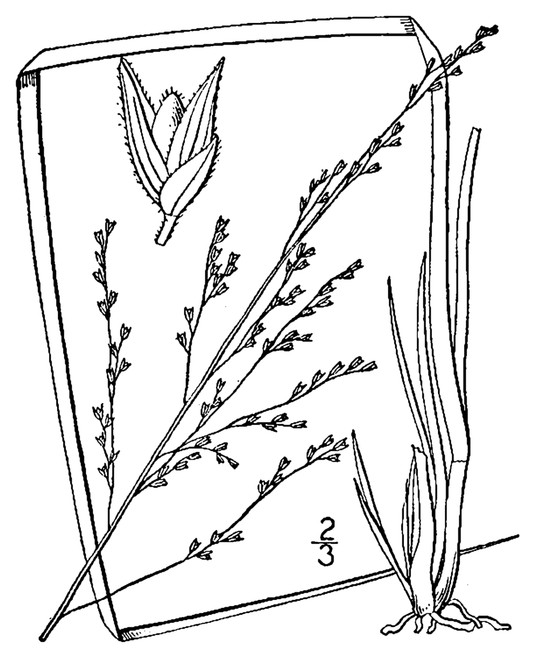
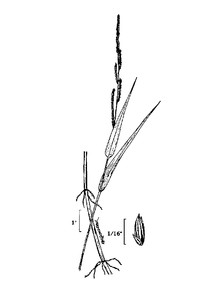
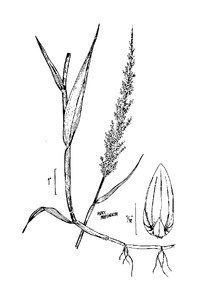
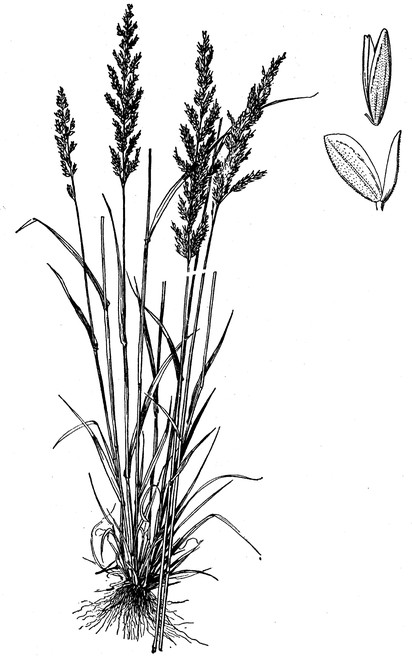
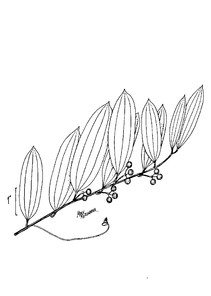
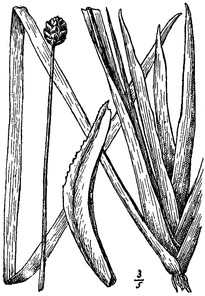
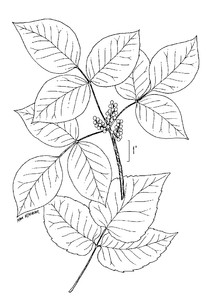
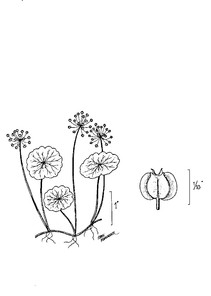
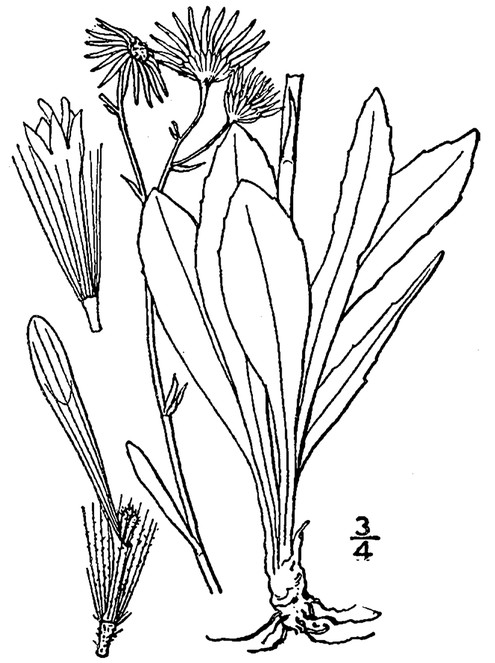
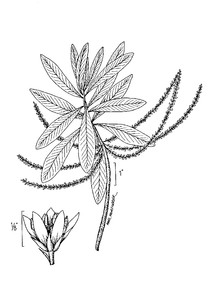
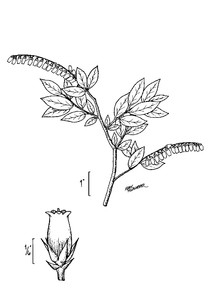
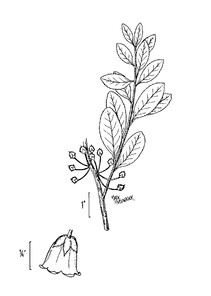
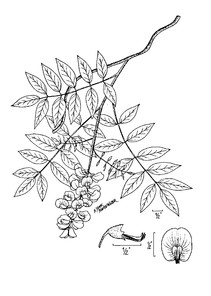
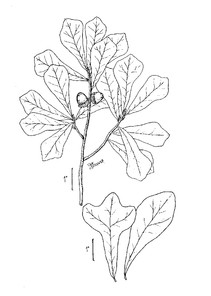
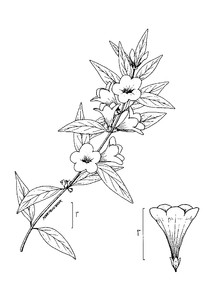
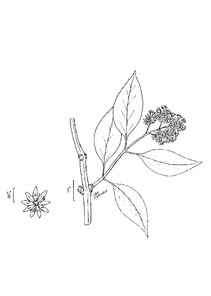
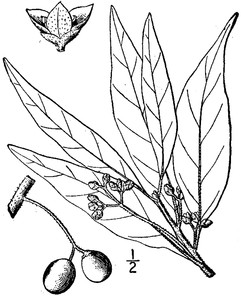
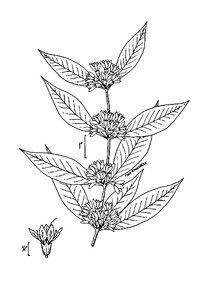
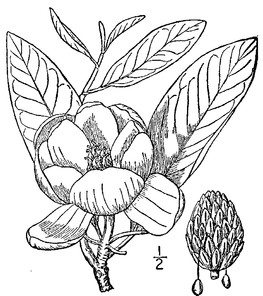
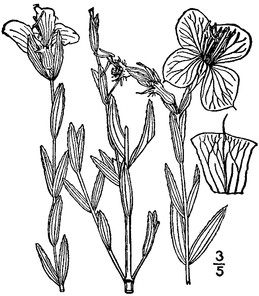
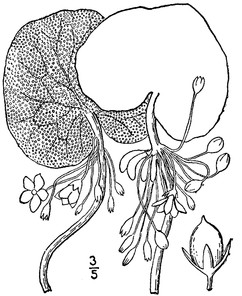
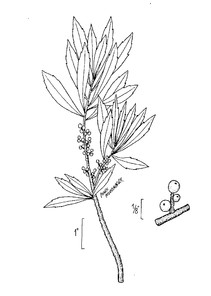
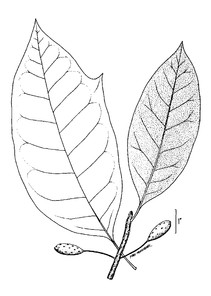
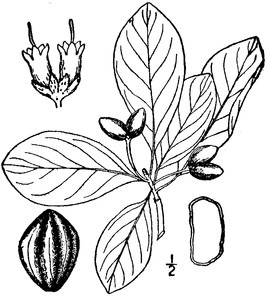
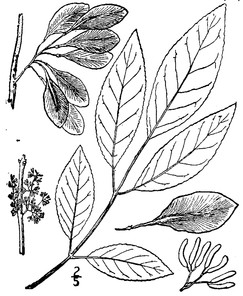
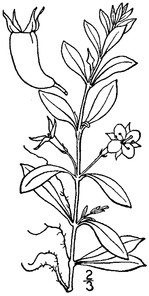
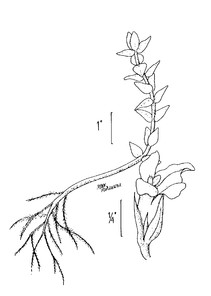
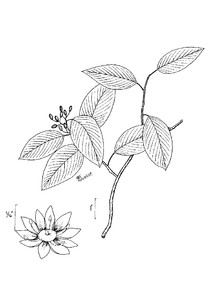
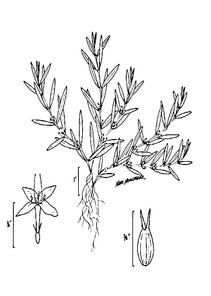
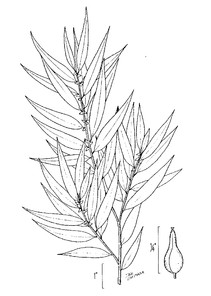
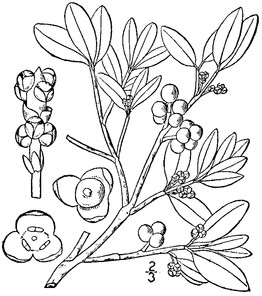
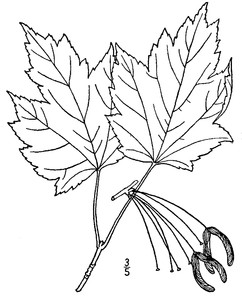
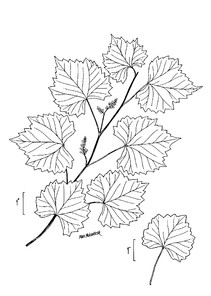
Perennial herbs. Upper eulittoral zone; typically found in saturated soils or rooted on logs, stumps, and other debris (NLSS–C, NLSS–LW, NLSM–T). Jun–Sep. Fig.
Lorinseria areolata
Basionym: Acrostichum areolatum L.
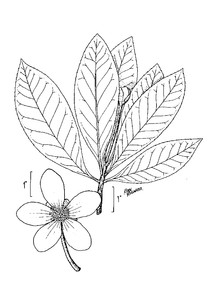
Taxon concept: [= Woodwardia areolata (L.) T. Moore − RAB, FNA, Weakley]
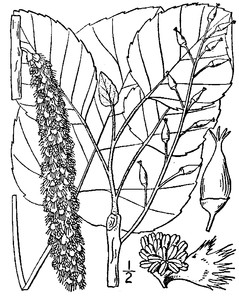
Bay Tree Lake (Occasional): Howell BATR−5, 26 (NCSC!)
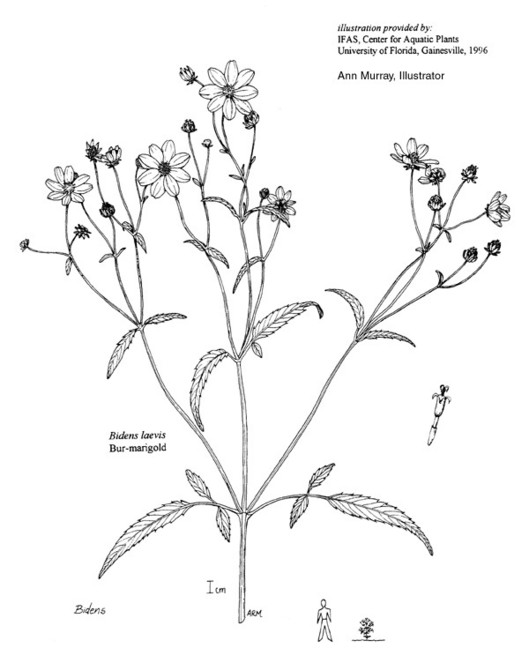
Lake Waccamaw: Wilbur 84200 (DUKE!)
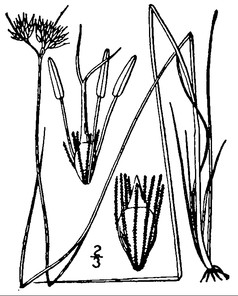
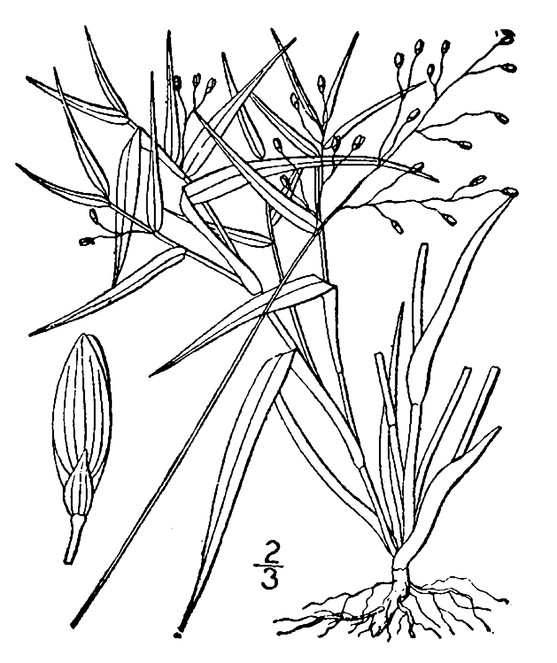
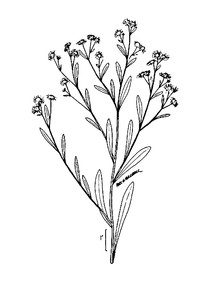
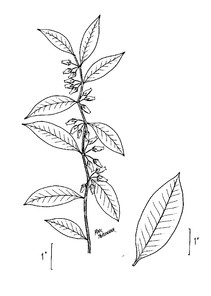
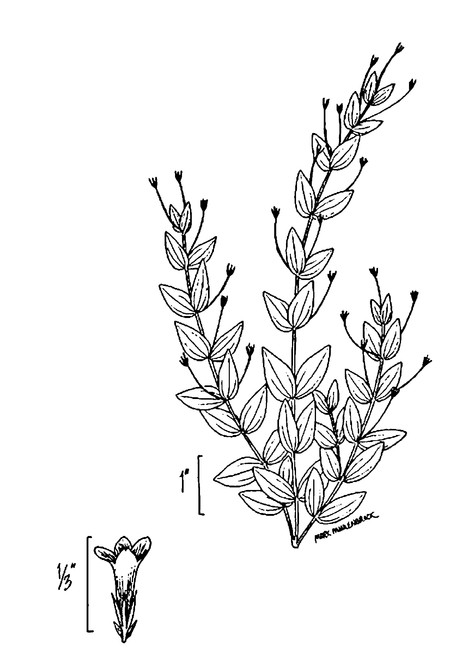
Little Singletary Lake (Occasional): Howell LISI–6 (NCSC!)
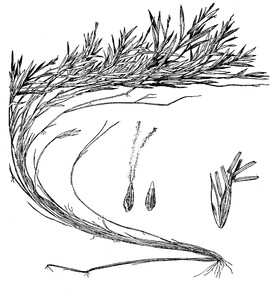
Singletary Lake: Hueske s.n. (NCU!)
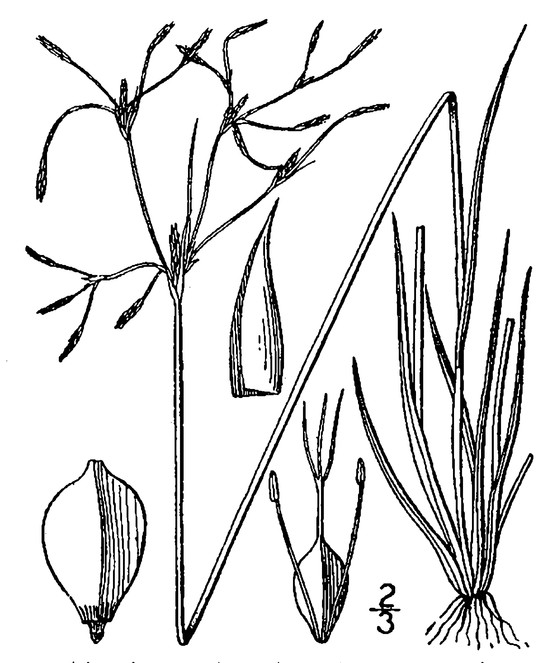
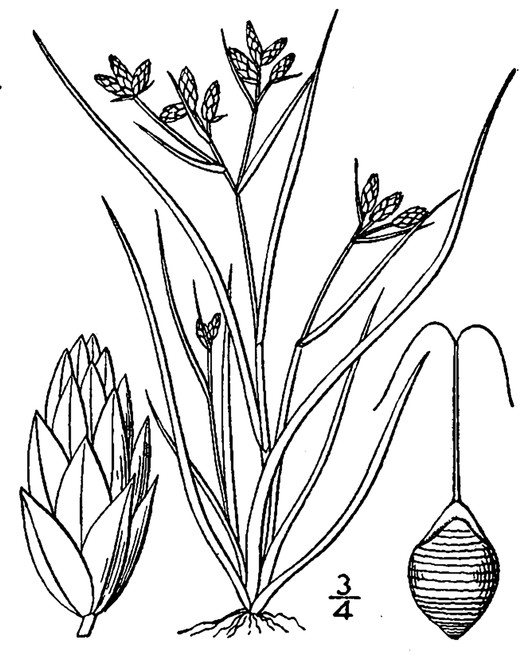
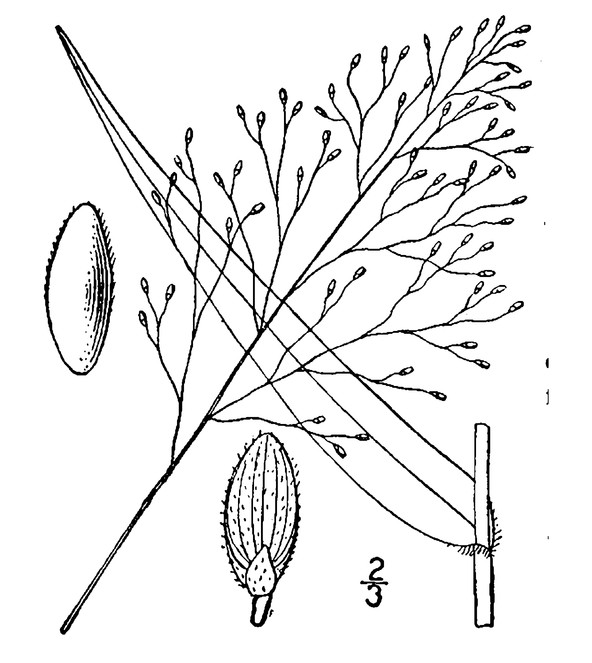
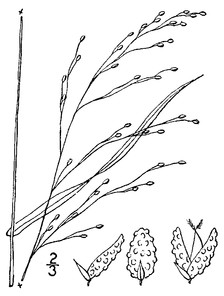
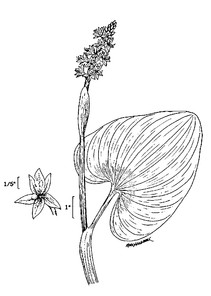
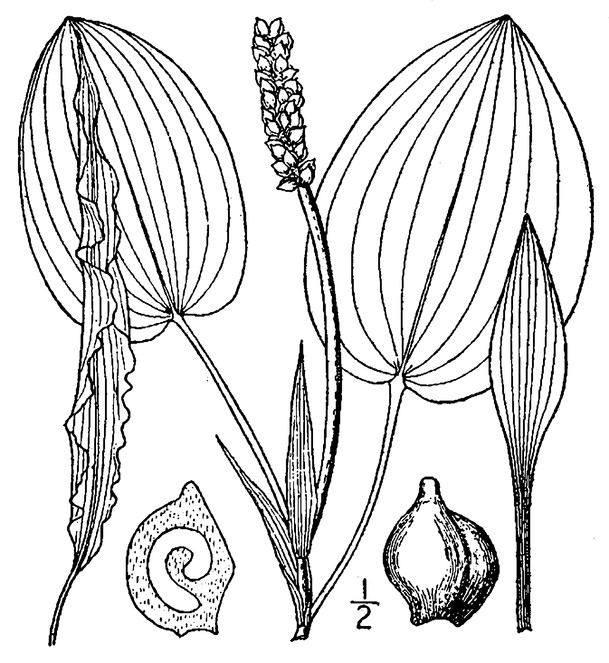
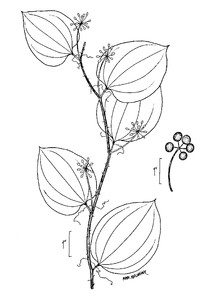
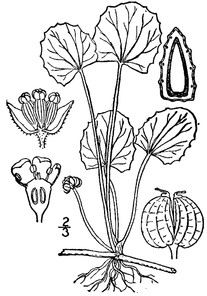
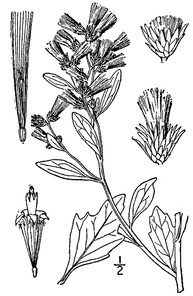
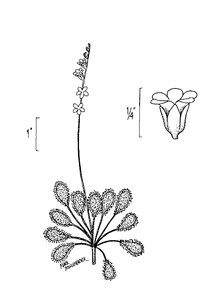
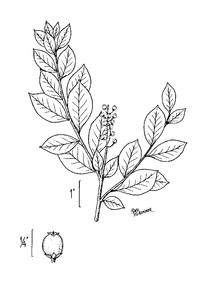
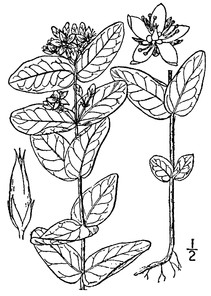
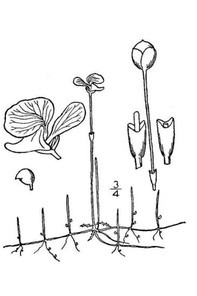
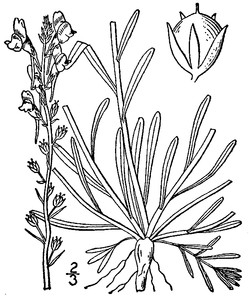
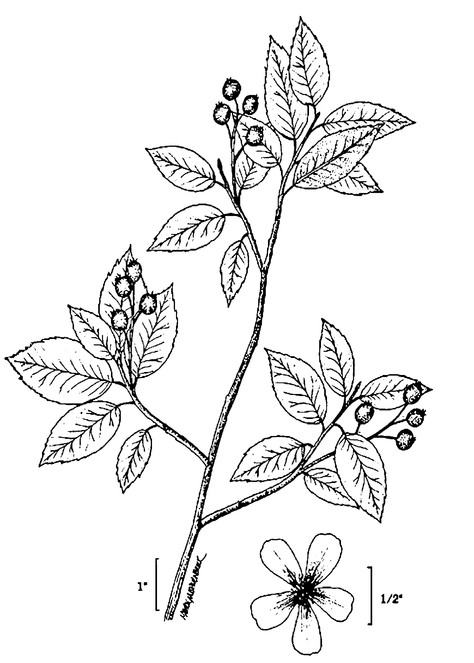
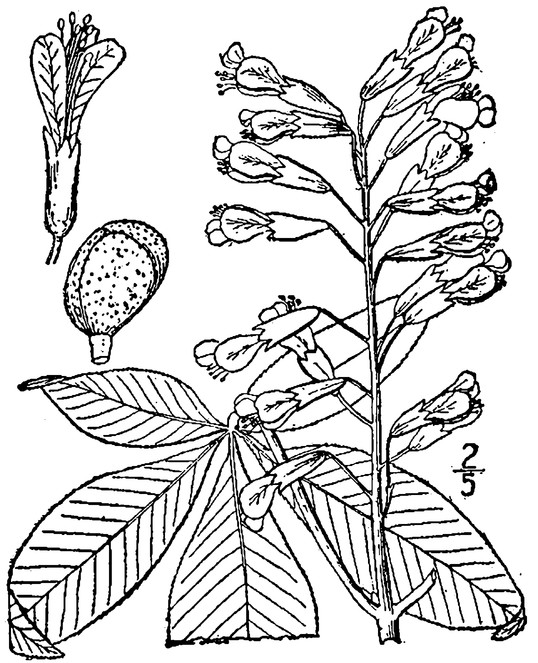
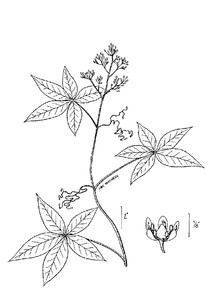
Perennial herbs. Upper eulittoral zone; typically found in saturated soils or rooted on logs, stumps, and other debris (NLSS–C, NLSS–LW, NLSM–T) . May–Sep. Fig.
Dryopteridaceae
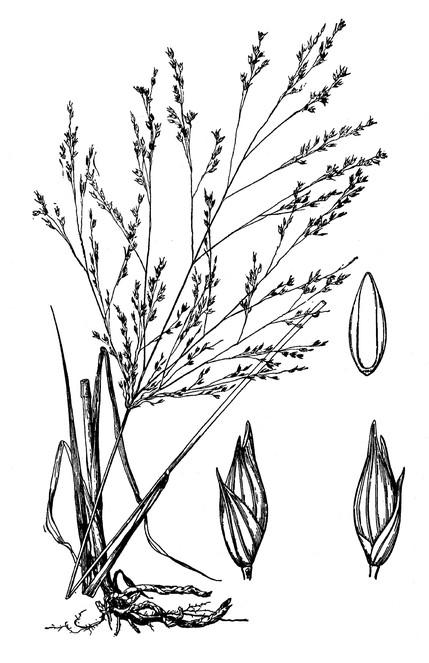
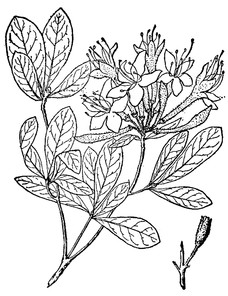
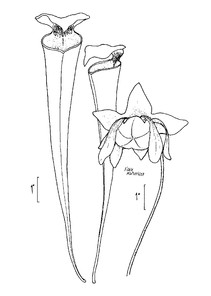
Dryopteris ludoviciana
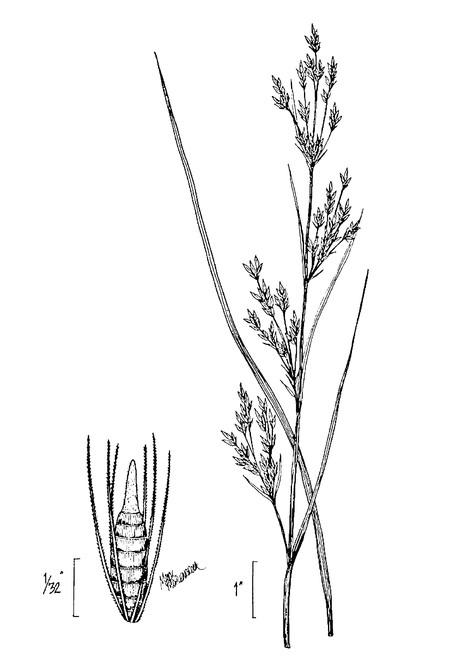
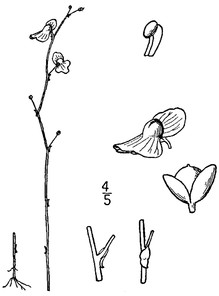
Basionym: Aspidium ludovicianum Kunze
Taxon concept: [= RAB, FNA, Weakley]
Lake Waccamaw: Bennedict 1247 & 2298 (NCU!); Blomquist & Correll 7625 (NCU!)
Perennial herbs. Juncture of eulittoral and supralittoral zones (NLSS–LW). Jun–Sep. This species was not encountered by the first author, but voucher specimens (see above) place it within close proximity of Lake Waccamaw’s shoreline (i.e., it has the potential to occur at the uppermost portions of the littoral zone where the swamp forest adjoins the shoreline community on the southwest side of the lake). Fig.
Lycopodiaceae
Lycopodiella appressa
Basionym: Lycopodium inundatum var. appressum Chapm.
Taxon concept: [= Lycopodium appressum (Chapm.) F.E. Lloyd & Underw. − RAB; = FNA, Weakley]
Bay Tree Lake: Wilbur 48656 (DUKE!)
Horseshoe Lake (Infrequent): Howell HOLA−52 (NCSC!)
Lake Waccamaw (Infrequent): Howell LAWA−110 (NCSC!)
Perennial herbs. Upper eulittoral zone; usually in association with saturated peaty to sandy soils (NLSS–LW, CPSI–CG). Jul–Sep. Fig.
Onocleaceae
Onoclea sensibilis
Taxon concept: [< O. sensibilis L. – RAB, FNA; = Weakley]
Lake Waccamaw: Wilbur 84220 (DUKE!)
Perennial herbs. Upper eulittoral zone (NLSS−LW). May−Jun. This species was not encountered by the first author in the field, but a single voucher (see above) places it within close proximity to Lake Waccamaw’s southwest shoreline. Fig.
Osmundaceae
Osmunda spectabilis
Taxon concept: [< O. regalis var. spectabilis (Willd.) A. Gray − RAB, FNA; = Weakley]
Lake Waccamaw (Infrequent): Howell LAWA−58, 87, 90 (NCSC!)
Polypodiaceae
Pleopeltis polypodioides var. michauxiana
Basionym: Polypodium polypodioides var. michauxianum Weath.
Taxon concept: [< Polypodium polypodioides (L.) Watt – RAB; = FNA, Weakley]
Lake Waccamaw (Infrequent): Howell LAWA−47 (NCSC!)
Salters Lake (Infrequent): Howell SALA−1 (NCSC!)
Perennial, frequently epiphytic, herbs. Eulittoral zone; commonly on large limbs and trunks of Taxodium and Nyssa (NLSS−C, NLSS−LW). Jun−Oct. Fig.
GYMNOSPERMS
Families represented: 2
Cupressaceae
Chamaecyparis thyoides
Basionym: Cupressus thyoides L.
Taxon concept: [= RAB, FNA, Weakley]
Bay Tree Lake (Infrequent): Howell BATR−2 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−2, 13 (NCSC!)
Jones Lake (Occasional): Brown s.n. (NCSC!); Howell JOLA−1, 23 (NCSC!); Lance s.n. (NCU!); Russell 1304 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−8, 26 (NCSC!)
Singletary Lake (Infrequent): Howell SILA−14 (NCSC!)
Trees. At or just below the juncture of the supralittoral and eulittoral zones; often in saturated peaty or sandy soil (NLSS–C, NLSS–LW, NLSM–T). Mar–Apr; Oct– Nov. Fig.
Taxodium ascendens
Taxon concept: [= RAB; < T. distichum var. imbricarium (Nutt.) Croom − FNA; = Weakley]
Bakers Lake (Abundant): Howell BALA−15 (NCSC!)
Bay Tree Lake (Abundant): Howell BATR−7 (NCSC!)
Horseshoe Lake (Abundant): Howell HOLA−10 (NCSC!)
Jones Lake (Abundant): Howell JOLA−3, 22 (NCSC!); Krings 508 (NCSC!); Wilbur 57584 (DUKE!)
Lake Waccamaw (Abundant): Howell LAWA−13 (NCSC!)
Little Singletary Lake (Abundant): Howell LISI−4, 20 (NCSC!)
Salters Lake (Abundnat): Howell SALA−8 (NCSC!)
Singletary Lake (Abundant): Howell SILA−13 (NCSC!); Wilbur 27966 (DUKE!)
Taxodium distichum
Basionym: Cupressus disticha L.
Taxon concept: [= RAB; < T. distichum var. distichum – FNA; = Weakley]
Bay Tree Lake: Wilbur 61464 (DUKE!)
Jones Lake: Stone 3704 (DUKE!)
Lake Waccamaw: ♦
Salters Lake: Beckman & Linnenburger 38 (DUKE!)
Singletary Lake: Crosby 4032 (DUKE!)
Pinaceae
Pinus serotina
Taxon concept: [= RAB, FNA, Weakley]
Jones Lake (Rare): Howell JOLA−14 (NCSC!)
Singletary Lake (Rare): Howell SILA−37 (NCSC!)
Trees. Juncture of supralittoral and eulittoral zones (NLSS–C). Apr–Aug (or any time of the year in response to fire). Fig.
Pinus taeda
Taxon concept: [= RAB, FNA, Weakley]
Lake Waccamaw (Rare): Howell LAWA−71 (NCSC!)
Little Singletary Lake (Infrequent): Howell LISI−27 (NCSC!)
Singletary Lake (Rare): Howell SILA−12 (NCSC!)
MONOCOTYLEDONS
Families represented: 17
Alismataceae
Sagittaria filiformis
Taxon concept: [= S. stagnorum Small – GW; S. subulata var. gracillima (S. Watson) J.G. Sm.; = FNA, Weakley]
SR−P; SH, G4G5.
Lake Waccamaw: Blomquist & Schuster 16191 (DUKE!)
Perennial herbs. Eulittoral and infralittoral zones (NLSS−LW, NLSM−LWP). May−Sep. The first author has not encountered this taxon in the field, but a single voucher specimen (see above) confirms its historic presence within the lake. Fig.
Sagittaria graminea
Taxon concept: [= S. graminea var. graminea – RAB, GW; = S. graminea ssp. graminea – FNA; = Weakley]
Lake Waccamaw (Frequent): Howell LAWA−19, 57 (NCSC!); Radford s.n. (NCU!); ♦
Sagittaria isoetiformis
Taxon concept: [< S. teres S. Watson (misapplied) – RAB; = GW, FNA, Weakley]
State T; S2, G4?.
Horseshoe Lake (Infrequent): Grant s.n. (NCU!); Howell HOLA−34 (NCSC!)
Lake Waccamaw: LeBlond 5792D (NCU!)
Perennial herbs. Eulittoral zone (NLSS−LW, NLSM−LWP, CPSI−CG, FB). Jun−Sep. Fig.
Sagittaria weatherbiana
Taxon concept: [= S. graminea var. weatherbiana – RAB, GW; = S. graminea ssp. weatherbiana – FNA; = Weakley]
State E, FSC; S2, G3G4.
Lake Waccamaw: Adams s.n. (NCSC!)
Perennial herbs. Eulittoral zone (NLSS−LW, NLSM−LWP). Apr−Jun.
Araceae
* Colocasia esculenta
Basionym: Arum esculentum L.
Taxon concept: [= GW, FNA, Weakley]
Lake Waccamaw (Infrequent): Howell LAWA−93 (NCSC!)
Perennial herbs. Eulittoral zone (NLSS−LW, NLSM−LWP). “Generally infertile in our area” (
Genus Wolffia
The first author has not encountered taxa within this genus in the field; however, the Carolina Vegetation Survey reported “Wolffia spp.” from the southwest side Lake Waccamaw. Although a species-level identification has not been made, a key to the two species most likely to inhabit this location is provided in the Identification Keys section below.
Bromeliaceae
Tillandsia usneoides
Basionym: Renealmia usneoides L.
Taxon concept: [= RAB, FNA, Weakley]
Bakers Lake (Occasional): •
Bay Tree Lake (Frequent): Howell BATR−49 (NCSC!)
Horseshoe Lake (Occasional): •
Jones Lake (Occasional): Howell JOLA–33 (NCSC!)
Lake Waccamaw (Abundant): Howell LAWA−46, 84 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−18 (NCSC!)
Salters Lake (Occasional): Howell SALA−9 (NCSC!)
Singletary Lake (Frequent): Howell SILA−6, 20 (NCSC!)
Perennial, epiphytic herbs. Eulittoral zone; common in low-hanging limbs of Taxodium or Nyssa (NLSS−C, NLSS−LW, NLSM−T, CPSI−CG). Apr−Jun. Fig.
Burmanniaceae
Burmannia capitata
Basionym: Vogelia capitata Walter ex J.F. Gmel.
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw: LeBlond & Franklin 6578 (NCU!)
Cyperaceae
Carex alata
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw (Rare): Howell LAWA−98 (NCSC!)
Carex longii
Taxon concept: [< C. albolutescens Schwein. – RAB, GW; = FNA, Weakley]
Bay Tree Lake (Rare): Howell BATR−34 (NCSC!)
Carex lupulina
Taxon concept: [= RAB; < C. lupulina Muhl. ex Willd. – GW (see C. lupuliformis); = FNA, Weakley]
Lake Waccamaw (Rare): Howell LAWA−136 (NCSC!)
Perennial herbs. Juncture of the eulittoral and supralittoral zones (NLSS−LW). Jun−Sep. A taxon of bottomland forests throughout the state, this large-fruited sedge occurs where bottomland swamp forests abut the shoreline of Lake Waccamaw. Fig.
Carex striata var. brevis
Taxon concept: [< C. walteriana L.H. Bailey – RAB, GW; = FNA, Weakley]
Horseshoe Lake: Buell 2279 (DUKE!, NCSC!)
Cladium mariscoides
Basionym: Schoenus mariscoides Muhl.
Taxon concept: [= RAB, FNA, Weakley]
SR–O; S3, G5.
Lake Waccamaw (Abundant): Howell LAWA−16, 146 (NCSC!); LeBlond 3862 (NCU!); Wilbur 49778, 49789 (DUKE!)
Perennial herbs. Eulittoral zone (NLSS−LW). Jul−Sep. This taxon is the principal sedge component of the natural shoreline community of Lake Waccamaw. Fig.
Cyperus erythrorhizos
Taxon concept: [= RAB, GW, FNA, Weakley]
Horseshoe Lake: Buell 2263 (DUKE!); Rothfels, Burge, Duke Natural History Society 2403 (DUKE!)
Annual herbs. floating bogs; saturated, acidic, peaty soil (FB). Jul−Sep. Fig.
Cyperus odoratus var. odoratus
Taxon concept: [= C. odoratus L. – RAB, GW; < C. odoratus L. – FNA; = Weakley]
Bay Tree Lake (Rare): Howell BATR−63 (NCSC!)
Cyperus polystachyos
Taxon concept: [> C. polystachyos var. texensis (Torr.) Fernald – RAB; < C. polystachyos – GW; = FNA, Weakley]
Jones Lake (Rare): Howell JOLA−43 (NCSC!)
Perennial herbs. Eulittoral zone; usually in sandy moist soil just below the maximum annual high water mark (NLSM−T). Jul−Oct. Fig.
Dulichium arundinaceum var. arundinaceum
Basionym: Cyperus arundinaceus L.
Taxon concept: [< D. arundinaceum (L.) Britton – RAB, GW; = FNA, Weakley]
Horseshoe Lake (Occasional): Beal 4345 (NCSC!); Buell s.n. (DUKE!); Howell HOLA−32 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−26, 77 (NCSC!)
Little Singletary Lake (Infrequent): Howell LISI−41 (NCSC!)
Perennial herbs. Eulittoral zone; calm, quiet waters along shorelines or on floating bogs (NLSS−C, NLSS−LW, CPSI−CG, FB). Jul–Oct. Fig.
Eleocharis baldwinii
Taxon concept: [= RAB, GW, FNA, Weakley]
Bay Tree Lake (Infrequent): Howell BATR−36, 40 (NCSC!)
Horseshoe Lake: • (The first author has observed Eleocharis baldwinii/vivipara around the peripheries of floating bogs and along saturated peaty shores, but voucher specimens were not collected. These two species are unidentifiable from a distance and the use of a hand lens is needed to distinguish one from the other.)
Little Singletary Lake (Rare): Howell LISI−43 (NCSC!)
Annual (?) herbs. Eulittoral zone and infralittoral zones; typically submersed in shallow water or on sarurated organic to sandy soils above current lake levels (NLSS−C, NLSM−T). Jul−Sep. Fig.
Eleocharis equisetoides
Basionym: Scirpus equisetoides Elliott
Taxon concept: [= RAB, GW, FNA, Weakley]
W1; S3, G4.
Lake Waccamaw (Infrequent): Howell LAWA−67, 155 (NCSC!)
Little Singletary Lake (Rare): Howell LISI−38 (NCSC!)
Perennial herbs. Eulittoral and infralittoral zones; calm, quiet waters along shorelines (NLSS−C, NLSS−LW). Jun−Sep. Fig.
Eleocharis olivacea var. olivacea
Taxon concept: [< E. flavescens (Poir.) Urb. – RAB; < E. olivacea Torr. – GW; < E. flavescens var. olivacea (Torr.) Gleason – FNA; = Weakley]
Lake Waccamaw (Rare): Howell LAWA−78 (NCSC!); LeBlond 3987 (NCU!)
Eleocharis vivipara
Taxon concept: [= RAB, GW, FNA, Weakley]
State E; S1, G5.
Horseshoe Lake: • (The first author has observed Eleocharis baldwinii/vivipara around the peripheries of floating bogs and along saturated peaty shores, but voucher specimens were not collected. These two species are unidentifiable from a distance and the use of a hand lens is needed to distinguish one from the other.)
Little Singletary Lake (Rare): Howell LISI−53 (NCSC!)
Fimbristylis autumnalis
Basionym: Scirpus autumnalis L.
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw: Radford 677 (NCU!)
Fuirena pumila
Basionym: Fuirena squarrosa var. pumila Torr.
Taxon concept: [= RAB, GW, FNA, Weakley]
Bay Tree Lake (Rare): Howell BATR−62 (NCSC!); Wilbur 57396 (DUKE!)
Rhynchospora alba
Basionym: Schoenus albus L.
Taxon concept: [= RAB, GW, FNA, Weakley]
SR−P; S2, G5.
Horseshoe Lake (Occasional): Howell HOLA−45 (NCSC!)
Rhynchospora corniculata
Basionym: Schoenus corniculatus Lam.
Taxon concept: [= RAB, GW, FNA; < R. corniculata var. corniculata − Weakley]
Lake Waccamaw (Occasional): Howell LAWA−135, 163 (NCSC!)
Rhynchospora distans
Basionym: Schoenus distans Michx.
Taxon concept: [< R. fascicularis (Michx.) Vahl – RAB, GW, FNA; = Weakley]
Bakers Lake (Rare): Howell BALA−2 (NCSC!)
Lake Waccamaw: Wilbur 49814 (DUKE!)
Little Singletary Lake (Rare): Howell LISI−33 (NCSC!)
Rhynchospora elliottii
Taxon concept: [= R. schoenoides (Elliott) Wood – RAB; = GW, FNA, Weakley]
Lake Waccamaw: ♦
Perennial herbs. Eulittoral zone (NLSS−LW). Jul−Sep.
Rhynchospora inexpansa
Basionym: Schoenus inexpansus Michx.
Taxon concept: [= RAB, GW, FNA, Weakley]
Jones Lake: Beal 799 (NCSC!)
Rhynchospora inundata
Basionym: Ceratoschoenus macrostachyus var. inundatus Oakes
Taxon concept: [= RAB, GW, FNA, Weakley]
W1; S3, G4?
Horseshoe Lake (Infrequent): Howell HOLA−53 (NCSC!); Grant s.n. (NCU!); Rothfels, Burge, Duke Nat. Hist. Soc. 2401 (DUKE!)
Rhynchospora latifolia
Basionym: Dichromena latifolia Baldwin
Taxon concept: [= Dichromena latifolia Baldwin ex Elliott – RAB, GW; = FNA, Weakley]
Lake Waccamaw: Radford 723 (NCU!)
Rhynchospora macrostachya
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw (Infrequent): Howell LAWA−130 (NCSC!)
Rhynchospora nitens
Basionym: Scirpus nitens Vahl
Taxon concept: [= Psilocarya nitens (Vahl) Alph. Wood – RAB, GW; = FNA, Weakley]
W1; S3, G4?
Lake Waccamaw: Wilbur 49781 (DUKE!)
Scirpus cyperinus
Basionym: Eriophorum cyperinum L.
Taxon concept: [= RAB, GW, FNA, Weakley]
Bay Tree Lake (Occasional): Howell BATR−58 (NCSC!)
Jones Lake (Occasional): Howell JOLA−4, 45 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−166 (NCSC!)
Little Singletary Lake (Occasional): •
Eriocaulaceae
Eriocaulon aquaticum
Basionym: Cespa aquatica Hill
Taxon concept: [> E. pellucidum Michx. – RAB; = E. septangulare – GW; = FNA, Weakley]
SC−V; S2, G5.
Lake Waccamaw (Abundant): Howell LAWA−5, 52 (NCSC!); Lynch 185 (NCSC!); Wilbur 49802 (DUKE!)
Perennial herbs. Eulittoral and infralittoral zones (NLSS−LW, NLSM−LWP). Jul−Oct. A dominant species in the littoral zone of Lake Waccamaw. Fig.
Haemodoraceae
Lachnanthes caroliniana
Basionym: Dilatris caroliniana Lam.
Taxon concept: [= RAB, GW, FNA, Weakley]]
Bay Tree Lake (Infrequent): Howell BATR−50, 51 (NCSC!)
Horseshoe Lake (Infrequent): Howell HOLA−51 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−107 (NCSC!)
Little Singletary Lake (Infrequent): Howell LISI−25, 51 (NCSC!)
Perennial herbs. Eulittoral zone; typically in saturated soils at or below the maximum annual high water mark (NLSS–C, NLSS–LW, NLSM–T, CPSI–CG). Jun–early Sep; Sep–Nov. Fig.
Hydrocharitaceae
* Hydrilla verticillata
Basionym: Serpicula verticillata L.f.
Taxon concept: [= GW, FNA, Weakley]
Lake Waccamaw: ¤
Perennial herbs. Infralittoral zone (NLSS−LW, NLSM−LWP). Jun−Aug. This exotic, invasive taxon is native to warm climates of the Old World. Hydrilla verticillata was introduced to Florida in 1950 as an ornamental and has since become a terrible aquatic invasive throughout the Southeast. Where introduced, H. verticillata chokes out native submersed aquatic vegetation (e.g., Ceratophyllum, Myriophyllum, Najas, Potomogeton, Vallisneria), negatively impacts recreational activities and alters natural hydrology and water chemistry (
Najas guadalupensis var. guadalupensis
Basionym: Caulinia guadalupensis Spreng.
Taxon concept: [< N. guadalupensis (Spreng.) Magnus – RAB, GW; = N. guadalupensis ssp. guadalupensis – FNA; = Weakley]
Lake Waccamaw: Blomquist & Schuster 16190 (DUKE!)
Hypoxidaceae
Hypoxis curtissii
Taxon concept: [= H. hirsuta var. leptocarpa (Engelm. & A. Gray) Fernald – RAB; = H. leptocarpa (Engelm. & A. Gray) Small – GW; = FNA, Weakley]
Lake Waccamaw (Rare): Howell LAWA−60 (NCSC!)
Juncaceae
Juncus acuminatus
Taxon concept: [= RAB, GW, FNA, Weakley]
Bay Tree Lake (Rare): Howell BATR−15 (NCSC!)
Perennial herbs. Eulittoral zone. May−Aug. Fig.
Juncus biflorus
Taxon concept: [= RAB; < J. marginatus Rostk. – GW, FNA; = Weakley]
Little Singletary Lake (Rare): Howell LISI−58 (NCSC!)
Singletary Lake: Beal 796 (NCSC!)
Juncus canadensis
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw (Infrequent): Howell LAWA−32, 170 (NCSC!)
Perennial herbs. Eulittoral zone (NLSS−LW). Jul−Oct. Fig.
Juncus coriaceus
Taxon concept: [= RAB, GW, FNA, Weakley]
Horseshoe Lake: Beal 828 (NCSC!)
Juncus effusus subsp. solutus
Taxon concept: [< J. effusus – RAB, GW, FNA; = Weakley]
Bay Tree Lake (Occassional): Howell BATR−6 (NCSC!)
Little Singletary Lake (Occassional): Howell LISI−3 (NCSC!)
Horseshoe Lake (Occassiona): Howell HOLA−8 (NCSC!)
Perennial herbs. Eulittoral zone (NLSS−C, NLSM−T, CPSI−CG). Jun−Sep. Fig.
Juncus pelocarpus
Taxon concept: [> J. abortivus Chapm. – RAB, GW; = FNA, Weakley]
Bay Tree Lake (Frequent): Howell BATR−61 (NCSC!); Wilbur 57415 (DUKE!)
Horseshoe Lake: Wilbur 2264, 81465 (DUKE!)
Jones Lake (Occasional): Howell JOLA−17, 35 (NCSC!); Wilbur 57582 (DUKE!)
Lake Waccamaw (Frequent): Howell LAWA−3 (NCSC!); Wilbur s. n., 84188 (DUKE!)
Singletary Lake (Occasional): Howell SILA−31 (NCSC!)
Juncus repens
Taxon concept: [= RAB, GW, FNA, Weakley]
Bay Tree Lake (Occasional): Howell BATR−8 (NCSC!); Wilbur 57395 (DUKE!)
Horseshoe Lake (Occasional): Beal 4348 (NCSC!); Howell HOLA−14 (NCSC!); Wilbur & Menchi Ho 83792 (DUKE!)
Lake Waccamaw (Occasional): Howell LAWA−30, 31 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−19, 44 (NCSC!)
Singletary Lake (Occasional): Howell SILA−1, 32 (NCSC!)
Perennial herbs. Eulittoral zone (NLSS−C, NLSS−LW, NLSM−T, CPSI−CG). Jun−Oct. Fig.
Juncus repens (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Habit
d: Habit
Juncus scirpoides var. scirpoides
Taxon concept: [< J. scirpoides Lam. – RAB, GW, FNA, Weakley]
Bay Tree Lake (Infrequent): Howell BATR−27, 66 (NCSC!)
Little Singletary Lake (Infrequent): Howell LISI−55 (NCSC!)
Mayacaceae
Mayaca fluviatilis
Taxon concept: [> M. aubletii Michx. – RAB; > M. fluviatilis Aubl. – RAB; = GW, FNA, Weakley]
Lake Waccamaw: ¤
Orchidaceae
Calopogon tuberosus var. tuberosus
Basionym: Limodorum tuberosum L.
Taxon concept: [< C. pulchellus R. Brown − RAB; < C. tuberosus (L.) Britton, Sterns, & Poggenb. – GW; = FNA, Weakley]
Horseshoe Lake (Occasional): Howell HOLA−24, 39 (NCSC!)
Epidendrum magnoliae
Taxon concept: [< E. conopseum R. Br. – RAB; = FNA, Weakley]
T; S1S2, G4.
Lake Waccamaw: Correll & Blomquist 4900 (DUKE!)
Perennial, epiphytic herbs. Eulittoral zone; typically on limbs and trunks of Taxodium ascendens, Taxodium distichum, Nyssa aquatica, Nyssa biflora, Liquidambar styraciflua, and possibly other bottomland tree species in the shoreline of Lake Waccamaw (NLSS– LW, NLSS–C). Jul–Oct. This species usually co-occurs with Pleopeltis polypodiodes. The first author observed a vegetative specimen on the edge of Big Creek ca. 50–70 meters from the shoreline of Lake Waccamaw. The specimen was on a large Nyssa aquatica limb, ca. 25–30 meters above the water, and was co- occuring with Pleopeltis polypodioides.
Habenaria repens
Taxon concept: [= RAB, GW, FNA, Weakley]
W1; S2, G5.
Lake Waccamaw: ►
Perennial herbs. Eulittoral zone (NLSS–LW). Apr–Nov.
Pogonia ophioglossoides
Basionym: Arethusa ophioglossoides L.
Taxon concept: [= RAB, GW, FNA, Weakley]
Horseshoe Lake (Occasional): Howell HOLA−30 (NCSC!)
Spiranthes laciniata
Basionym: Gyrostachys laciniata Small
Taxon concept: [= RAB; < S. × laciniata – GW; = FNA, Weakley]
SC−V; S2, G4,G5.
Lake Waccamaw (Occasional): Howell LAWA−105, 106, 116 (NCSC!)
Poaceae
Agrostis hyemalis
Basionym: Cornucopiae hyemalis Walter
Taxon concept: [< A. hyemalis (Walter) Britton, Sterns & Poggenb. – RAB; = A. hiemalis (Walter) Britton, Sterns & Poggenb. – GW; = FNA, Weakley]
Little Singletary Lake (Rare): Howell LISI−37 (NCSC!)
Perennial herbs. Juncture of supralittoral and eulittoral zones; typically in moist sandy soils (NLSM−T). Mar−Jul. Fig.
Andropogon glaucopsis
Taxon concept: [< A. virginicus L. – RAB; = GW; = A. glomeratus var. glaucopsis (Elliott) C. Mohr − FNA; = Weakley]
Horseshoe Lake: Buell s.n. (DUKE!, NCSC!)
Jones Lake (Infrequent): Howell JOLA–16 (NCSC!)
Andropogon virginicus var. virginicus
Taxon concept: [< A. virginicus L. – RAB; = FNA, Weakley]
Lake Waccamaw: ♦
Arundinaria tecta
Basionym: Arundo tecta Walter
Taxon concept: [< A. gigantea (Walter) Muhl. – RAB, GW; = FNA, Weakley]
Lake Waccamaw: Bennedict 4350 (DUKE!)
Arborescent herbs. Eulittoral zone; at or just below the mean annual high water mark (NLSS−LW). Apr−Jul. The first author has not encountered this taxon in the field, but a single voucher specimen (see above) places it within the immediate vicinity. Fig.
Coleataenia longifolia var. longifolia
Basionym: Panicum longifolium Torr.
Taxon concept: [= Panicum longifolium var. longifolium – RAB; < Panicum longifolium Torr. – GW; = Panicum rigidulum ssp. pubescens (Vasey) Freckmann & Lelong − FNA; = Weakley]
Bay Tree Lake (Infrequent): Howell BATR – 68 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA −145, 147, 164, 168 (NCSC!)
Coleataenia tenera
Basionym: Panicum tenerum Bey. ex Trin.
Taxon concept: [= Panicum tenerum Bey. ex Trin. – RAB, GW, FNA; = Weakley]
Lake Waccamaw: ►
Perennial herbs. Eulittoral zone (NLSS−LW). Jun−Sep.
Dichanthelium boreale
Basionym: Panicum boreale Nash
Taxon concept: [> Panicum bicknellii Nash– RAB; > D. boreale (Nash) Freckmann – FNA; = Weakley]
Lake Waccamaw: Blomquist 957 (DUKE!)
Dichanthelium dichotomum var. roanokense
Basionym: Panicum roanokense Nash
Taxon concept: [< D. dichotomum (L.) Gould – RAB, GW; < D. dichotomum (L.) Gould) ssp. roanokense (Ashe) Freckmann & Lelong – FNA; = Weakley]
Lake Waccamaw: Ashe s.n. (NCU!)
Perennial herbs. Eulittoral zone; moist to peaty lakeshores (NLSS−LW). May−Sep.
Dichanthelium erectifolium
Basionym: Panicum erectifolium Nash
Taxon concept: [= Panicum erectifolium Nash – RAB, GW; = FNA, Weakley]
W2; S2, G4.
Lake Waccamaw (Infrequent): Howell LAWA−111 (NCSC!)
Dichanthelium species 3 = lancearium
Taxon concept: [= Panicum lancearium Trinius – RAB; < D. portoricense ssp. patulum (Scribner & Merrill) Freckmann & Lelong – FNA; = Weakley]
Lake Waccamaw: Blomquist & Correll 9383 (NCU!)
Perennial herbs. Eulittoral zone (NLSS−LW). May−Sep.
Dichanthelium mattamuskeetense
Basionym: Panicum mattamuskeetense Ashe
Taxon concept: [< Panicum dichotomum L. – RAB, GW; < D. dichotomum ssp. mattamuskeetense (Ashe) Freckmann & Lelong – FNA; = Weakley]
Lake Waccamaw: Blomquist & Correll 9385 (DUKE!)
Dichanthelium portoricense
Basionym: Panicum portoricense Desv. ex Ham.
Taxon concept: [= Panicum portoricense Desv. ex Ham. – RAB; = D. portoricense ssp. portoricense – FNA; = Weakley]
Bay Tree Lake (Infrequent): Howell BATR – 52 (NCSC!)
Lake Waccamaw: Blomquist & Correll 9383 (NCU!)
Eragrostis elliottii
Taxon concept: [= RAB, GW, FNA, Weakley]
Bay Tree Lake (Rare): Howell BATR−67 (NCSC!)
Lake Waccamaw: ►
Eragrostis refracta
Basionym: Poa refracta Muhl. ex Elliott
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw: ►
Perennial herbs. Eulittoral zone (NLSS−LW). Jul−Oct.
Luziola fluitans var. fluitans
Basionym: Zizania fluitans Michx.
Taxon concept: [= Hydrochloa carolinensis P. Beauv. – RAB, GW; = FNA, Weakley]
Lake Waccamaw (Occasional): Bolser MEH107 (NCU!); Howell LAWA−51 (NCSC!)
Panicum hemitomon
Taxon concept: [= RAB, GW, FNA, Weakley]
Bay Tree Lake (Abundant): Howell BATR−18 (NCSC!)
Horseshoe Lake (Infrequent): Howell HOLA−23 (NCSC!)
Lake Waccamaw (Abundant): Blomquist 1399 (DUKE!); Blomquist & Correll 9379 (DUKE!, NCU!); Howell LAWA−79 (NCSC!)
Little Singletary Lake (Infrequent): Howell LISI−35 (NCSC!)
Salters Lake (Occasional): Howell SALA−14 (NCSC!)
Singletary Lake (Occasional): Blomquist 1400 (DUKE!); Howell SILA−17 (NCSC!); Wilbur 60947 (DUKE!)
Perennial herbs. Eulittoral and infralitoral zones (NLSS−C, NLSS−LW, NLSM−T, CPSI−CG). Jun−Jul. Fig.
Panicum hemitomon (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Habit
d: Inflorescences
Panicum verrucosum
Taxon concept: [= RAB, GW, FNA, Weakley]
Bay Tree Lake (Infrequent): Howell BATR−53 (NCSC!)
Jones Lake (Rare): Howell JOLA−40, 41 (NCSC!)
Little Singletary Lake (Rare): Howell LISI−50 (NCSC!)
Panicum virgatum var. virgatum
Taxon concept: [< P. virgatum – RAB, GW, FNA; = Weakley]
Bay Tree Lake: Wilbur 57420 (DUKE!)
Saccharum giganteum
Basionym: Anthoxanthum giganteum Walter
Taxon concept: [= Erianthus giganteus (Walter) P. Beauv. – RAB, GW; = FNA. Weakley]
Jones Lake (Rare): Howell JOLA−37 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−7, 160 (NCSC!)
Perennial herbs. Eulittoral zone (NLSS−C, NLSS−LW). Sep−Oct. Fig.
Saccharum giganteum (digital photographs taken by Nathan Howell; illustration from
b: lllustration
c: Culm and leaf blade
d: Inflorescence
Sacciolepis striata
Basionym: Holcus striatus L.
Taxon concept: [= RAB, GW, FNA, Weakley]
Bay Tree Lake (Occasional): Howell BATR−43, 54, 55 (NCSC!); Wilbur 48657, 57394 (DUKE!)
Horseshoe Lake: Rothfels, Burge, Duke Natural History Society 2398 (DUKE!)
Lake Waccamaw (Occasional): Howell LAWA−131 (NCSC!)
Singletary Lake (Occasional): Beal 3225 (NCSC!); Frey s.n. (NCU!); Howell SILA−38 (NCSC!)
Perennial herbs. Eulittoral zone (NLSS−C, NLSS−LW, NLSM−T, CPSI−CG). Jul−Oct. Fig.
Sacciolepis striata (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Leaf sheath
d: Inflorescence
Sphenopholis obtusata
Basionym: Aria obtusata Michx.
Taxon concept: [= RAB, FNA, Weakley]
Lake Waccamaw: Blomquist 1492 (DUKE!)
Pontederiaceae
Pontederia cordata var. cordata
Taxon concept: [< P. cordata − RAB; = GW; < P. cordata – FNA; = Weakley]
Bay Tree Lake (Rare): •Lake Waccamaw (Occasional): Howell LAWA−15, 50, 159 (NCSC!); Matthews s.n. (NCU!); Wilbur 59382 (DUKE!)
Perennial herbs. Eulittoral zone (NLSS−LW). May−Oct. Fig.
Pontederia cordata var. cordata (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Leaves
d: Inflorescence
Pontederia cordata var. lancifolia
Taxon concept: [< P. cordata – RAB; = GW; < P. cordata – FNA; = Weakley]
Lake Waccamaw: ♦
Perennial herbs. Eulittoral zone (NLSS−LW). May−Oct.
Potamogetonaceae
Potamogeton pulcher
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw: ►
Potamogeton pusillus
Taxon concept: [< P. berchtoldii Fieber – RAB; = GW; > P. pusillus ssp. pusillus – FNA; > P. pusillus var. pusillus − Weakley]
Lake Waccamaw: ¤
Annual herbs. Eulittoral and infralittoral zones (NLSS−LW, NLSM−LWP). May−Sep.
Smilacaceae
Smilax glauca
Taxon concept: [= RAB, GW, FNA, Weakley]
Bay Tree Lake (Rare): Howell BATR−29 (NCSC!)
Perennial vines. Juncture of eulittoral and supralittoral zones. Late Apr−Early Jun; Sep−Nov and persisting. Fig.
Smilax laurifolia
Taxon concept: [= RAB, GW, FNA, Weakley]
Bakers Lake (Frequent): Howell BALA−13 (NCSC!)
Bay Tree Lake (Occasional): Howell BATR−44 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−3 (NCSC!)
Jones Lake (Frequent): Howell JOLA−7 (NCSC!)
Lake Waccamaw (Infrequent): Howell LAWA−34 (NCSC!)
Salters Lake (Frequent): Howell SALA−4, 15 (NCSC!)
Singletary Lake (Frequent): Howell SILA−9 (NCSC!)
Perennial vines. Eulittoral zone; typically at the maximum annual high water mark in saturated organic to sandy soils (NLSS−C, NLSS−LW, NLSM−T, CPSI−CG). Jul−Aug; Sep−Oct and persisting. Fig.
Smilax laurifolia (digital photographs taken by Nathan Howell; illustrations from
b: Illustration
c: Habit
d: Infructescence
Smilax rotundifolia
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw: ♦
Perennial vines. Juncture of eulittoral and supralittoral (NLSS−LW). Apr−May; Sep−Oct and persisting. Fig.
Smilax rotundifolia (digital photographs taken by Alexander Krings; illustration from
b: Leaf (adaxial surface)
c: Stem (with prickles), petiole, and withered stipular tendrils (at base of petiole)
d: Denticulations at base of leaf blade (arrowed)
Smilax walteri
Taxon concept: [= RAB, GW, FNA, Weakley]
Horseshoe Lake (Occasional): Howell HOLA−13, 21, 25 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−29, 55, 162 (NCSC!)
Perennial vines. Eulittoral zone (NLSS−LW, CPSI−CG). Late Apr−May; Sep−Nov and persisting. Fig.
Xyridaceae
Xyris fimbriata
Taxon concept: [= RAB, GW, FNA, Weakley]
Horseshoe Lake: Rothfels, Burge, Duke Natural History Society 2400, 2404 (DUKE!)
Lake Waccamaw: ►
Singletary Lake: Frey s.n. (NCU!)
Perennial herbs. Eulittoral zone (NLSS−C, NLSM−T, NLSS−LW, CPSI–CG). Sep−Oct. Fig.
Xyris iridifolia
Taxon concept: [= RAB, GW; < X. laxifolia var. iridifolia (Chapm.) Kral – FNA; = Weakley]
W7; S2, G4G5T4T.
Salters Lake: ♦
Perennial herbs. Eulittoral zone (NLSS–C). Jul–Sep.
Xyris jupicai
Taxon concept: [= RAB, GW, FNA, Weakley]
Little Singletary Lake (Infrequent): Howell LISI−46 (NCSC!)
Xyris smalliana
Taxon concept: [= RAB, GW, FNA, Weakley]
W1; S3, G5.
Horseshoe Lake: R.L Wilbur 81092 (DUKE!)
Jones Lake (Occasional): Howell JOLA−42 (NCSC!)
Lake Waccamaw (Abundant): Howell LAWA−114, 125, 142, 144 (NCSC!); LeBlond 3996 (NCU)
Salters Lake: Beckman & Linnenburger 24 (DUKE!); Grant s.n. (NCU)
Singletary Lake (Occasional): Howell SILA−29 (NCSC!); Rothfels & O’ Reilly, Shaw Lab s.n. (DUKE!)
BASAL ANGIOSPERMS, MAGNOLIIDS, and EUDICOTYLEDONS
Families represented: 55 (BA: 2; M: 2; E: 51)
Altingiaceae
Liquidambar styraciflua
Taxon concept: [= RAB, GW, FNA, Weakley]
Bay Tree Lake (Infrequent): Howell BATR−47 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−45, 133 (NCSC!)
Trees. Juncture of eulittoral and supralittoral zones (NLSS–LW). Apr–May; Aug–Sep. Fig.
Liquidambar styraciflua (digital photographs taken by Nathan Howell [leaves] and Alexander Krings [twig, staminate flowers, fruit]; illustration from
b: Illustration
c: Twig, bud, and leaf scars
d: Leaves
e: Staminate flowers
f: Fruit
Amaranthaceae
* Alternanthera philoxeroides
Basionym: Bucholzia philoxeroides Mart.
Taxon concept: [= RAB, FNA, Weakley]
Lake Waccamaw (Infrequent): Beal 543, 1776 (DUKE!); Howell LAWA−65 (NCSC!)
Perennial herbs. Eulittoral zone; calm, quiet waters (NLSS–LW). Apr–Oct. Fig.
Alternanthera philoxeroides (digital photographs taken by Nathan Howell [habit, node] and Alexander Krings [leaf, inflorescence]; illustration from
b: Illustration
c: Habit
d: Node
e: Leaf
f: Inflorescence
Anacardiaceae
Rhus copallinum var. copallinum
Taxon concept: [< R. copallina L. – RAB; = Weakley]
Bakers Lake (Rare): Howell BALA−10 (NCSC!)
Shrubs or small trees. Juncture of eulittoral and supralittoral zones (NLSS−C). Jul−Sep; Aug−Oct. Fig.
Rhus copallinum (digital photographs taken by Nathan Howell [infructescence] and Alexander Krings [stem, leaf, infructescence detail]; illustration from
b: Illustration
c: Stem
d: Imparipinnate leaf (with winged rachis)
e: Infructescence
f: Infructescence (detail)
Toxicodendron radicans var. radicans
Taxon concept: [< Rhus radicans L. – RAB; < T. radicans (L.) Kuntze – GW; = Weakley]
Bay Tree Lake (Infrequent): •
Lake Waccamaw (Occasional): Howell LAWA−82, 152 (NCSC!)
Shrubs or lianas. Eulittoral zone; typically growing on woody shrubs and trees at or just below the high water mark (NLSS−LW, NLSM−LWP). Late Apr−May; Aug−Oct. Fig.
Toxicodendron radicans (digital photographs taken by Nathan Howell; illustration from
b: lllustration
c: Habit
d: Climbing stem with adventitious roots
e: Inflorescence
f: Fruits
Apiaceae
Centella asiatica
Basionym: Hydrocotyle asiatica L.
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw (Frequent): Howell LAWA−25, 115 (NCSC!)
Cicuta maculata var. maculata
Taxon concept: [= C. maculata L. – RAB, GW; = Weakley]
Lake Waccamaw (Rare): Howell LAWA−121 (NCSC!)
Perennial herbs. Eulittoral zone; moist soils at or just below the mean annual high water mark, also on sandbars and peninsular islands stranded in the littoral zone (NLSS–LW). May−Aug; Jul−Sep. Fig.
Cicuta maculata (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Leaf
d: Inflorescence
Aquifoliaceae
Ilex coriacea
Basionym: Prinos coriaceus Pursh
Taxon concept: [= RAB, GW, Weakley]
Bay Tree Lake (Rare): Howell BATR−16 (NCSC!)
Jones Lake (Occasional): Howell JOLA−10, 32 (NCSC!)
Ilex glabra
Basionym: Prinos glaber L.
Taxon concept: [= RAB, GW, Weakley]
Bakers Lake (Rare): Howell BALA−9 (NCSC!)
Bay Tree Lake (Rare): Howell BATR−3, 59 (NCSC!)
Lake Waccamaw (Infrequent): Howell LAWA−9, 153 (NCSC!)
Shrubs. Juncture of eulittoral and supralittoral zones (NLSS−C, NLSS−LW). May−Jun; Sep−Nov. Fig.
Ilex glabra (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Leaves
d: Fruits
Araliaceae
Hydrocotyle umbellata
Taxon concept: [= RAB, GW, RAB, Weakley]
Bay Tree Lake (Rare): Howell BATR−45 (NCSC; this specimen is sterile and tentatively referred here)
Lake Waccamaw (Frequent): Howell LAWA−24, 53 (NCSC; these specimens are sterile and tentatively referred here)
Perennial herbs. Eulittoral zone (NLSS–LW). Apr–Sep. Fig.
Asteraceae
Baccharis halimifolia
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw (Infrequent): Howell LAWA−88 (NCSC!)
Shrubs. Eulittoral zone; can be found on saturated soil at or just below the mean annual high water mark or growing from the bases of Taxodium in the littoral zone (NLSS−LW). Aug−Oct. Fig.
Bidens laevis
Basionym: Helianthus laevis L.
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw: Radford 683 (NCU!)
Annual herbs. Eulittoral zone; wet sandy beaches and sand bars (NLSS−LW). (Aug−Nov). The first author did not encounter this taxon in the field, but a single voucher confirms its historical presence. Fig.
Boltonia asteroides var. glastifolia
Basionym: Matricaria glastifolia Hill
Taxon concept: [< B. asteroides (L.) L’Hér – RAB; < Boltonia spp. – GW (formal treatment of the genus lacking); < B. asteroides var. asteroides – FNA; = Weakley]
SR−O; S2,G5TNR.
Lake Waccamaw (Infrequent): Howell LAWA−1, 158 (NCSC!)
Perennial herbs. Eulittoral zone (NLSS−LW). Aug−Sep. Fig.
Boltonia asteroides var. glastifolia (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Stem and leaf
d: Radiate head
Erigeron vernus
Basionym: Aster vernus L.
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw: ♦
Eupatorium capillifolium
Basionym: Artemisia capillifolia Lam.
Taxon concept: [= E. capillifolium var. capillifolium – RAB; = GW, FNA, Weakley]
Lake Waccamaw (Infrequent): Howell LAWA– 141 (NCSC!)
Perennial herbs. Eulittoral zone; usually in a stunted form where detritus has washed ashore just under the maximum annual high water mark (NLSS−LW). Sep−Nov. Fig.
Eupatorium capillifolium (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Leaf
d: Leaf
Eupatorium mohrii Greene × paludicola
Lake Waccamaw (Rare): Howell LAWA−6 (NCSC!)
Euthamia caroliniana
Basionym: Erigeron carolinianus L.
Taxon concept: [> Solidago microcephala (Nutt.) Bush – RAB; > Solidago tenuifolia Pursh – RAB; < E. tenuifolia – GW (also see E. hirtipes); > E. minor (Michx.) Greene – GW; = FNA, Weakley]
Lake Waccamaw (Rare): Howell LAWA−12 (NCSC!)
Perennial herbs. Eulittoral zone (NLSS−LW). Sep−Dec. Fig.
* Hypochaeris radicata
Taxon concept: [= Hypochoeris radicata L. – RAB; = FNA, Weakley]
Bay Tree Lake (Rare): Howell BATR−32 (NCSC!)
Krigia virginica
Basionym: Hyoseris virginica L.
Taxon concept: [= RAB, GW, FNA, Weakley]
Bay Tree Lake (Rare): Howell BATR−20 (NCSC!)
Mikania scandens
Basionym: Eupatorium scandens L.
Taxon concept: [= RAB, GW, Weakley]
Lake Waccamaw (Infrequent): Howell LAWA−161 (NCSC!)
Perennial, sometimes lianescent, vines. Eulittoral zone; usually sprawling and climbing on small shrubs and trees (NLSS−LW). Jun−Oct. Fig.
Mikania scandens (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Leaves
d: Capitulescence
Pluchea baccharis
Basionym: Conyza baccharis Mill.
Taxon concept: [= P. rosea R.K. Godfrey – RAB; = P. rosea var. rosea – GW; = FNA, Weakley]
Lake Waccamaw (Frequent): Howell LAWA−2, 101, 148 (NCSC!)
Sclerolepis uniflora
Basionym: Ethulia uniflora Walter
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw (Frequent): Howell LAWA−18, 23, 103, 108 (NCSC!)
Solidago fistulosa
Taxon concept: [= RAB, GW, FNA, Weakley]
Bay Tree Lake (Rare): Howell BATR−64, 65 (NCSC!)
Horseshoe Lake: Buell 2266 (NCSC!)
Perennial herbs. Eulittoral zone; saturated peaty to sandy soils at or just below the mean annual high water mark (CPSI−CG). Aug−Nov. Fig.
Betulaceae
Alnus serrulata
Basionym: Betula serrulata Aiton
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw (Occasional): Howell LAWA−39, 170 (NCSC!); Matthews 683 (NCU!)
Shrubs. Eulittoral zone (NLSS–LW). Feb–Mar; Aug–Oct. Fig.
Alnus serrulata (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Twig and axillary bud
d: Leaf
e: Staminate catkin
f: Pistillate catkin
Betula nigra
Taxon concept: [= RAB, GW, FNA, Weakley]
Bay Tree Lake (Occasional): Howell BATR−13, 19 (NCSC!)
Lake Waccamaw (Occasional): Blomquist 15004 (DUKE!); Howell LAWA−37, 63 (NCSC!)
Little Singletary Lake (Infrequent): Howell LISI−29 (NCSC!)
Trees. Eulittoral zone (NLSS–LW). Mar–Apr; May–Jun. Fig.
Betula nigra (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Bark (young tree on right)
d: Pistillate catkin
Bignoniaceae
Campsis radicans
Basionym: Bignonia radicans L.
Taxon concept: [= RAB, GW, Weakley]
Lake Waccamaw (Infrequent): Howell LAWA−81 (NCSC!)
Lianas. Eulittoral zone; climbing on young trees and shrubs at or just below the mean annual high water mark (NLSS−LW). Jun−Jul; Sep−Oct. Fig.
Campsis radicans (digital photographs taken by Alexander Krings; illustration from
b: lllustration
c: Flower
d: Fruit
Cabombaceae
Brasenia schreberi
Taxon concept: [= RAB, GW, FNA, Weakley]
Horseshoe Lake (Infrequent): Howell HOLA−43 (NCSC!)
Perennial herbs. Infralittoral zone (CPSI–CG). Jun–Oct. Fig.
Cabomba caroliniana
Taxon concept: [= RAB, GW, FNA, Weakley]
Horseshoe Lake (Infrequent): Howell HOLA−26 (NCSC!)
Campanulaceae
Lobelia glandulosa
Taxon concept: [= RAB, GW, Weakley]
Lake Waccamaw: ¤
Perennial herbs. Eulittoral zone (NLSS−LW). Sep−Oct.
Lobelia nuttallii
Taxon concept: [= RAB, GW, Weakley]
Horseshoe Lake (Rare): Howell HOLA−47 (NCSC!)
Perennial herbs. Eulittoral zone; moist sandy soil at or just below the high water mark (CPSI−CG). May−Nov. Fig.
Caryophyllaceae
Stipulicida setacea var. setacea
Taxon concept: [< S. setacea Michx. – RAB; = FNA, Weakley]
Bay Tree Lake (Rare): Howell BATR−21 (NCSC!)
Clethraceae
Clethra alnifolia
Taxon concept: [< C. alnifolia var. alnifolia – RAB; = GW, FNA, Weakley]
Bay Tree Lake (Occasional): Howell BATR−12 (NCSC!)
Jones Lake (Occasional): Howell JOLA−5 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−42, 132, 150 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−7, 57 (NCSC!)
Singletary Lake (Occasional): Howell SILA−15, 28 (NCSC!)
Shrubs. Juncture of supralittoral and eulittoral zones; can also establish itself on stumps, logs, and tree bases in the eulittoral zone (NLSS−C, NLSS−LW, NLSM−T, CPSI−CG). Jun−Jul; Sep−Oct. Fig.
Clethra alnifolia (digital photographs taken by Nathan Howell [leaves and flowers] and Alexander Krings [twig and fruits]; illustration from
b: Illustration
c: Twig, showing leaf scar
d: Leaves
e: Flowers
f: Fruits
Cyrillaceae
Cyrilla racemiflora
Taxon concept: [= RAB, GW, FNA, Weakley]
Bay Tree Lake (Frequent): Howell BATR−38, 41, 60 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−37 (NCSC!)
Jones Lake (Occasional): •
Lake Waccamaw (Frequent): Howell LAWA−100 (NCSC!)
Little Singletary Lake (Frequent): Howell LISI−32 (NCSC!)
Singletary Lake (Frequent): Howell SILA−11, 23 (NCSC!)
Shrubs or small trees. Juncture of eulittoral and supralittoral zones (NLSS−C, NLSM−T, NLSS−LW, CPSI−CG). May−Jul; Sep−Oct. Fig.
Cyrilla racemiflora (digital photographs taken by Nathan Howell [inflorescence and infructescence] and Alexander Krings [twig and leaves]; illustration from
b: Illustration
c: Twig, leaf scar, and bud
d: Leaves
e: Inflorescence
f: Infructescence
Droseraceae
Drosera intermedia
Taxon concept: [= RAB, GW, Weakley]
Horseshoe Lake (Frequent): Howell HOLA−28, 40, 49 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−52, 56 (NCSC!)
Perennial herbs. Eulittoral zone and floating bogs (NLSS−C, NLSM−T, CPSI−CG, FB). Jul–Sep. Fig.
Drosera intermedia (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Habit
d: Inflorescences
Ebenaceae
Diospyros virginiana
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw (Rare): Howell LAWA−80 (NCSC!)
Trees. Juncture of eulittoral and supralittoral zones (NLSS−LW). May−Jun; Sep−Dec. Fig.
Diospyros virginiana (digital photographs taken by Nathan Howell [leaves and fruit] and Alexander Krings [twig and flowers]; illustration from
b: Illustration
c: Twig, showing the typical dark bud and a single vascular bundle scar in the leaf scar
d: Leaves
e: Flowers
f: Fruit
Ericaceae
Chamaedaphne calyculata
Basionym: Andromeda calyculata L.
Taxon concept: [= Cassandra calyculata (L.) D. Don − RAB; = FNA, Weakley]
Bakers Lake (Occasional): Howell BALA−1 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−1, 6, 42 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−15, 24 (NCSC!)
Singletary Lake: Fox, Wells, Sharp, Whitford, Fairchild s. n. (NCSC!)
Shrubs. Eulittoral zone, either in shallow water or in saturated organic soils at the high water mark (NLSS–C, NLSM-T, CPSI–CG, FB). Mar–Apr; Jun–Oct. Fig.
Chamaedaphne calyculata (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Inflorescences
d: Inflorescences
Eubotrys racemosa
Basionym: Andromeda racemosa L.
Taxon concept: [= Leucothoe racemosa (L.) A. Gray − RAB; = FNA, Weakley]
Horseshoe Lake (Occasional): Howell HOLA−12 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−40, 151 (NCSC!); Matthews s.n. (NCU!)
Jones Lake (Occasional): Howell JOLA−30 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−1 (NCSC!)
Salters Lake (Occasional): Howell SALA−12 (NCSC!)
Singletary Lake (Occasional): Howell SILA−7 (NCSC!)
Shrubs. Juncture of eulittoral and supralittoral zones; sometimes on the bases of large Taxodium trunks (NLSS–C, NLSS–LW, NLSM–T, CPSI–CG). Late Mar–early Jun; Sept–Oct. Fig.
Eubotrys racemosa (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Inflorescence
d: Infructescence
Lyonia ligustrina var. foliosiflora
Basionym: Andromeda paniculata var. foliosiflora Michx.
Taxon concept: [< L. ligustrina (L.) DC. – RAB; = GW, FNA, Weakley]
Bakers Lake (Infrequent): Howell BALA−17 (NCSC!)
Jones Lake (Infrequent): Howell JOLA−31 (NCSC!)
Salters Lake (Infrequent): Howell SALA−17 (NCSC!)
Shrubs. Juncture of eulittoral and supralittoral zones; sometimes growing from the bases of large Taxodium (NLSS–C). Late Apr–Jul; Sep–Oct. Two varieties of Lyonia ligustrina are commonly recognized: var. foliosiflora (Michx.) Fernald, with numerous and conspicuous leaf-like bracts in the inflorescence, and var. ligustrina, with no or few leaf-like bracts in the inflorescence. The material collected by the first author is var. foliosiflora, the more common variety found in the North Carolina Coastal Plain. Fig.
Lyonia ligustrina (digital photographs taken by Nathan Howell [leaves] and Alexander Krings [abaxial leaf surface, flower, infructescence]; illustration from
b: Illustration
c: Leaves
d: Abaxial leaf surface
e: Flower
f: Fruits
Lyonia lucida
Basionym: Andromeda lucida Lam.
Taxon concept: [= RAB, GW, FNA, Weakley]
Bakers Lake (Occasional): Howell BALA−8 (NCSC!)
Bay Tree Lake (Occasional): Howell BATR−17 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−7 (NCSC!)
Jones Lake (Frequent): Howell JOLA−11, 19 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−35 (NCSC!)
Little Singletary (Occasional): Howell LISI−5, 36 (NCSC!)
Salters Lake (Frequent): Howell SALA−5, 11 (NCSC!)
Singletary Lake (Frequent): Howell SILA−3 (NCSC!)
Shrubs. Juncture of eulittoral and supralittoral zones; sometimes growing from the bases of mature Taxodium (NLSS–C, NLSS–LW, NLSM–T, CPSI–CG). Apr–early Jun; Sep–Oct. Fig.
Lyonia lucida (digital photographs taken by Nathan Howell [inflorescence] and Alexander Krings [leaf, flowers, fruits]; illustration from
b: Illustration
c: Leaf (abaxial surface)
d: Inflorescence
e: Flowers
f: Fruits
Rhododendron viscosum var. serrulatum
Basionym: Azalea serrulata Small
Taxon concept: [= RAB; < R. viscosum – GW, FNA; = Weakley]
Singletary Lake (Rare): Howell SILA−33, 34 (NCSC!)
Shrubs. Juncture of eulittoral and supralittoral zones (NLSS–C). Late May–Jun; Jul–Oct. Fig.
Vaccinium formosum
Taxon concept: [< V. corymbosum L. – RAB; = V. australe Small – GW; < V. corymbosum L. – FNA; = Weakley]
Bay Tree Lake (Occasional): Howell BATR−33 (NCSC!)
Jones Lake (Occasional): Howell JOLA−13, 24, 26 (NCSC!)
Salters Lake (Occasional): Howell SALA−10 (NCSC!)
Singletary Lake (Occasional): Howell SILA−18 (NCSC!)
Shrubs. Juncture of eulittoral and supralittoral zones (NLSS−C, NLSM−T, NLSS−LW). Late Feb−May; Jun−Aug. Fig.
Vaccinium fuscatum
Taxon concept: [= V. atrococcum (Gray) Heller – RAB; = GW; < V. corymbosum L. –FNA; Weakley]
Bakers Lake (Occasional): Howell BALA−7 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−49 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−14, 39, 40 (NCSC!)
Salters Lake (Occasional): Howell SALA−16 (NCSC!)
Singletary Lake (Occasional): Howell SILA−21 (NCSC!)
Shrubs. Juncture of eulittoral and supralittoral zones (NLSS−C, NLSM−T, NLSS−LW). Late Feb−May; Jun−Aug. Fig.
Zenobia pulverulenta
Basionym: Andromeda pulverulenta W. Bartram ex Willd.
Taxon concept: [= RAB, GW, FNA, Weakley]
Bakers Lake (Infrequent): Howell BALA−5 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−17, 22, 35 (NCSC!)
Jones Lake (Occasional): Howell JOLA−25 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−10, 23 (NCSC!)
Singletary Lake: Fox & Boyce 3781 (NCSC!); Fox, Wells, Sharp, Whitford, Fairchild 1708 (NCSC!)
Shrubs. Juncture of supralittoral and eulittoral zones; sometimes growing on the bases of mature Taxodium (NLSS–C, NLSM–T, CPSI–CG, FB). Apr–Jun; Sep–Oct. Fig.
Zenobia pulverulenta (digital photographs taken by Nathan Howell [inflorescence, flowers within] and Alexander Krings [leaves, flower]; illustration from
b: Illustration
c: Leaves
d: Inflorescence
e: Flowers within
f: Flower
Euphorbiaceae
* Triadica sebifera
Basionym: Croton sebifer L.
Taxon concept: [= Sapium sebiferum (L.) Roxb. – RAB, GW; = Weakley]
Lake Waccamaw (Infrequent): Howell LAWA−92 (NCSC!)
Fabaceae
Wisteria frutescens
Basionym: Glycine frutescens L.
Taxon concept: [= RAB, GW, Weakley]
Bay Tree Lake (Occasional): Howell BATR−37 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−99, 117 (NCSC!)
Singletary Lake (Infrequent): Howell SILA−35 (NCSC!)
Lianas. Eulittoral zone (NLSS–LW). Apr–May; Jun–Sep. Fig.
Wisteria frutescens (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Imparipinnate leaf
d: Fruit
Fagaceae
Quercus nigra
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw: Godfrey 6320 (NCSC!)
Trees. Juncture of eulittoral and supralittoral zones (NLSS–LW). Apr; Sep–Nov. Fig.
Quercus nigra (digital photographs taken by Alexander Krings; illustration from
b: Bark
c: Leaves
d: Fruit
Gelsemiaceae
Gelsemium sempervirens
Basionym: Bignonia sempervirens L.
Taxon concept: [= RAB, GW, Weakley]
Bay Tree Lake (Occasional): Howell BATR−10, 25 (NCSC!)
Horseshoe Lake (Rare): Howell HOLA−4 (NCSC!)
Jones Lake (Infrequent): Howell JOLA−34 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−41 (NCSC!)
Little Singletary Lake (Infrequent): Howell LISI−34 (NCSC!)
Singletary Lake (Infrequent): Howell SILA−36 (NCSC!)
Lianas. Eulittoral zone (NLSS–C, NLSS–LW, NLSM–T, CPSI–CG). Mar–early May; Sept–Nov. Fig.
Gelsemium sempervirens (digital photographs taken by Alexander Krings; illustration from
b: lllustration
c: Leaves
d: Flower
e: Fruit
f: Seed
Hydrangeaceae
Decumaria barbara
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw (Rare): Dumond 1621 (NCU!); Godfrey 52278 (NCSC!); Howell LAWA−86 (NCSC!)
Lianas. Eulittoral zone; climbing on trees and shrubs at or just below the maximum annual high water mark (NLSS−LW, NLSM−LWP). May−Jun; Jul−Oct. Fig.
Decumaria barbara (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Leaves
d: Fruits
Hypericaceae
Hypericum canadense
Taxon concept: [= RAB, GW, Weakley]
Horseshoe Lake (Rare): Howell HOLA−48 (NCSC!)
Annual or perennial herbs. Eulittoral zone; moist sandy soils at or just below the maximum annual high water mark (CPSI−CG). Jul−Sep. Fig.
Hypericum mutilum var. mutilum
Taxon concept: [< H. mutilum L. – RAB, GW; = Weakley]
Lake Waccamaw (Rare): Howell LAWA−139 (NCSC!)
Hypericum virginicum
Taxon concept: [= RAB; = Triadenum virginicum (L.) Raf. – GW; = Weakley]
Bay Tree Lake (Occasional): Howell BATR−9, 56 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−33 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−54 (NCSC!)
Hypericum walteri
Taxon concept: [= RAB; = Triadenum walteri (J.F. Gmel.) Gleason – GW; = Weakley]
Lake Waccamaw (Occasional): Howell LAWA−20, 134, 149 (NCSC!); Wilbur 9363 (DUKE!)
Iteaceae
Itea virginica
Taxon concept: [= RAB, GW, FNA, Weakley]
Bakers Lake (Infrequent): Howell BALA−6 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−9 (NCSC!)
Jones Lake (Occasional): Howell JOLA−27 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−85 (NCSC!)
Singletary Lake (Occasional): Howell SILA−2 (NCSC!)
Shrubs. Juncture of eulittoral and supralittoral zones; sometimes establishing itself on stumps, logs, and bases of trees in the eulittoral zone (NLSS−C, NLSS−LW, CPSI−CG). May−Jun. Fig.
Itea virginica (digital photographs taken by Nathan Howell [habit and inflorescence] and Alexander Krings [leaf and fruits]; illustration from
b: Illustration
c: Habit
d: Leaf
e: Inflorescence
f: Fruits
Juglandaceae
Carya glabra
Basionym: Juglans glabra Mill.
Taxon concept: [= RAB, GW; < C. glabra (Mill.) Sweet – FNA; = Weakley]
Lake Waccamaw (Rare): Howell LAWA−96 (NCSC!); Matthews s.n. (DUKE!)
Lamiaceae
Lycopus angustifolius
Taxon concept: [< L. rubellus var. angustifolius (Elliott) H.E. Ahles – RAB, GW; = Weakley]
SR−P; S1, G4?Q.
Lake Waccamaw (Infrequent): Howell LAWA−4, 156, 157 (NCSC!)
Perennial herbs. Eulittoral zone (NLSS–LW). Jun–Sep. Fig.
Lycopus angustifolius (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Flowers
d: Fruits
Lauraceae
Persea palustris
Basionym: Tamala palustris Raf.
Taxon concept: [< P. borbonia – RAB; = GW, FNA, Weakley]
Bakers Lake (Occasional): Howell BALA−12 (NCSC!)
Jones Lake (Occasional): Howell JOLA−6, 18 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−61, 69 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−6 (NCSC!)
Salters Lake (Occasional): Buell s.n. (DUKE!, NCSC!); Howell SALA−6, 20 (NCSC!)
Singletary Lake (Occasional): Howell SILA−10, 25, 27 (NCSC!)
Shrubs or small trees. Juncture of eulittoral and supralittoral zones (NLSS−C, NLSS−LW). May−Jun; Sep−Oct. Fig.
Lentibulariaceae
Utricularia cornuta
Taxon concept: [= RAB, GW, Weakley]
Lake Waccamaw (Rare): Howell LAWA−109 (NCSC!)
Utricularia gibba
Taxon concept: [= RAB; = U. biflora Lam. – GW; = Weakley]
Horseshoe Lake (Occasional): Howell HOLA−19 (NCSC!)
Annual or perennial herbs. Eulittoral and infralittoral zones; Godfrey and Wooten (1981) described the habit as “very much intertwined, forming large floating bunches or mats” (CPSI−CG). May−Sep. Fig.
Utricularia purpurea
Taxon concept: [= RAB, GW, Weakley]
Horseshoe Lake (Occasional): Howell HOLA−36 (NCSC!)
Utricularia resupinata
Taxon concept: [= GW, Weakley]
Lake Waccamaw (Rare): Howell LAWA−123 (NCSC!)
Annual or perennial herbs. Eulittoral zone; commonly in saturated sandy to peaty soils above current lake levels or in 1−4 inches of water (NLSS−LW). Jun−Aug. Fig.
Utricularia striata
Taxon concept: [= U. fibrosa Walter – RAB, GW; = Weakley]
Bay Tree Lake (Occasional): Howell BATR−35, 42 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−27 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−14, 122 (NCSC!)
Utricularia subulata
Taxon concept: [= RAB, GW, Weakley]
Lake Waccamaw: ♦
Little Singletary Lake (Infrequent): Howell LISI−28, 49 (NCSC!)
Annual or perennial herbs. Eulittoral zone; typically found in saturated sands and peats (NLSS−C, NLSS−LW, NLSM−T). Mar−Aug. Fig.
Utricularia subulata (digital photographs taken by Nathan Howell [habit, flower front, flower back] and Alexander Krings [flower side]; illustration from
b: Illustration
c: Habit
d: Flower (front)
e: Flower (back)
f: Flower (side)
Linderniaceae
Lindernia dubia var. dubia
Basionym: Gratiola dubia L.
Taxon concept: [= L. dubia (L.) Pennell – RAB, GW; = Weakley]
Lake Waccamaw: Radford & Stewart 679 (NCU!)
Annual or biennial herbs. Eulittoral zone; saturated sandy soils (NLSS−LW). May−Nov. (Fig. 159). The first author did not encounter this taxon in the field, but a single voucher confirms its historic presence (see above). Fig.
Loganiaceae
Mitreola petiolata
Basionym: Cynoctonum petiolatum Walter ex J.F. Gmelin
Taxon concept: [= Cynoctonum mitreola (L.) Britton – RAB; = GW, Weakley]
Lake Waccamaw (Infrequent): Howell LAWA−140, 154 (NCSC!)
Annual herbs. Eulittoral zone; shallow water (1−6 inches) or saturated soils above current lake levels (NLSS−LW). Jul−Sep; Sep−Nov. Fig.
Lythraceae
Decodon verticillatus
Basionym: Lythrum verticillatum L.
Taxon concept: [= RAB, GW, Weakley]
Bay Tree Lake (Infrequent): •
Horseshoe Lake (Occasional): Howell HOLA−31 (NCSC!)
Jones Lake (Infrequent): •
Salters Lake (Infrequent): •
Shrubs. Eulittoral zone (NLSS−C, NLSM−T, FB, CPSI−CG). Jul−Sep. Fig.
Decodon verticillatus (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Leaves
d: Fruits
Magnoliaceae
Magnolia virginiana var. virginiana
Taxon concept: [< M. virginiana – RAB, GW, FNA; = Weakley]
Bakers Lake (Occasional): Howell BALA−3 (NCSC!)
Jones Lake (Occasional): Howell JOLA−8, 29 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−62 (NCSC!)
Salters Lake (Occasional): Howell SALA−13 (NCSC!)
Singletary Lake (Occasional): Howell SILA−5, 19 (NCSC!)
Trees. Juncture of eulittoral and supralittoral zones (NLSS−C, NLSS−LW, NLSM−T). Fig.
Melastomataceae
Rhexia aristosa
Taxon concept: [= RAB, GW, Weakley]
SC–V, FSC; S3, G3G4.
Horseshoe Lake: ►
Rhexia cubensis
Taxon concept: [= RAB, GW, Weakley]
W1; S3, G4G5.
Lake Waccamaw (Occasional): Howell LAWA–113, 126, 129 (NCSC!); LeBlond 3990 (NCU!)
Rhexia mariana var. exalbida
Taxon concept: [= RAB; < R. mariana var. mariana – GW; = Weakley]
Horseshoe Lake (Rare): Howell HOLA−46 (NCSC!)
Rhexia nashii
Taxon concept: [< R. mariana var. purpurea Michx. – RAB; = GW, Weakley]
Bay Tree Lake (Occasional): Howell HOLA−39, 57 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−44 (NCSC!)
Jones Lake (Infrequent): Howell JOLA−38,39 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−45 (NCSC!)
Singletary Lake (Infrequent): Howell SILA−26 (NCSC!)
Rhexia virginica
Taxon concept: [> R. virginica var. purshii – RAB; > R. virginica var. virginica; = GW, Weakley]
Little Singletary Lake (Infrequent): Howell LISI – 47 (NCSC!)
Menyanthaceae
Nymphoides aquatica
Basionym: Villarsia aquatica J.F. Gmel.
Taxon concept: [= RAB, GW, Weakley]
Lake Waccamaw (Occasional): Harper 954 (NCU!); Howell LAWA−28, 54 (NCSC!)
Perennial herbs. Eulittoral zone (NLSS−LW). Late Apr−Sep. Fig.
Myricaceae
Morella cerifera
Basionym: Myrica cerifera L.
Taxon concept [< Myrica cerifera var. cerifera – RAB; < Myrica cerifera L. – GW, FNA; = Weakley]
Bay Tree Lake (Infrequent): Howell BATR−11 (NCSC!)
Jones Lake (Infrequent): Howell JOLA−15 (NCSC!)
Lake Waccamaw (Occasional): Dennis 66-15 (DUKE!); Howell LAWA−36, 169 (NCSC!)
Salters Lake (Infrequent): Howell SALA−3 (NCSC!)
Shrubs or small trees. Juncture of eulittoral and supralittoral zones (NLSS−C, NLSS−LW). Apr; Aug–Oct. Fig.
Morella cerifera (digital photographs taken by Nathan Howell; illustration from
b: lllustration
c: Habit
d: Leaf
e: Staminate inflorescence
f: Fruits
Nelumbonaceae
Nelumbo lutea
Taxon concept: [= RAB, GW, FNA, Weakley]
W7; S2, G4.
Lake Waccamaw: Bell 12836 (NCU!); Leonard, Burnham & Ripperton 1748 (NCU!); Radford 6078 (NCU!); Schallert 10662 (DUKE!)
Nymphaeaceae
Nuphar sagittifolia
Basionym: Nymphaea sagittifolia Walter
Taxon concept: [< N. luteum ssp. sagittifolium (Walter) E.O. Beal – RAB, GW; = FNA, Weakley]
W1, FSC; S2, G5T2.
Lake Waccamaw (Rare along south and southwest shorelines; frequent elsewhere): Buell & Godfrey 3505 (NCSC!); Fox 1878 (NCSC!); Godfrey & Buell 3505 (NCU!); Howell LAWA−83 (NCSC!); Leconte 1085 (DUKE!); Matthews s.n. (DUKE!, NCU!); Radford 681, 4348 (NCU!)
Perennial herbs. Infralittoral zone; encountered around dam, northern shorelines, and offshore (NLSS–LW, NLSM–LWP). Apr–Oct. Fig.
Nymphaea odorata var. odorata
Taxon concept: [< N. odorata – RAB, GW; = FNA, Weakley]
Horseshoe Lake (Frequent): Beal 4349 (NCSC!); Buell & Whitford 1851 (DUKE!, NCSC!); Howell HOLA−18 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−17, 27, 76 (NCSC!)
Singletary Lake: Wilbur 60946 (DUKE!)
Perennial herbs. Eulittoral and infralittoral zones (NLSS–LW, NLSM–T, NLSM–LWP, CPSI–CG). Jun–Sep. Fig.
Nymphaea odorata var. odorata (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Leaves
d: Flower
Nyssaceae
Nyssa aquatica
Taxon concept: [= RAB, GW, Weakley]
Lake Waccamaw (Infrequent): Howell LAWA−56, 89 (NCSC!)
Trees. Eulittoral zone (NLSS−LW). Apr−May; Sep−Oct. Fig.
Nyssa aquatica (digital photographs taken by Nathan Howell [leaves, fruits] and Alexander Krings [bark, twig]; illustration from
b: Illustration
c: Bark
d: Twig
e: Leaves
f: Fruit
Nyssa biflora
Taxon concept: [= N. sylvatica var. biflora (Walter) Sarg. – RAB, GW; = Weakley]
Bakers Lake (Frequent): Howell BALA−11 (NCSC!)
Bay Tree Lake (Occasional): Howell BATR−48 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−15 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−10 (NCSC!); Totten s.n. (NCU!)
Little Singletary Lake (Occasional): Howell LISI−12, 17 (NCSC!)
Salters Lake (Occasional): •
Singletary Lake (Occasional): Howell SILA−4 (NCSC!)
Trees. Eulittoral zone (NLSS–C, NLSS–LW, NLSM–T, CPSI–CG). Apr–Jun; Aug–Oct. Fig.
Oleaceae
Fraxinus caroliniana
Taxon concept: [= RAB, GW, Weakley]
Lake Waccamaw (Infrequent): Howell LAWA−70, 75 (NCSC!)
Onagraceae
Ludwigia brevipes
Basionym: Ludwigiantha brevipes Long
Taxon concept: [= RAB, GW, Weakley]
SR–T, FSC; S1S2, G2G3.
Lake Waccamaw (Infrequent): Howell LAWA−102, 118 (NCSC!)
Ludwigia sphaerocarpa
Taxon concept: [= RAB, GW, Weakley]
E; S1, G5.
Lake Waccamaw (Occasional): Howell LAWA−33, 143 (NCSC!)
Plantaginaceae
Bacopa caroliniana
Basionym: Obolaria caroliniana Walter
Taxon concept: [= RAB, GW, Weakley]
T; S1, G4G5.
Lake Waccamaw (Rare): Howell LAWA−66, 120 (NCSC!); LeBlond 3984 (NCU!)
Perennial herbs. Eulittoral zone; calm, quiet waters (NLSS–LW). May–Sep. Fig.
Bacopa caroliniana (digital photographs by Nathan Howell; illustration from
b: Illustration
c: Habit
d: Flower
Nuttallanthus canadensis
Basionym: Antirrhhinum canadense L.
Taxon concept: [< Linaria canadensis (L.) Dum. Cours.; = Weakley]
Bay Tree Lake (Rare): Howell BATR–28 (NCSC!)
Annual or biennial herbs. Juncture of eulittoral and supralittoral zones. Mar−Jul. Fig.
Platanaceae
Platanus occidentalis
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw (Infrequent): Howell LAWA−68 (NCSC!)
Trees. Eulittoral zone; on saturated soils of sandbars and shorelines (NLSS−LW). Apr−May; Sep−Nov. Fig.
Polygalaceae
Polygala lutea
Taxon concept: [= RAB, GW, Weakley]
Horseshoe Lake (Rare): Howell HOLA−50 (NCSC!)
Lake Waccamaw (Rare): Howell LAWA−127 (NCSC!)
Biennial herbs. Eulittoral zone; moist sandy soils at or below the maximum annual high water mark (NLSS−LW, CPSI−CG). Apr−Oct. Fig.
Polygonaceae
Rumex hastatulus
Taxon concept: [= RAB, GW, FNA, Weakley]
Bay Tree Lake (Infrequent): Howell BATR−14, 23, 30 (NCSC!)
Ranunculaceae
Clematis crispa
Taxon concept: [= RAB, GW, FNA. Weakley]
Lake Waccamaw: Matthews s.n. (NCU!)
Rhamnaceae
Berchemia scandens
Basionym: Rhamnus scandens Hill
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw (Occasional): Howell LAWA−38 (NCSC!)
Lianas. Eulittoral zone (NLSS–LW). Apr–May; Aug–Oct. Fig.
Berchemia scandens (digital photographs taken by; illustration from
b: Illustration
c: Habit
d: Stem and leaves
Rosaceae
Amelanchier canadensis
Basionym: Mespilus canadensis L.
Taxon concept: [=RAB, GW, FNA, Weakley]
Bay Tree Lake: Radford 1354 (NCU!)
Shrubs or small trees. Juncture of eulittoral and supralittoral zones. Mar−Apr; May−Jun. Fig.
Amelanchier obovalis
Basionym: Mespilus canadensis var. obovalis Michx.
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw (Rare): Howell LAWA−48 (NCSC!)
Shrubs. Eulittoral zone (NLSS−LW). Mar−Apr; May−Jun. The only specimen encountered by the current author was found in a shallow concave depression in the middle of two boles of Taxodium ascendens arising from the same stump. The shrub established itself in the small amount of soil that had accumulated in the depression through the years. Fig.
Aronia arbutifolia
Basionym: Mespilus arbutifolia L.
Taxon concept: [= Sorbus arbutifolia var. arbutifolia; = RAB; = GW, FNA, Weakley]
Salters Lake: Buell s.n. (NCSC!)
Singletary Lake (Rare): Howell SILA−24 (NCSC!)
Shrubs. Juncture of eulittoral and supralittoral zones (NLSS−C). Mar−May; Sep−Nov. Fig.
Aronia arbutifolia (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Inflorescence
d: Infructescence
Rosa palustris
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw (Infrequent): LAWA−74, 112 (NCSC!)
Singletary Lake: Fox, Wells, Sharp, Whitford, Fairchild s. n. (NCSC!)
Shrubs. Eulittoral zone; sandy to peaty soils at or just below the maximum annual high water mark (NLSS−C, NLSS−LW). May−Jul; Sep−Oct. Rosa palustris can be distinguished from R. multiflora, a common exotic in the North Carolina Coastal Plain, by its large (adnate portion 13–30 mm long), entire, stipules. Those of R. multiflora are up to 21 mm long (adnate portion 3–15 mm long) and pectinate- fringed. Fig.
Rubus pensilvanicus
Taxon concept: [> R. argutus Link – RAB, GW; > R. betulifolius Small – RAB; = Weakley]
Lake Waccamaw (Infrequent): Howell LAWA−73, 97 (NCSC!)
Rubiaceae
Cephalanthus occidentalis
Taxon concept: [= RAB; < C. occidentalis var. occidentalis – GW; = Weakley]
Lake Waccamaw (Occasional): Howell LAWA−104, 119, 165 (NCSC!)
Shrubs. Eulittoral zone (NLSS–LW). Jun–Jul. Fig.
Cephalanthus occidentalis (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Leaves
d: Inflorescence
Diodia virginiana
Taxon concept: [= RAB, GW, Weakley]
Bay Tree Lake (Rare): Howell BATR−22 (NCSC!)
Annual or perennial herbs. Eulittoral zone; sandy soils at or just below the maximum annual high water mark. Jun–Dec. Fig.
Diodia virginiana (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Flower (adaxial)
d: Flower (lateral)
Galium obtusum var. obtusum
Taxon concept: [= RAB; < G. obtusum – GW; = Weakley]
Lake Waccamaw (Rare): Howell LAWA−138 (NCSC!)
Salicaceae
Populus heterophylla
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw (Infrequent): Howell LAWA−91 (NCSC!)
Trees. Eulittoral zone; saturated soils at or just below the maximum annual high water mark (NLSS−LW). Mar−Apr. Fig.
Populus heterophylla (digital photographs taken by Nathan Howell [young tree, leaves, inflorescences] and Alexander Krings [abaxial leaf surface]; illustration from
b: Illustration
c: Young tree
d: Leaves
e: Abaxial leaf surface
f: Inflorescences
Salix caroliniana
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw: Harper 970 (NCU!); Matthews s.n. (DUKE!, NCU!)
Trees. Eulittoral zone; sandbars and sandy shorelines (NLSS−LW). Mar−Apr. This taxon was not encountered by the first author, but voucher specimens confirm its historical presence. Fig.
Salix caroliniana (digital photograph taken by Alexander Krings; illustration from
b: Leaves (abaxial surface [top], adaxial surface [bottom])
Salix nigra
Taxon concept: [= RAB, GW, FNA, Weakley]
Lake Waccamaw (Infrequent): Howell LAWA−72 (NCSC!)
Santalaceae
Phoradendron leucarpum var. leucarpum
Basionym: Viscum leucarpum Raf.
Taxon concept: [< P. serotinum (Raf.) M.C. Johnst. – RAB; = Weakley]
Jones Lake (Infrequent): Howell JOLA−12 (NCSC!)
Salters Lake (Infrequent): Howell SALA−7, 19 (NCSC!)
Epiphytic shrubs. Eulittoral zone; typically on limbs of Acer or Nyssa (NLSS–C). Oct–Nov; Nov–Jan. Fig.
Sapindaceae
Acer rubrum var. rubrum
Taxon concept: [< A. rubrum L. – RAB, GW; = Weakley]
Bakers Lake (Rare): Howell BALA−4 (NCSC!)
Trees. Eulittoral zone; typically in saturated organic to sandy soils at or just below the maximum annual high water mark (NLSS−C). Jan−Mar; Apr−July. Fig.
Acer rubrum var. trilobum
Taxon concept: [< A. rubrum L. – RAB, GW; = Weakley]
Bay Tree Lake (Occasional): Howell BATR−1 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−5, 20 (NCSC!)
Jones Lake (Occasional): Howell JOLA−9, 21 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−22, 43, 44 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−11, 21 (NCSC!)
Salters Lake (Occasional): Beckman & Linnenburger 27 (DUKE!); Howell SALA−2, 18 (NCSC!)
Singletary Lake (Occasional): Howell SILA−8 (NCSC!)
Trees. Eulittoral zone; typically in saturated organic to sandy soils at or just below the maximum annual high water mark (NLSS−C, NLSS−LW, NLSM−T, CPSI−CG). Jan−Mar; Apr−Jun. Fig.
Aesculus pavia var. pavia
Taxon concept: [< A. pavia L. − RAB; = Weakley]
Lake Waccamaw: Harbison 6084 (NCU!); Harper s. n., 955, 965 (NCU!); Matthews s.n. (NCU!); Oosting 3498 (DUKE!); Reed & Stites 275 (NCU!)
Sarraceniaceae
Sarracenia flava
Taxon concept: [= RAB, GW, FNA, Weakley]
Horseshoe Lake (Abundant): Buell & Whitford s.n. (NCSC!); Howell HOLA−16, 41 (NCSC!)
Perennial herbs. floating bogs (CPSI-CG, FB). Mar–Apr; May–Jun. Fig.
Sarracenia flava (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Habit
d: Flower (petals removed)
Theaceae
Gordonia lasianthus
Basionym: Hypericum lasianthus L.
Taxon concept: [= RAB, GW, FNA, Weakley]
Bakers Lake (Infrequent): Howell BALA−16 (NCSC!)
Horseshoe Lake: Buell 2262 (NCSC!)
Jones Lake (Occasional): Howell JOLA−2 (NCSC!)
Little Singletary Lake (Infrequent): Howell LISI−30, 48 (NCSC!)
Singletary Lake (Infrequent): Howell SILA−30 (NCSC!)
Trees. Juncture of eulittoral and supralittoral zones (NLSS−C). Jul−Sep; Sep−Oct. Fig.
Gordonia lasianthus (digital photographs taken by Nathan Howell; illustration from
b: Illustration
c: Twig and leaves
d: Leaves
e: Flower
f: Fruit
Ulmaceae
Ulmus americana var. americana
Taxon concept: [< U. americana L. – RAB, GW, FNA; = Weakley]
Lake Waccamaw (Rare): Bell 12839 (NCU!); Godfrey 6318 (NCSC!); Howell LAWA−95 (NCSC!)
Vitaceae
Muscadinia rotundifolia var. rotundifolia
Basionym: Vitis rotundifolia Michx.
Taxon concept: [< Vitis rotundifolia Michx. – RAB, GW; = Weakley]
Bay Tree Lake (Occasional): Howell BATR−46 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−64, 137 (NCSC!)
Salters Lake (Infrequent): Howell SALA−21 (NCSC!)
Lianas. Upper eulittoral zone; typically at the high water mark forming dense tangles along the waters edge (NLSS–C, NLSS–LW, NLSM–T). Late Apr–May; late Jul–Sep. Fig.
Muscadinia rotundifolia (digital photographs taken by Nathan Howell [all, except flowers] and Alexander Krings [flowers]; illustration from
b: Illustration
c: Habit
d: Leaf
e: Flowers
f: Fruits
Parthenocissus quinquefolia
Basionym: Hedera quinquefolia L.
Taxon concept: [= RAB, Weakley]
Lake Waccamaw (Infrequent): Howell LAWA−94 (NCSC!)
Lianas. Eulittoral zone; growing on fallen trees, shrubs, and erect trees at or just below the maximum annual high water mark (NLSS−LW). May−Jul; Jul−Aug. Fig.
Parthenocissus quinquefolia (digital photographs taken by Nathan Howell [fruits] and Alexander Krings [flowers]; illustration from
b: Illustration
c: Flowers
d: Fruits
Identification keys
|
Keys to the major vascular plant groups |
||
| 1 | Plant reproducing by spores | Pteridophytes |
| – | Plant reproducing by seeds | 2 |
| 2 | Seeds borne in woody cones; leaves needle-like or scale-like, < 3 mm wide | Gymnosperms |
| – | Seeds borne in fruits; leaves various | 3 |
| 3 | Plant exhibiting ≥ 2 of the following characters: Cotyledon 1; stem vascular bundles scattered; leaves parallel veined; floral parts in 3s | Monocotyledons |
| – | Plant exhibiting ≥ 2 of the following characters: Cotyledons 2; stem vascular bundles in a ring; leaves without parallel venation; floral parts in 4s and 5s | Basal Angiosperms, Magnoliids, and Eudicotyledons |
|
PTERIDOPHYTES Key adapted from Note: Successful keying of ferns is greatly facilitated by a basic understanding of fern morphology. |
||
| 1 | Leaves simple, scale-like, < 2 cm long, each leaf with 1, unbranched vein; sporangia borne in strobili at the tips of shoots |
Lycopodiaceae [Lycopodiella appressa] Fig. |
| – | Leaves pinnatifid to 2-pinnate, “ferny”, > 2 cm long, each leaf bearing numerous pinately-branched veins; sporangia borne in sori on the undersides of modified or unmodified pinnae | 2 |
| 2 | Plant epiphytic, growing on large limbs and tree trunks along shorelines; leaves (not including the petiole) 3−25 × 2.5−5 cm, evergreen, undersides with peltate, gray scales |
Polypodiaceae [Pleopeltis polypodioides ssp. michauxiana]Fig. |
| – | Plant not epiphytic, growing in inundated, saturated, or moist soils of shorelines; leaves (not including the petiole) > 25 cm × 5 cm, deciduous or evergreen, undersides lacking peltate, gray scales | 3 |
| 3 | Stipules present, wing-like; leaves 2-pinnate or more divided, pinnae divided to their midribs; sori and indusia lacking |
Osmundaceae [Osmunda spectabilis] Fig. |
| – | Stipules absent; leaves 1-pinnate-pinatifid or less divided; sori and indusia present | 4 |
| 4 | Leaves 1-pinnatifid, the rachis winged by leaf tissue throughout most or allof its length | 5 |
| – | Leaves 1-pinnate-pinnatifid, the pinnae fully divided from one another (the rachis not winged by leaf tissue throughout most or all of its length) | 6 |
| 5 | Fertile leaf woody, with bead-like segments; margins of sterile leaves entire, wavy, the lowermost pinnae sometimes becoming slightly lobed; pinnae with obtuse apices |
Onocleaceae [Onoclea sensibilis] Fig. |
| – | Fertile leaf herbaceous, not woody or with bead-like segments; margins of sterile pinnae finely serrulate; pinnae mostly with acute apices |
Blechnaceae [Lorinseria areolata] Fig. |
| 6 | Rhizomes long-creeping; leaves deciduous, monomorphic, 28–60 cm long, scattered along the rhizome, forming clonal patches; petiole dark purple to black proximally; sori elongate, borne end to end along both sides of main veins, pinnae lobes of sterile leaves with reticulate, chain-like venation on either side of the central vein |
Blechnaceae [Anchistea virginica] Fig. |
| – | Rhizomes short-creeping; leaves evergreen, somewhat dimorphic (fertile pinnae in distal half of leaves), 35–120 cm long, clustered on the rhizome, not forming clonal patches; petiole not purple to black proximally; sori circular, not borne end to end along the main veins, located midway between main vein and pinnae lobe margins; pinnae lobes of sterile portions of leaves lacking a chain-like venation pattern on either side of the central vein |
Dryopteridaceae [Dryopteris ludoviciana] Fig. |
|
GYMNOSPERMS Key adapted from |
||
| 1 | Leaves scale-like or needle-like, < 1.5 cm long, not in fascicles; seed cone scales valvate or imbricate, if imbricate then leaves opposite and scale-like; seeds 1−3 per scale | Cupressaceae |
| – | Leaves needle-like, (10−) 12−45 cm long, in fascicles of 2−3 leaves; seed cone scales imbricate; seeds 2 per scale | Pinaceae [Pinus] |
|
Cupressaceae Key adapted from |
||
| 1 | Leaves scale-like, 1−3 mm long, opposite or whorled, evergreen; mature seed cones woody, 4−9 mm broad, scales imbricate; seeds 1–2 (−3) per scale |
Chamaecyparis thyoides Fig. |
| – | Leaves linear, 3−17 mm long, alternate, deciduous; mature seed cones woody, 1.3–3.6 cm broad, scales valvate; seeds (1−) 2 per scale | Taxodium |
|
Taxodium Rich. Key adapted from Note: “In the following key, leaf and branchlet characters of T. ascendens refer to mature trees; foliage of juvenile trees often mimics that of T. distichum. Leaf and branchlet characters of T. distichum refer to both mature and juvenile trees; however, in the crowns of mature T. distichum, leaf and branchlet characters sometimes mimic those of T. ascendens. For these reasons, accurate identification of the two species often requires observation of other, non-foliage features, including the stature of the “knees”, the thickness and texture of the bark, and the habitat in which the trees grow” ( |
||
| 1 | Leaves mostly vertically ascending, appressed and overlapping, spirally arranged; branchlets ascending from twigs, secundly erect; bark 1–2.5 cm thick, furrowed, dark- brown, not exfoliating; larger knees short, rarely > 4 dm tall, with thick, compact bark on top; trees of isolated depressions, natural lakes, wet savannas, pocosins, other wet peaty habitats, and, less commonly, blackwater swamps |
Taxodium ascendens Fig. |
| – | Leaves pendent to horizontally spreading to laterally divergent, spirally arranged but generally appearing distichous (“featherlike”); branchlets not ascending from twigs; bark < 1 cm thick, exfoliating in shreddy, orange-brown strips; larger knees often tall, frequently > 4 dm tall, with thin, shreddy bark on top; trees of blackwater swamps, brownwater swamps, natural lakes, and millponds; usually in riverine situations |
Taxodium distichum Fig. |
|
Pinaceae Key adapted from |
||
| 1 | Open seed cones about as broad as long, “top-shaped”, 3–6 cm long, serotinous; trunks typically producing epicormic branches, especially in response to fire |
Pinus serotina Fig. |
| – | Open seed cones distinctly longer than broad, not top-shaped, 6–18(−20) cm long, not serotinous; trunk not producing epicormic branches |
Pinus taeda Fig. |
|
MONOCOTYLEDONS Key adapted from |
||
| 1 | Plant an epiphyte, growing on the trunks and limbs of trees in the littoral zone | 2 |
| – | Plant not epiphytic, rooted in soil or freely floating | 3 |
| 2 | Plant green, erect, not scurfy; leaves lanceolate; roots present, fibrous; flowers in racemes, petals dimorphic (two similar in size, the third differentiated into a broad lip) | Orchidaceae [Epidendrum magnoliae] |
| – | Plants gray, pendent (often in masses), scurfy; leaves filiform; roots absent; flowers solitary, petals monomorphic |
Bromeliaceae [Tillandsia usneoides Fig. |
| 3 | Plant diminutive ≤ 1.5 mm long in any dimension, floating or submersed in water, sometimes left stranded on mud or debris by receding water levels, plants thallus-like, not differentiated into stems and leaves, rootless or with few simple roots | Araceae [Wolffia] |
| – | Plant not diminutive or thallus-like, > 2 mm in any dimension, differentiated into stems and leaves, rooted in soil or floating on water surface | 4 |
| 4 | Stems woody | 5 |
| – | Stems herbaceous | 6 |
| 5 | Leafy stem erect, smooth, lacking prickles; internodes hollow |
Poaceae [Arundinaria tecta Fig. |
| – | Leafy stems climbing by stipular tendrils, armed with prickles; internodes solid | Smilacaceae [Smilax] |
| 6 | Flowers borne in a single compact head terminating an elongate scape | 7 |
| – | Flowers not borne in single compact heads atop elongated scapes | 8 |
| 7 | Flowering head involucrate, white to gray, hemispheric, “button-like”, < 1 cm tall; flowers 2−3-merous, unisexual, 1.5−4 mm long, pale to grayish, not subtended by a scale- like bract, sepals and petals partially coated with club-shaped hairs; anthers black, 2-locular | Eriocaulaceae |
| – | Flowering head not involucrate, brown, globose to cylindrical, “cone-like”, 0.5−3.5 cm tall; flowers 3-merous, bi-sexual, individual petals 3−6 mm long, yellow, subtended by a conspicuous scale-like bract, sepals and petals not coated with white club-shaped hairs; anthers yellow, 2−4-locular | Xyridaceae |
| 8 | Flowers and fruits subtended by imbricate or distichous bracts or scales and for the most part hidden by them, usually only the stamens and styles protruding at anthesis; fruit 1-seeded | 9 |
| – | Flowers and fruits not subtended by imbricate or distichous scales, or if so, then the flowers exceeding or equalling the bracts or scales and not hidden; fruit > 1- seeded | 10 |
| 9 | Leaves usually 3-ranked, sheaths typically closed; culms typically triangular in cross- section and solid; fruit an achene | Cyperaceae |
| – | Leaves usually 2-ranked, sheaths open (split lengthwise on the side opposite the blade); culms terete in cross-section, usually hollow; fruit a caryopsis | Poaceae |
| 10 | Plants aquatic, wholly submersed (except for Mayaca fluviatilis, which may be found wholly submersed or growing erect in saturated soils along shorelines); inflorescences submersed, floating, or just above the water surface | 11 |
| – | Plants terrestrial, or if growing in shallow water then the inflorescences well above the water surface (except during infrequent flooding events) | 13 |
| 11 | Leaves opposite or whorled (if opposite but appearing whorled, then leaf bases dilated and sheathlike); flowers either lacking perianth parts as in Najas or inconspicuous as in Hydrilla | Hydrocharitaceae |
| – | Leaves alternate; perianth parts present or not, if so, then conspicuous | 12 |
| 12 | Plant moss-like, habit ranging from wholly submersed to completely emersed; not heterophyllous; leaves 20−200 (−300) × 0.5−1 mm, very numerous and tightly spaced, spirally arranged, apices sometimes slightly bifid; flowers solitary in the leaf axils, petals rose to maroon to lilac, sometimes white basally, obovate |
Mayacaceae [Mayaca fluviatilis Fig. |
| – | Plant not moss-like, habit restricted to wholly submersed; heterophyllous or not, if heterophyllous, then the submersed leaves transluscent and with a soft, fragile, texture, the floating leaves coriaceous; leaves 10−160 × 0.5−85 mm, diffusely spaced, somewhat spirally arranged in P. pusillus, no so in P. pulcher, apices entire; flowers in axillary spikes, perianth lacking | Potamogetonaceae |
| 13 | Inflorescence a spadix surrounded by a yellow spathe; leaves 17−70 × 10−40 cm, peltate, bases cordate to sagittate to hastate, adaxial surface glaucous blue-green, typically with a red or purple spot where the petiole attaches to the blade | Araceae [Colocasia esculenta] |
| – | Plant not with the above combination of characters | 14 |
| 14 | Perianth segments densely pubescent abaxially | 15 |
| – | Perianth segments not densely pubescent abaxially | 16 |
| 15 | Leaves linear, equitant; corolla yellow; ovary inferior |
Haemodoraceae [Lachnanthes caroliniana Fig. |
| – | Leaves cordate to lanceolate, not equitant; corolla blue to purple; ovary superior | Pontederiaceae [Pontederia cordata] |
| 16 | Corolla stellate, petals white, female flowers exhibiting an apocarpous gynoecium, each pistil ripening into an achene; phyllodia present | Alismataceae [Sagittaria] |
| – | Corolla not stellate (or, if so, then petals not white), female flowers not exhibiting an apocarpous gynoecium, 1 pistil restricted to each flower, ripening into a capsule; phyllodia absent | 17 |
| 17 | Plant annual, diminutive, 5−20 cm tall, stems filiform; leaves minutely scale-like |
Burmanniaceae [Burmannia capitata Fig. |
| – | Plant perennial, not diminutive, > 20 cm tall, stems not filiform; leaves not scale-like (though blades not well-developed in Juncus effusus) | 18 |
| 18 | Ovary superior; perianth parts bract-like, dry, scarious, persistent, not petal-like; leaves septate or not, terete, or flat and blade-like | Juncaceae |
| – | Ovary inferior, perianth parts petal-like, neither bract-like, hard, nor scarious, not persistent; leaves flat and blade-like, never septate | 19 |
| 19 | Flowers radially symmetric; androecium and gynoecium in separate whorls, not borne in a column; pollen free |
Hypoxidaceae [Hypoxis curtisii Fig. |
| – | Flowers strongly bilaterally symmetric; androecium and gynoecium borne in a column; pollen in pollinia (pollen sacs) | Orchidaceae |
|
Alismataceae Key adapted from |
||
| 1 | Leaf blades floating, cordate basally |
Sagittaria filiformis Fig. |
| – | Leaf blades not floating, without basal lobes, linear to lanceolate, or modified asbladeless phyllodia, these with a spongy texture | 2 |
| 2 | Stalks of the pistillate flowering heads stout and reflexed in fruit; stamen filaments glabrous | Sagittaria filiformis |
| – | Stalks of the pistillate flowering heads not overly stout and either spreading or ascending in fruit; stamen filaments roughened with minute scales | 3 |
| 3 | Mature leaves all phyllodial, phyllodia terete or very nearly so |
Sagittaria isoetiformis Fig. |
| – | Mature leaves with blades and petioles, or phyllodia flattened on the adaxial surface or triangular in cross-section | 4 |
| 4 | Plant with corms or stolons, coarse rhizomes lacking; blades of emersed leaves < 3 (−4) mm wide; flowers ≤ 1.3 cm in diam. | Sagittaria isoetiformis |
| – | Coarse rhizomes present, stolons and corms absent; blades of emersed leaves > 1 cm wide; flowers ≤ 2.3 cm in diameter | 5 |
| 5 | Larger phyllodes ≤ 1 cm wide, apices acute; pistillate pedicels 1−4 cm long; median resin duct of mature achene club-shaped, 2× the width of the posterior duct |
Sagittaria graminea Fig. |
| – | Larger phyllodes 0.8−2.5 cm wide, apices blunt; pistillate pedicels 2−5 (−6.5) cm long; median resin duct of mature achene linear, about as wide as the posterior duct (or ducts absent) | Sagittaria weatherbiana |
|
Araceae Key adapted from |
||
| 1 | Plant terrestrial, stems present, rooted in moist to saturated soils; leaf blades to 70 cm long |
Colocasia esculenta Fig. |
| – | Plant floating, diminutive, thallus-like, stems absent, dropping water levels sometimes leaving some plants stranded; leaf blades < 0.2 cm long | Wolffia spp. |
|
Wolffia Horkel ex Schleid. Key adapted from Note: The first author did not encountered taxa within this genus in the field; however, the Carolina Vegetation Survey reported “Wolffia spp.” from the southwest side of Lake Waccamaw. Although a species-level identification has not been made, a key to the two species most likely to inhabit this location is provided below. |
||
| 1 | Fronds nutshell-like, upper surface flattened, 0.5−1 × as deep as wide, a small portion not flattened and with minute central papillae, fronds brownish punctate above (best seen in dead fronds), cells of fronds inflated in the lower portions and becoming progressively smaller and more compact toward the upper surface |
Wolffia brasiliensis Fig. |
| – | Fronds globoid to ovoid, upper surface convex, 1−1.5 × as deep as wide, a small portion slightly flattened and roughened with minute central papillae, fronds not brownish punctate above, cells of frond uniformly inflated throughout | Wolffia columbiana |
|
Cyperaceae Key adapted from |
||
| 1 | Achenes enclosed in a perigynium; flowers unisexual | Carex |
| – | Achenes not enclosed within a perigynium; flowers unisexual or bisexual | 2 |
| 2 | Leaves absent; spikelets 1 per culm, terminal | Eleocharis |
| – | Leaves present; spikelets ≥ 1 per culm, terminal or axillary | 3 |
| 3 | Spikelet scales distichous (two−ranked) | 4 |
| – | Spikelet scales spirally arranged, imbricate | 5 |
| 4 | Leaves not 3−ranked, predominantly basal; inflorescence terminal; perianth bristles lacking | Cyperus |
| – | Leaves prominently 3−ranked, cauline; inflorescence axillary; perianth bristles 6−9 |
Dulichium arundinaceum Fig. |
| 5 | Base of style hardened, differentiated from achene body, persistent as a tubercle at apex of achene | Rhynchospora |
| – | Base of style not hardened; tubercle absent from apex of achene | 6 |
| 6 | Perianth bristles present | 7 |
| – | Perianth bristles absent | 8 |
| 7 | Perianth scales 3, stalked, paddle−shaped; perianth bristles 3 |
Fuirena pumila Fig. |
| – | Perianth scales lacking; perianth bristles typically 4−8 |
Scirpus cyperinus Fig. |
| 8 | Style entire along margins; culms obtusely angled, 50−80 cm tall; leaf blade margins scaberulous; perennial |
Cladium mariscoides Fig. |
| – | Style fringed along margins; culms flattened, to 40 cm tall; leaf blade margins glabrous; annual |
Fimbristylis autumnalis Fig. |
|
Carex L. Key adapted from |
||
| 1 | Achene lenticular (biconvex); stigmas 2; perigynia wing-margined | 2 |
| – | Achene trigonous (three-sided); stigmas 3; perigynia not wing-margined | 3 |
| 2 | Pistillate scales in middle to lower portions of spike 2.8−3.5 (3.8) mm long, apices short-aristate; leaf blades 3−7 per fertile culm, 11−50 × 0.25−0.6 cm; spikes 6−20 × 4−9 mm; perigynia faintly 3−8 nerved on each face, obovate, 4−5.5 × 2.5−3.8 mm; achenes oblong, 1.7−2 × 0.9−1.1 mm, 0.3−0.4 mm thick |
Carex alata Fig. |
| – | Pistillate scales in middle to lower portions of spike 2.2−3.7 mm long, apices mostly obtuse, not short-aristate; leaf blades 2−4 (−6) per fertile culm, 8−30 × 0.25−0.4 cm; spikes 6−13 (−17) × 3.8−7 mm; perigynia conspicuously 5−many- nerved on each face, obovate, 3−4.6 × 1.6−2.6 (2.8) mm; achenes oblong, 1.3−1.7 × 0.7−1 mm, 0.4−0.5 mm thick |
Carex longii Fig. |
| 3 | Style jointed near the base, disarticulating at the joint; culms erect 20−100 (−130) cm; pistillate spikes 1.5−6.5 × 1.3−3 cm; perigynia 11−19 × 3−6 mm; pistillate scales about as long as the body of the perigynia; achenes 3−4 (−4.5) × 1.7−2.6 (−2.8) mm |
Carex lupulina Fig. |
| – | Style not jointed near the base, hardened and persistent, remaining attached to the mature achene; culms erect 40−90 cm; pistillate spikes 2−4 × 0.7−0.8 cm; perigynia 3.9−7 × 2−3.3 mm; lower pistillate scales about as long as the body of the perigynia, upper about 1⁄2 as long; achenes 2−2.5 ×1.5−2 mm |
Carex striata Fig. |
|
Cyperus L. Key adapted from |
||
| 1 | Stigmas 2; achenes lenticular |
Cyperus polystachyos Fig. |
| – | Stigmas 3; achenes trigonous | 2 |
| 2 | Mature spikelets shedding scales and achenes individually, leaving the rachilla intact (for at least a short while); roots and lower sheaths conspicuously reddish-purple; culms trigonous to roundly trigonous, (0.5−) 5−25 (−105) cm × 0.1−0.25 (0.75) cm; spikelets 3−8 (−11) × 1−1.5 mm; pistillate scales deciduous, laterally light brown with red speckles and ribless, medially greennish and 3-ribbed, 1.3−1.5 × 0.8−1.2 mm, apex obtuse, mucronulate; achenes sessile, ovoid, (0.4−) 0.7−1 × 0.4−0.6 mm, surface glabrous |
Cyperus erythrorhizos Fig. |
| – | Mature spikelets disarticulating into segemets, each comprised of a scale, an achene, and a cartilaginously thickened section of the rachilla; roots and lower sheaths not conspicuously reddish-purple; culms trigonous (4−) 10−50 (−130) × (0.05−) 0.1−0.4 cm; spikelets (5−) 8−15 (−38) × 0.8−1.3 (−1.9) mm; floral scales medially green and 2−5 ribbed, laterally straw-colored to reddish and 1−3 ribbed, (2−) 2.2−2.8 (−3.2) × (1.2−) 1.4−1.6 (−1.8) mm, apex entire or emarginate; achene stipitate, narrowly ellipsoid to oblong, (1−) 1.2−1.5 (−1.9) × 0.5−0.6 (−0.75) mm, surface finely papillose |
Cyperus odoratus Fig. |
|
Eleocharis R.Br. Key adapted from Note: Achene measurements in this key do not include the tubercle. Eleocharis baldwinii and E. vivipara can be difficult to distinguish in the field when they are both in their vegetative forms. One should pay particular attention to the sheaths encircling the culms; the differences are highlighted in the key below. |
||
| 1 | Culm as broad or broader than width of terminal spike, nodose-septate |
Eleocharis equisetoides Fig. |
| – | Culm narrower than width of terminal spike, not nodose-septate | 2 |
| 2 | Culms strictly producing fertile spikelets, vegetative proliferations absent; achenes lenticular or biconvex; styles 2−branched |
Eleocharis olivacea var. olivacea Fig. |
| – | Culms producing vegetative proliferations or fertile spikelets; achenes trigonous or nearly terete; styles 3−branched | 3 |
| 3 | Upper portion of sheath thin and scarious, lacking a noticeable red-dotted band encircling the apex of sheath (i.e, the apex of the sheath is not differently colored than the lower potions of sheath); sheath tips 1−2 mm long; culms usually more thin and capillary; scales of spikes 2-ranked (distichous); spike usually 2−4 flowered; achenes trigonous, smooth, grayish-olive, 0.6−0.9 × 0.4−0.6 mm, apex constricted proximal to tubercle; tubercle pyramidal, trigonous, 0.2−0.3 (−0.4) × 0.2−0.5 mm |
Eleocharis baldwinii Fig. |
| – | Upper portion of sheath firm, a noticeable red-dotted band encircling the sheath apex present (i.e., the sheath apex a different color than the lower sheath); sheath tips <1 mm long; culms usually more robust and less capillary than E. baldwinii; scales of spike spirally imbricate, not 2-ranked; spike with > 4 flowers; achenes trigonous, finely reticulate, gray to greenish, 0.6−0.9 × 0.55−0.8 mm, apex constricted proximal to tubercle; tubercle pyramidal, trigonous, 0.2−0.5 × 0.4−0.5 mm |
Eleocharis vivipara Fig. |
|
Rhynchospora Vahl Key adapted from Note: A voucher (Wilbur 49814, DUKE) for Rhynchospora fascicularis (Michx.) Vahl was collected from the shoreline of Lake Waccamaw; however, this specimen appears referable to R. distans (Michx.) Vahl. Nonetheless, though not otherwise reported from the littoral zone of Carolina bay lakes, R. fascicularis has the potential to occur in these sites and is therefore included in the key below. Achene measurements in this key do not include the tubercle (i.e., the tubercle and achene should be measured as two separate entities). |
||
| 1 | Tubercle 3−23 mm long; style simple or bifid only at tip | 2 |
| – | Tubercle < 3 mm long; style divided into 2 slender branches | 4 |
| 2 | Longest perianth bristles shorter than the achene body |
Rhynchospora corniculata Fig. |
| – | Longest perianth bristles equaling or exceeding the achene body | 3 |
| 3 | Plants rhizomatous; primary clusters with 1−6 loosely clustered spikelets; achene (3.5−) 4.0−4.8 mm long |
Rhynchospora inundata Fig. |
| – | Plants cespitose; primary clusters with 10−50 densely clustered spikelets; achene (4.5−) 5−6 mm long |
Rhynchospora macrostachya Fig. |
| 4 | Inflorescence bracts several, bright white basally |
Rhynchospora latifolia Fig. |
| – | Inflorescence bracts 0−several, not white basally | 5 |
| 5 | Perianth bristles retrorsely barbellate (at least distally) |
Rhynchospora alba Fig. |
| – | Perianth bristles antrorsely barbellate | 6 |
| 6 | Surface of achene smooth, minutely pitted, or finely striate | 7 |
| – | Surface of achene transversely ridged, rugose, or honeycomb-reticulate | 8 |
| 7 | Bristles > 1⁄2 as long or exceeding the achene body; larger basal leaves 1.3−2.5 mm wide, achene elliptic, 1.1−1.3 mm wide, tubercle triangular−attenuate |
Rhynchospora distans Fig. |
| – | Bristles virtually non-existent to 1⁄2 as long as the achene body (rarely > 1⁄2 as long as the achene body); larger basal leaves 2−4 mm wide; achene suborbicular, 1.2−1.5 mm wide, tubercle triangular | [Rhynchospora fascicularis] |
| 8 | Achenes biconvex, not flat or concave on one side |
Rhynchospora nitens Fig. |
| – | Achene faces flat or concave, when one face is concave, the other slightly convex | 9 |
| 9 | Achene < 2× as long as wide, obovate, tubercle triangular, 0.2−0.9 mm long | Rhynchospora elliottii |
| – | Achene at least 2× as long as wide, elliptic−oblong, tubercle subulate, 0.8−1.2 mm long |
Rhynchospora inexpansa Fig. |
|
Eriocaulaceae Key adapted from Note: Although the first author has only encountered E. aquaticum in the field, E. compressum was reported from the NCSU Crop Science Department (Rob Richardson and Justin Nawrocki, pers. comm., April 9, 2015) and is therefore included in the key below. |
||
| 1 | Plant 4−21 cm tall (−100 cm when submersed); receptacle/base of flowers glabrous or sparingly hairy; heads overall appearing dark gray to white, 4−10 mm in diam. when in full flower and fruit; seeds light-brown or red-brown, ovoid to broadly ellipsoid, faintly reticulate, not papillate; of sandy to peaty shorelines, bogs, and streams |
Eriocaulon aquaticum Fig. |
| – | Plant 20−70 cm tall; receptacle/base of flowers copiously hairy; heads overall appearing white, 10−20 mm in diam. when in full flower or fruit; seeds dark lustrous brown, broadly ovoid to round but asymmetric, minutely spiny papillate; of seasonally floooded depression ponds, savannas, flatwoods, ditches | [Eriocaulon compressum] |
|
Hydrocharitaceae Key adapted from |
||
| 1 | Leaves noticeably rough to the touch, in whorls of (3−) 4−8, 1.2−4 mm wide, lacking sheaths, margins conspicuously serrulate, each serration tipped with 1-celled sharp teeth, 1- nerved, mid-vein keeled below, keels bearing conical protrusions, each armed with sharp teeth; plants dioecious, flowers unisexual (only female plants found in the southeastern United States) |
Hydrilla verticillata Fig. |
| – | Leaves not rough, opposite or sometimes crowded and appearing whorled, 0.2−2.1 mm wide, sheaths present, margins minutely serrulate, 1-nerved, midvein lacking an abaxial keel and conical protrusions; plants monoecious, flowers unisexual |
Najas guadalupensis var. guadalupensis Fig. |
|
Juncaceae Key adapted from |
||
| 1 | Inflorescence bract exceeding the inflorescence, inflorescence thus appearing lateral | 2 |
| – | Inflorescence bract not exceeding the inflorescence, inflorescence appearing terminal | 3 |
| 2 | Basal sheaths (or at least a few) producing elongate well-developed blades; inflorescence bract channeled on one side; capsules subglobose |
Juncus coriaceus Fig. |
| – | Basal sheaths not producing elongate blades; inflorescence bract not channeled on one side; capsules more or less oblong, 3-sided |
Juncus effusus ssp. solutus Fig. |
| 3 | Leaf blades not septate | 4 |
| – | Leaf blades septate | 5 |
| 4 | Stems erect and with a hardened base, never creeping or forming mats; perianth < 6 mm long; plant not cinfined to aquatic settings, may occur in uplands as well as wetland margins, never submersed |
Juncus biflorus Fig. |
| – | Stems soft, weak, creeping and rooting at the nodes, often forming homogeneous mats or stands in shallow water or saturated soils above current water level; perianth 6−10 mm long; plant strictly aquatic, submersed and sterile or emersed/stranded and fertile |
Juncus repens Fig. |
| 5 | Flowers or fruits borne singly (solitary) on the branches of the inflorescence; inflorescence diffuse, with slender flexuous branches; flowers often aborted; seeds without tail-like appendages |
Juncus pelocarpus Fig. |
| – | Flowers or fruits borne in heads of 3 or more, heads often spherical; inflorescence not diffuse, branches not slender and flexuous; flowers seldom aborted; seeds with or without tail-like appendages | 6 |
| 6 | Mature seeds with elongate tail-like appendages, body of seeds 1.2−2.2 mm long |
Juncus canadensis Fig. |
| – | Mature seeds lacking elongate tail-like appendages; body of seeds < 0.7 mm long | 7 |
| 7 | Heads turbinate to hemispherical, 3−15-flowered; capsules 2.8−3.5 (−4) mm long, straw-colored, exerted, abruptly contracting at the summit, apex acute, valves separating (dehiscing) at maturity, equaling or just exceeding the perianth; stamens 3 or 6; seeds ellipsoid, clear amber |
Juncus acuminatus Fig. |
| – | Heads spherical, 15−60-flowered; capsules 2−3 mm long, straw-colored, exerted, apex gradually tapering to the summit, remaining attached at the tip, valves not separating (dehiscing) at maturity, subulate tips of the capsules exceeding the perianth when fully mature; stamens 3; seeds oblong, dark to clear yellow amber |
Juncus scirpoides var. compositus Fig. |
|
Orchidaceae Key adapted from |
||
| 1 | Plant an epiphyte, typically found on bases, boles, and large limbs of Taxodium, Nyssa, Liquidambar, and other deciduous hardwoods | Epidendrum magnoliae |
| – | Plant not epiphytic, found in the littoral zone and on floating bogs | 2 |
| 2 | Corolla greenish-colored, lip with a spur, spur deeply divided into 3 linear segments; leaves 3−5, basally disposed | Habenaria repens |
| – | Corolla white, pink, purple or magenta, lip not spurred; leaves basally disposed or cauline | 3 |
| 3 | Flowers arranged in distinct spirals (often appearing 3–4 ranked if spiral is “tight”, white, relatively small, 3–5 mm wide |
Spiranthes laciniata Fig. |
| – | Flowers not in distinct spirals, pink, magenta, purple, larger, typically ≥ 1 cm wide | 4 |
| 4 | Flowers not resupinate, lip oriented upwards, bearing numerous orange or yellow clavellate trichomes reminiscent of stamens |
Calopogon tuberosus var. tuberosus Fig. |
| – | Flowers resupinate, lip oriented downwards, not bearing numerous stamen-like trichomes |
Pogonia ophioglossoides Fig. |
|
Poaceae Key adapted from |
||
| 1 | Culm perennial, woody, developing complex branching systems from upper culm nodes; [Bambuseae] |
Arundinaria tecta Fig. |
| – | Culm annual or facultatively perennial, herbaceous, not developing complex branching systems from upper culm nodes | 2 |
| 2 | Spikelets almost always with 2 florets, lower floret in spikelet always sterile or staminate, frequently absent or reduced to lemma, upper floret bisexual, staminate, or sterile, unawned or awned from the lemma apices; [Andropogoneae and Paniceae] | 3 |
| – | Spikelets either not with 2 florets or with two florets and the lower bisexual or upper floret awned from lemma backs or bases [various tribes] | 10 |
| 3 | Spikelets in sessile-pedicellate pairs, not arranged in conspicuous rows on one side of the rachis; glumes stiff, indurate; usually subequal in length, one or usually both exceeding the floret (excluding the lemma awn); lemmas hyaline; paleas hyaline or absent; [Andropogoneae] | 4 |
| – | Spikelets solitary, or if paired, then forming 2–4 obvious rows on one side of rachis; glumes membranous, lower usually shorter than upper or absent entirely, upper glumes shorter than or nearly equaling upper floret; lower lemmas membranous, upper lemmas typically stiff and indurate, occasionally membranous; upper paleas of similar texture to upper lemmeas; [Paniceae] | 5 |
| 4 | Plant to 1 m tall; spikelets of the pair unalike, sessile bisexual, pedicellate sterile, vestigial, or absent | Andropogon |
| – | Plant to 3 m tall; spikelets of the pair alike, pedicellate spikelet perfect |
Saccharum giganteum Fig. |
| 5 | Base of spikelets with rounded, distended, swellings (gibbous) |
Sacciolepis striata Fig. |
| – | Spikelets not gibbous | 6 |
| 6 | Plant producing simple culms with terminal “spring” paniculate inflorescences before mid-summer, the culms branching and producing lateral “autumnal” inflorescences from mid to lower culm nodes in the summer and autumn, these often his by the fascicles of smaller “autumnal” leaves; upper florets not disarticulating at maturity | Dichanthelium |
| – | Plant producing terminal panicles in late summer and fall; culms usually not branching from mid to lower culm nodes, or, if so, the branches seldom further branched; upper florets disarticulating or not at maturity | 7 |
| 7 | Plant annual, lacking rhizomes or hard knotty crowns; spikelets verrucose | Panicum [in part] |
| – | Plant a perennial, with rhizomes or hard knotty crowns; spikelets not verrucose | 8 |
| 8 | Plant with hard, knotty crowns, lacking rhizomes; upper lemmas 1.2−1.6 mm long | Coleataenia [in part] |
| – | Plant with rhizomes; upper lemmas 1.6−4 mm long | 9 |
| 9 | Culms slightly compressed below; ligules ≤ 0.5 mm tall; spikelets subsecund, usually obliquely bent above the first glume, pedicels appressed; upper lemma apices lacking papillae, with minute tuft of hair | Coleataenia [in part] |
| – | Culms terete, not slightly compressed below; ligules 2−6 mm tall; spikelets not secund, not obliquely bent above first glume, pedicels spreading; upper lamma apices with simple or compound papillae, glabrous | Panicum [in part] |
| 10 | Plant seldom seen in flower; spikelets composed of a single floret, florets imperfect; culms ≤ 2 mm wide, slender, flexuous, prostrate; leaves conspicuously clustered at the culm apices, floating (lentic system) or streaming (lotic system) on the water surface, or emergent after receding water levels; glumes absent; [Oryzeae] |
Luziola fluitans Fig. |
| – | Plants regularly seen in flower; spikelets composed of ≥ 1 floret, florets imperfect or perfect; culms > 2 mm wide, slender, flexuous, or prostrate; leaves not conspicuously clustered at the culm apices, not floating or emergent after receding water levels; glumes present | 11 |
| 11 | Spikelets with (4−) 6−30 florets; [Cynodonteae] | Eragrostis |
| – | Spikelets with ≤ 3 florets; [Poaeae] | 12 |
| 12 | Culm 1.5-8.2 dm; sheaths glabrous; ligules (0.7−) 1.2−4 mm tall; blades 3−10 × 0.1−0.2 cm; panicles (5−) 10−25 (36) × (3) 4−24 cm, diffuse, the whole panicle detaching at the base at maturity, the resulting detached panicle resemblig a “tumbleweed” |
Agrostis hyemalis Fig. |
| – | Culm (0.9−) 2−13 dm; sheaths glabrous, hairy, or scabridulous; ligules (1−) 1.5−2.5 mm tall; blades 5−14 × (0.1-) 0.2-0.8 cm; panicles (2−) 5−15 (−25) × 0.5−2 cm, compact, spike-like, the panicle not detaching at the base at maturity |
Sphenopholis obtusata Fig. |
|
Andropogon L. Key adapted from |
||
| 1 | Leaves strongly glaucous (appearing powdery-white and leaving white residue on fingers when rubbed), glabrous; ligules (0.9−) 1.5 (−2) mm tall |
Andropogon glaucopsis Fig. |
| – | Leaves green, not glaucous (never powdery-white), pubescent (at least on the margin near the collar; ligules 0.2−1 mm tall |
Andropogon virginicus var. virginicus Fig. |
|
Coleataenia Griseb. Key adapted from |
||
| 1 | Cauline leaf blades 2−8 mm wide; glumes and sterile lemmas keeled along midvein; apices of fertile lemmas with a minute tuft of hairs |
Coleataenia longifolia var. longifolia Fig. |
| – | Cauline leaf blades 1−4 mm wide; glumes and sterile lemmas not keeled along midvein; apices of fertile lemmas lacking a minute tuft of stiff hairs | Coleataenia tenera |
|
Dichanthelium (Hitchc. et Chase) Gould Key adapted from |
||
| 1 | Spikelets 0.8−2.0 mm long | 2 |
| – | Spikelets 2.1−3.2 mm long | 5 |
| 2 | Internodes glabrous | 3 |
| – | Internodes crisp-puberulent | 4 |
| 3 | Plants with hard knotty crowns; culms to 100 cm; nodes without a distinct constricted yellow ring; vernal cauline leaves 15−20× as long as wide (5−12 cm long); ligules < 1 mm tall; spikelets 1.7−2.3 mm, glabrous | Dichanthelium dichotomum var. roanokense |
| – | Plants cespitose; culms 30−75 cm; nodes with a distinct constricted yellow ring; vernal cauline leaves < 15× as long as wide (5−10 cm long); ligules 0.2−0.5 mm tall; ligules 0.2−0.5 mm; spikelets 0.9−1.2 mm, puberulent to subglabrous |
Dichanthelium erectifolium Fig. |
| 4 | Spikelets 1.5−1.8 mm; first glume 0.5−0.8 mm; lower culm blades 2−5 mm wide |
Dichanthelium portoricense Fig. |
| – | Spikelets (1.8−) 1.9−2.2 (−2.3) mm; first glume 0.8−1.2 mm; lower culm blades 4−8 mm wide | Dichanthelium species 3 (=lancearium) |
| 5 | Larger culm blades usually 6−15 mm wide; spikelets 2−3 mm, pubescent; internodes glabrous | 6 |
| – | Larger culm blades usually 3.5−8 mm wide; spikelets 1.7−2.3 mm, pubescent or glabrous; internodes crisp-puberulent or glabrous | 7 |
| 6 | Larger culm blades 6−12 mm wide; lower culm nodes not bearded; spikelets 2−3 mm long; first glumes 0.5–1 mm long |
Dichanthelium boreale Fig. |
| – | Larger culm blades 13−25 mm wide; lower culm nodes bearded (often retrorsely); spikelets (2−) 2.2−2.8 mm long; first glumes 0.5–1.3 mm long |
Dichanthelium mattamuskeetense Fig. |
| 7 | Spikelets 1.7−2.3 mm long, glabrous; first glume 0.6−1.1 mm; largest vernal blades 15−20× as long as wide; internodes glabrous | Dichanthelium dichotomum var. roanokense |
| – | Spikelets (1.8) 1.9−2.2 (2.3) mm long, pubescent; first glume 0.8−1.2 mm; largest vernal blades < 15× as long as wide; internodes crisp-puberulent | Dichanthelium species 3 (=lancearium) |
|
Eragrostis Wolf Key adapted from |
||
| 1 | Lateral spikelets with widely spreading pedicels; lower pedicles longer than spikelets; disarticulation of the lemmas only, paleas are persistent |
Eragrostis elliottii Fig. |
| – | Lateral spikelets with appressed pedicels, lower pedicels shorter than spikelets; disarticulation usually of the whole floret | Eragrostis refracta |
|
Panicum L. Key adapted from |
||
| 1 | Glumes and lower lemmas verrucose; ligules 0.2−0.5 mm tall |
Panicum verrucosum Fig. |
| – | Glumes and lower lemmas smooth, not verrucose; ligules 0.5−6 mm tall | 2 |
| 2 | Panicle < 1 cm wide at maturity; upper glume and lower lemma 3−5 veined; ligule <1 mm tall |
Panicum hemitomon Fig. |
| – | Panicle 4−20 cm wide at maturity; upper glume and lower lemma 7−11 veined; ligule 2−6 mm tall |
Panicum virgatum Fig. |
|
Pontederiaceae Key adapted from |
||
| 1 | Outside of floral tube villous when young, essentially glabrous to sparsely glandular at maturity; leaves ovate to triangular−lanceolate, 2.2−21 cm wide, base usually cordate or truncate |
Pontederia cordata var. cordata Fig. |
| – | Outside of floral tube persistently pubescent with glandular hairs; leaves lanceolate, 0.4−8.3 cm wide, base usually cuneate to truncate | Pontederia cordata var. lancifolia |
|
Potamogetonaceae Key adapted from Note: The first author has not encountered Potamogeton in the field, but Potamogeton pusillus L. and Potamogeton pulcher were reported from Lake Waccamaw by the NCSU Crop Science Department (Rob Richardson and Justin Nawrocki, pers. comm., April 9, 2015) and Richard LeBlond with the North Carolia Natural Heritage Program (see specimen label of LeBlond 3382, NCU!). A key to these reported taxa is provided below. |
||
| 1 | Plant with floating and submersed leaves; submersed to 30 mm wide, linear to narrowly- lanceolate to lanceolate, mid to upper stem leaves translucent, with 4−8 rows of lacunae aon either side of midvein, floating to 85 mm wide, coriaceous, ovate to oblong-elliptic, bases rounded or slightly cordate |
Potamogeton pulcher Fig. |
| – | Plant with submersed leaves only, leaves linear, thread-like, or ribbonlike, to 3 mm wide, obvious lacunae absent on either side of midvein | Potamogeton pusillus |
|
Smilacaceae Key adapted from |
||
| 1 | Leaves evergreen, blades more or less oblong to linear or narrowly lanceolate, thick, coriaceous, midvein (as seen from the abaxial leaf surface) much more pronounced than the secondary veins, which are not noticeably evident (except perhaps at base of leaf blade) |
Smilax laurifolia Fig. |
| – | Leaves deciduous or evergreen, blades ovate to suborbicular, membraneous, midvein (as seen from the abaxial leaf surface) little if any more pronounced than the secondary veins, which are noticeably evident | 2 |
| 2 | Abaxial surface of mature leaves strongly glaucous |
Smilax glauca Fig. |
| – | Abaxial surface of mature leaves not glaucous, usually paler green than the adaxial surface | 3 |
| 3 | Mature berries blue-black, seeds (1−) 2−3 per berry; perianth green; leaves semi- evergreen to evergreen, margins of mature leaf blades usually not revolute, typically with small, flat, tooth-like projections near the base; of various upland and wetland habitats, typically not restricted to sites that are inundated for much of the year |
Smilax rotundifolia Fig. |
| – | Mature berries bright red, seeds 2−4 per berry; perianth brownish-yellow; leaves deciduous; margins of mature leaf blades usually revolute, lacking small, flat, tooth-like projections near the base; restricted to sites with long hydroperiods |
Smilax walteri Fig. |
|
Xyridaceae Key adapted from |
||
| 1 | Keel of lateral sepals long–fimbriate towards apex, fimbriate tip conspicuously protruding beyond the subtending bract (sometimes eroded and less evident in older spikes) |
Xyris fimbriata Fig. |
| – | Keel of lateral sepals lacerate, not ciliate or long-fimbriate | 2 |
| 2 | Lateral sepals longer than and protruding from the subtending bracts; scapes 5−15 dm tall |
Xyris smalliana Fig. |
| – | Lateral sepals shorter than subtending bracts, hidden (except when spikes open during maturity); scapes 1.5−12 dm tall | 3 |
| 3 | Summit of the scape distinctly flattened and broad relative to the spike; scape ridges 2−3, the two more prominent ridges comprising the flattened edge of the scape, therefore the upper scape ellipsoidal or fusiform in cross-section | Xyris iridifolia |
| – | Summit of the scape not flattened and broad relative to the spike; scape ridges > 3 (at least on the mid to lower portions of the scape), scape much narrower than the spike, terete or slightly flattened in cross-section |
Xyris jupicai Fig. |
|
BASAL ANGIOSPERMS, MAGNOLIIDS, and EUDICOTYLEDONS |
||
| 1 | Plant epiphytic |
Santalaceae [Phoradendron leucarpum; Fig. |
| – | Plant terrestrial or aquatic, not epiphytic | 2 |
| 2 | Plants woody [trees, shrubs, and lianas] | Key 1 |
| – | Plants herbaceous [herbs and vines] | Key 2 |
|
Key 1: Woody plants (trees, shrubs, and lianas) Key adapted from |
||
| 1 | Plant a liana, climbing by means of tendrils, adventitious roots, or twining stems | 2 |
| – | Plant a tree or shrub, not climbing | 10 |
| 2 | Leaves compound | 3 |
| – | Leaves simple | 7 |
| 3 | Leaves opposite | 4 |
| – | Leaves alternate | 5 |
| 4 | Stems not ribbed; leaves 1-pinnate, leaflets (5−) 7−11 (−15), 4−8 cm long, ovate, unlobed, margins coarsely serrate, rounded at base, apices acute; inflorescence > 1-flowered, flowers erect or spreading, pedicels shorter to slightly longer than the calyx tube; calyx greenish-yellow or orange, campanulate to funnel-shaped, 1.5−2 cm long, lobes ascending, margins entire; corolla showy, orange to scarlet, funnelform, 6−8 cm long; fruit a fusiform, falcate, capsule |
Bignoniaceae [Campsis radicans; Fig. |
| – | Stems ribbed; leaves 1−2-pinnate or sometimes simple or trifoliolate, leaflets 4−10 plus a ± tendrilate terminal leaflet, (1.5−) 3−10 cm long, linear to ovate, unlobed or proximally 3−5 lobed, margins entire, bases broadly to narrowly cuneate, truncate, occasionally subcordate, apices acute, obtuse, or acuminate; inflorescence 1-flowered, flower pendent, pedicel > 2x length of the calyx tube; calyx violet-blue, campanulate, 2.5−5 cm long, lobes strongly recurved, margins crisped; corolla lacking; fruit an aggregate of achenes |
Ranunculaceae [Clematis crispa; Fig. |
| 5 | Leaves trifoliolate; plant climbing by adventitious roots, tendrils absent; fruit a drupe, white; plant containing contact poisons |
Anacardiaceae [Toxicodendron radicans; Fig. |
| – | Leaves palmately or pinnately compound but not trifoliolate; plant twining or tendrillate; fruit a legume and brown or a berry and blue; plant not containing contact poisons | 6 |
| 6 | Plant twining, tendrils absent; leaves 1-pinnate, leaflets 9−15, entire; fruit a legume |
Fabaceae [Wisteria frutescens; Fig. |
| – | Plant tendrillate; leaves palmately compound, leaflets (3−) 5 (−7), coarsely serrate on their distal margins; fruit a berry |
Vitaceae [Parthenocissus quinquefolia; Fig. |
| 7 | Leaves opposite | 8 |
| – | Leaves alternate | 9 |
| 8 | Plant twining; leaves evergreen, lanceolate to ovate, 3−9 × 1−2.5 cm, apices acute to acuminate, margins entire; flowers solitary or sometimes in 2−3-flowered axillary cymes; petals lemon yellow, connate; ovary superior |
Gelsemiaceae [Gelsemium sempervirens; Fig. |
| – | Plant climbing by means of aerial adventitous roots; leaves deciduous, ovate to orbicular 3−12 cm × 1−8 cm, apices abruptly short acuminate, acute, or obtuse, margins distally serrate; flowers numerous, borne in terminal compound cymes; petals white, not connate; ovary inferior |
Hydrangeaceae [Decumaria barbara; Fig. |
| 9 | Tendrils lacking; leaves 3−8 × 1.5−4 cm, elliptic to ovate, margins slightly wavy to entire; fruit a blue-black drupe, 5−7 mm long |
Rhamnaceae [Berchemia scandens; Fig. |
| – | Tendrils present, borne opposite the leaves; leaves 5−12 × 5−12 cm, orbicular to ovate, margins prominently dentate-serrate; fruit a blue-black berry, 1−2.5 cm long |
Vitaceae [Muscadinia rotundifolia; Fig. |
| 10 | Leaves opposite or whorled | 11 |
| – | Leaves alternate | 15 |
| 11 | Plant exhibiting varying degrees of both opposite and whorled leaf arrangement | 12 |
| – | Plant exhibiting strictly opposite leaf arrangement | 13 |
| 12 | Plant woody proximally and herbaceous distally, stem with a soft corky texture when under water, young stems strongly pubescent, green; petioles not connected by a central stipule or stipular scars; leaves lanceolate to elliptic, to 20 × 5 cm, bases and apices acute, glabrous adaxially and pubescent with branched hairs abaxially; flowers in cymose inflorescences; corolla majenta; stamens of 3 possible lengths, 2 of the 3 occuring in any one flower |
Lythraceae [Decodon verticillatus; Fig. |
| – | Plants woody entirely, stem not soft and corky when submersed, young stems sometimes short-pilose initially but becoming glabrous with age, reddish-brown; petioles connected by a central stipule or stipular scar; leaves oval, oblong oval, elliptic or ovate, to 15 × 10 cm, bases broadly rounded to cuneate, apices acute or acuminate, glabrous adaxially and short-pilose abaxially (at least on the principal veins); flowers in dense globose heads, corolla white; stamens of one length |
Rubiaceae [Cephalanthus occidentalis; Fig. |
| 13 | Leaves simple | Sapindaceae [Acer] |
| – | Leaves compound | 14 |
| 14 | Leaves 1-pinnate, imparipinnate, leaflets 5−7 (−9); inflorescences borne on old wood of previous growing seasons before the development of new shoots; corolla not scarlet red; fruit a samara |
Oleaceae [Fraxinus caroliniana; Fig. |
| – | Leaves palmately compound, leaflets 5−7; inflorescences borne on new shoots of current year; corolla scarlet red; fruit a capsule |
Sapindaceae [Aesculus pavia; Fig. |
| 15 | Leaves compound | 16 |
| – | Leaves simple | 18 |
| 16 | Stems arching, trailing, or erect to 2 m tall, armed with numerous prickles; leaves 1-pinnately or 1-palmately compound, leaflets 3−9; fruit an aggregate of drupes or an aggregate of achenes enclosed in a hip | Rosaceae [Rosa & Rubus] |
| – | Stems erect, > 2m in height, lacking prickles; leaves 1-pinnately compound, leaflets 5−23; fruit a nut or drupe | 17 |
| 17 | Plant a shrub to small tree, to 7 m tall; stems densely short pubescent; leaflets 9−11 (−23), 3−8 ×1−4 cm, rachis winged; fruit a drupe |
Anacardiaceae [Rhus copallinum var. copallinum; Fig. |
| – | Plant a medium to large tree (unless in juvenile stage of development), to 30 m tall; stems not densely pubescent; leaflets (3−) 5 (−7), 3−22.5 × 1.8−13 cm; rachis not winged; fruit a nut enclosed within a husk |
Juglandaceae [Carya glabra; Fig. |
| 18 | Flowers borne in heads subtended by an involucre of bracts |
Asteraceae [Baccharis halimifolia; Fig. |
| – | Flowers not borne in heads subtended by an involucre of bracts | 19 |
| 19 | Leaves palmately lobed, margins glandular-serrate; fruit a multiple of capsules |
Altingiaceae [Liquidambar styraciflua; Fig. |
| – | Leaves pinnately or palmately lobed, margins otherwise; fruit otherwise | 20 |
| 20 | Fruit a nut (acorn) bearing a basal cupule (“cap”); buds conspicuously clustered at twig tips, scales imbricate |
Fagaceae [Quercus nigra; Fig. |
| – | Fruit otherwise; axillary buds not clusted at twigs tips with scales imbricate | 21 |
| 21 | Stipular scars conspicuous, completely encircling the twig stems or nodes | 22 |
| – | Stipular scars (if present) not encircling the twig stems or nodes completely | 23 |
| 22 | Bark of mature specimens gray, intact; leaves 6−15 × 2−6 cm, tardily deciduous, long-elliptic to oblong, adaxial surface light green and glabrous, abaxial surface strongly glaucous, petiole base solid, not covering axillary bud; flowers very showy and fragrant, “magnolia-like”; fruit an aggregate of follicles, elongate, seeds red-arillate |
Magnoliaceae [Magnolia virginiana var. virginiana; Fig. |
| – | Bark of mature specimens brown, furrowed, and scaly proximally, sloughing away to reveal a bright white smooth inner bark distally; leaves to 35 cm long and wide, deciduous, as long as broad, both adaxial and abaxial surfaces green, petiole base hollow, covering axillary bud; flowers neither showy nor fragrant; fruit a multiple of achenes, spherical, seeds brown, not arillate |
Platanaceae [Platanus occidentalis; Fig. |
| 23 | Flowers unisexual and borne in catkins | 24 |
| – | Flowers bisexual and not borne in catkins | 26 |
| 24 | Leaves pleasantly aromatic when crushed, glandular punctate abaxially |
Myricaceae [Morella cerifera; Fig. |
| – | Leaves not aromatic when crushed; not glandular punctate abaxially | 25 |
| 25 | Leaves < 3× as long as broad, very “neatly” pinnately veined, lateral veins consistently parallel to one another, ovate-triangular, sub-rhombic, elliptic, obovate, or oblong, margins either doubly serrate or slightly wavy; fruit a nutlet, 1-seeded | Betulaceae |
| – | Leaves > 3× as long as broad, not so neatly pinnately veined, lanceolate, margins serrate; fruit a capsule, 2-valved, many-seeded | Salicaceae [Salix] |
| 26 | Leaves broadly ovate to rhombic-ovate | 27 |
| – | Leaves longer than broad | 28 |
| 27 | Plant exuding milky sap when injured; leaves to 7 (−9) cm long; fruit a 3-valved capsule, maturing after leaf maturation in late summer to fall |
Euphorbiaceae [Triadica sebiferum; Fig. |
| – | Plant lacking milky sap; larger leaves ≥ 10 cm long; fruit a 2−4-valved capsule, maturing prior to leaf emergence in the spring |
Salicaceae [Populus heterophylla; Fig. |
| 28 | Fruits dry (capsules, berry-like, samaras) | 29 |
| – | Fruits fleshy (berries, drupes, pomes) | 34 |
| 29 | Leaves 2-ranked on the twigs, bases markedly oblique; fruit a samara |
Ulmaceae [Ulmus americana; Fig. |
| – | Leaves ≥ 3-ranked on the twigs, bases not oblique; fruit not a samara | 30 |
| 30 | Fruit indehiscent, berry-like; stems typically sharply longitudinally ridged below point of attachment of leaf petioles; leaves spatulate to oblanceolate, margins entire |
Cyrillaceae [Cyrilla racemiflora; Fig. |
| – | Fruit a dehiscent capsule; stems not longitudinally ridged below point of attachment of leaf petioles; leaves obovate, elliptic, oblong, or lanceolate, margins toothed (if entire, then blades with a perimarginal vein, lepidote, or with margins ciliate) | 31 |
| 31 | Plant a tree, to 26 m tall; flowers solitary, axillary; stamens > 50 |
Theaceae [Gordonia lasianthus; Fig. |
| – | Plant a shrub, < 6 m tall; flowers numerous, borne in racemes or spikes; stamens ≤ 10 | 32 |
| 32 | Pith chambered; ovary 2-locular |
Iteaceae [Itea virginica; Fig. |
| – | Pith solid; ovary ≥ 3-locular | 33 |
| 33 | Young twigs, inflorescence rachises, pedicels, and calyces stellate-pubescent; leaves oblanceolate, widest above middle, margins serrate distally; corolla rotate, petals connate ≤ ½ their length, lobes 5−8 mm long; ovary 3-locular |
Clethraceae [Clethra alnifolia; Fig. |
| – | Young twigs, inflorescence rachises, pedicels, and calyces glabrous or pubescent, but not stellate-pubescent; leaves lanceolate, ovate, elliptic, oblanceolate, or narrowly obovate, widest at or below the middle (widest above middle in Rhododendron viscosum, but margins finely bristly-ciliate); corolla urceolate, campanulate, globose, rotate, or funnelform, petals connate ≥ ½ their length, lobes either < 4 mm long or 7−24 mm long; ovary 5-locular | Ericaceae [in part] |
| 34 | Fruit a pome | Rosaceae [Amelanchier and Aronia] |
| – | Fruit a drupe or berry | 35 |
| 35 | Leaves evergreen | 36 |
| – | Leaves deciduous or tardily deciduous | 37 |
| 36 | Leaves not aromatic when crushed; margins spinose, crenate, or sometimes entire, lacking deforming galls; drupes containing 4−8 seeds | Aquifoliaceae [Ilex] |
| – | Leaves with a spicy aromatic scent when crushed; margins entire, often with numerous deforming galls (galls a result of red bay psyllid activity); drupes containing 1 seed |
Lauraceae [Persea palustris; Fig. |
| 37 | Plant a shrub, typically < 4 m in height; flowers perfect; fruit a blue, purple, or black berry; seeds ≥ 10, ca. 1.2 mm long | Ericaceae [Vaccinium] |
| – | Plant a small to full sized tree, > 4 m in height; flowers imperfect or perfect; fruit a drupe or berry, if berry then yellow to orange (2−) 3−5 (−7.7) cm in diam, seeds 3−8, > 5 mm long | 38 |
| 38 | Vascular bundle scars 1 per leaf scar; leaves generally widest at or below the middle, margins lacking teeth, fruit a berry, orange at maturity, (2−) 3−5 (−7.7) cm in diam, subtended by a thick leathery calyx |
Ebenaceae [Diospyros virginiana; Fig. |
| – | Vascular bundle scars 3 per leaf scar; leaves generally widest at or above the middle, sometimes toothed (as in Nyssa aquatica); fruit a drupe, blue-black at maturity 0.7−1.2 cm in diam., a thick leathery calyx lacking | Nyssaceae [Nyssa] |
|
Key 2: Herbaceous plants (herbs and vines) Key adapted from |
||
| 1 | Flowers borne in ligulate, radiate, or discoid heads subtended by an involucre of bracts | Asteraceae |
| – | Flowers various but not borne in heads subtended by an involucre of bracts | 2 |
| 2 | Plant carnivorous, leaves modified into tube-like pitchers (Sarraceniaceae) or containing small inconspicuous “bladders” (Lentibulariaceae) or with obvious glandular trichomes (Droseraceae) | 3 |
| – | Plant not carnivorous, lacking the above carnivorous characters | 5 |
| 3 | Leaves modified into conspicuous water-storing, tubular pitchers; flowers with a conspicuous style disk, a strong odor of ammonia (somewhat like cat urine) present; stamens 50−100 |
Sarraceniaceae [Sarracenia flava; Fig. |
| – | Leaves not modified into water-storing, tubular pitchers; flowers lacking a style disk, lacking a strong odor of ammonia; stamens < 50 | 4 |
| 4 | Plants terrestrial (occuring in moist to saturated soils), leaves lacking bladder-like traps, instead exhibiting glandular trichomes; corolla actinomorphic, not 2-lipped, white |
Droseraceae [Drosera intermedia; Fig. |
| – | Plant terrestrial (occuring in moist to saturated soils) or aquatic (typically found floating on the water surface), leaves bearing small, subterranean, urn-like or bladder-like traps; corolla zygomorphic, 2-lipped, corolla yellow or purple-lavender | Lentibulariaceae [Utricularia] |
| 5 | Plant a rooted aquatic, having either submersed, floating, or both submersed and floating leaves [Plants included in this section are the “prototypical” truly aquatic plants, exhibiting submersed or floating leaves. However, fluctuating water levels can cause a small degree of ambiguity. Increasing water levels may flood emergent wetland plants and give them the appearance of having submersed or floating leaves. Similarly, receding water levels may leave “prototypical” aquatic plants stranded and give them the appearance of emergents. Taking this into consideration, certain families and genera are included both in this lead and the next to ensure a broad range of environmental conditions are covered] | 6 |
| – | Plant terrestrial, emergent, with only roots and/or basal leaves inundated | 11 |
| 6 | Leaves of two types: submersed cauline, opposite, and comprised of dichotomously dissected linear segments, floating alternate, simple, and peltate, blades elongate-rhombic |
Cabombaceae [Cabomba caroliniana; Fig. |
| – | Leaves of one type (two in Nuphar sagittifolia, but then submersed leaves not cauline and not dichotomously divided): floating (submersed, floating, or erect in Hydrocotyle umbellata), peltate or not, blades oval, orbicular, cordate, ovate, reniform, lanceolate, or oblong-lanceolate | 7 |
| 7 | Leaves peltate | 8 |
| – | Leaves not peltate, petiole attached to a cuneate, sagittate, or cordate base | 10 |
| 8 | Underwater portions of plant coated with transparent mucilaginous jelly; leaves elliptic |
Cabombaceae [Brasenia schreberi; Fig. |
| – | Underwater portions of plant lacking mucilaginous jelly; leaves orbicular | 9 |
| 9 | Leaves < 8 cm in diam., submersed, floating, or emersed at maturity, margins crenate; peduncle (inflorescence stalk) equaling or just exceeding the leaves |
Araliaceae [Hydrocotyle umbellata; Fig. |
| – | Leaves > 20 cm in diam., floating (sometimes emersed during falling water levels), margins entire; peduncle (inflorescence stalk) tall, commonly overtopping the leaves |
Nelumbonaceae [Nelumbo lutea; Fig. |
| 10 | Leaf 5−15 cm long, ovate to reniform; petiole often reddish purple-punctate; inflorescence borne amongst or immediately subtended by a cluster of stout, fleshy, tuber-like, bannana-shaped roots; flowers 4−5-merous (eudicot) |
Menyanthaceae [Nymphoides aquatica; Fig. |
| – | Leaf (5−) 10−50 cm long, orbicular or lanceolate to oblong-lanceolate; petiole not reddish purple-punctate; inflorescence not amongst or subtended by fleshy tuber-like roots; flowers > 5-merous (basal angiosperm) | Nymphaeaceae |
| 11 | Leaves peltate |
Araliaceae [Hydrocotyle umbellata; Fig. |
| – | Leaves not peltate | 12 |
| 12 | Plant exuding milky sap when injured | 13 |
| – | Plant exuding clear sap when injured | 14 |
| 13 | Plant 2−10 dm tall; cauline leaves .1−15 × .1−.8 cm, linear, narrowly elliptic, or oblanceolate, margins callose toothed; flowers single and relatively distant from one another on the racemes or raceme-like branches; sepals not composed of an inner two (enlarged and wing-like) and an outer three (reduced); corolla blue to bluish-white | Campanulaceae |
| – | Plant 0.5−4 dm tall; leaves 1.5−6 × 0.5−2 cm, spatulate to obovate, margins lacking callose teeth; flowers in dense racemose terminal heads; sepals 5, composed of an inner two (enlarged and wing-like) and on outer three (reduced); corolla orange |
Polygalaceae [Polygala lutea Fig. |
| 14 | Leaves basal (sprouting form the nodes of a stolon) and simple or cauline and 1−3-pinnately compound (Cicuta maculata; this plant is extremely poisonous and care should be taken when handling plant parts); inflorescence a single or compound umbel; fruit a schizocarp | Apiaceae |
| – | Leaves various, if basal then not sprouting from nodes of a stolon and if cauline then not compound; inflorescence not umbellate; fruits various but not a schizocarp | 15 |
| 15 | Cauline leaves alternate | 16 |
| – | Cauline leaves opposite | 18 |
| 16 | Perianth differentiated into sepals and petals; corolla zygomorphic, bluish-purple and white |
Plantaginaceae [Nuttallanthus canadensis Fig. |
| – | Perianth undifferentiated and comprised of green, pinkish, or red tepals, or comprised solely of sepals (petals lacking) | 17 |
| 17 | Mature stems to 8 dm tall, relatively dainty (herb-like), if submersed then not spongy and thickened, ocrea present; leaves primarily basal, those along stem stem much reduced and distant from one another, bases mostly hastate (sometimes cuneate due to relatively frequent wave disturbance); inflorescence composed of terminal paniculate racemes; sepals 6; fruit a single achene kept inside the inner calyces |
Polygonaceae [Rumex hastatulus Fig. |
| – | Mature stems to 10 dm tall, robust (shrub-like), submersed stems becoming very spongy and enlarged, apparently “splitting” in place, ocrea absent; leaves evenly distributed on the stem, not reduced and distant on the stem; inflorescence composed of a solitary flower in the leaf axils; sepals 4; fruit a capsule |
Onagraceae [Ludwigia sphaerocarpa Fig. |
| 18 | Stems dichotomously branched, “wiry” in overall appearance; cauline leaves ≤ 2 mm long, subulate, bases pectinately-fringed |
Caryophyllaceae [Stipulicida setacea Fig. |
| – | Stems not dichotomously branched, not “wiry” in overall appearance; cauline leaves > 2 mm long, not subulate, bases not pectinately-fringed | 19 |
| 19 | Stems 4-angled; flowers in dense axillary clusters; corolla < 7 mm long, connate most of length, white, somewhat bi-labiate, 5-lobed, pubescent within; fruit a nutlet |
Lamiaceae [Lycopus angustifolius Fig. |
| – | Stems not 4-angled (4-angled in Melastomataceae, but not with the above combination of floral characters); flowers not in dense axilary clusters; corolla not as above; fruits various but not a nutlet | 20 |
| 20 | Corolla ≤ 5 mm long; flowers secund on small branchlets; capsule swollen at the base with two incurving appendages distally, thus having the appearance of “horns” |
Loganiaceae [Mitreola petiolata Fig. |
| – | Corolla ≥ 5 mm long; flowers not secund on small branchlets; capsule not swollen at the base with horn-like appendages distally | 21 |
| 21 | Plants commonly creeping and forming small to large mats in shallow water; flowers solitary or in head-like inflorescences arising from leaf axils | 22 |
| – | Plants not creeping or forming small to large mats in shallow water; flowers and fruits not borne in leaf axils (except for Linderniaceae and Rubiaceae) | 24 |
| 22 | Leaves to 9 cm long, linear-elliptic, apices acute and tipped with a tiny spine; inflorescence a multi-flowered axillary or terminal white head; fruit an utricle |
Amaranthaceae [Alternanthera philoxeroides Fig. |
| – | Leaves to 2 cm long, ovate, oblanceolate, or sometimes elliptic, apices acute to rounded, lacking a tiny spine; inflorescence composed of a single axillary flower, corolla pale or bright blue to violet-blue or yellow; fruit a capsule | 23 |
| 23 | Plant lacking a pleasant citrus-spicy aroma when crushed; stems without spongy or succulent texture; leaves to 2.5 ×0.6 cm, oblanceolate, apices acute to obtuse, petals separate, ≤ 9 mm long yellow |
Onagraceae [Ludwigia brevipes Fig. |
| – | Plant with a very pleasant citrus-spicy aroma when crushed; stems with a spongy and succulent texture; leaves to 2 × 1.5 cm, ovate, apices obtuse to rounded; petals connate, 9−13 mm long, pale or bright blue to violet-blue |
Plantaginaceae [Bacopa caroliniana Fig. |
| 24 | Flowers axillary, solitary, usually in the axils of one of a given pair of leaves (sometimes one in each axil of the pair); sepals linear-attenuate scabrous; corolla funnelform, 5-lobed, upper lip erect and shallowly 2-lobed, lower lip deflexed and 3-lobed, lavender |
Linderniaceae [Lindernia dubia Fig. |
| – | Flowers axillary or not, if so, then sometimes having more than 1 flower per axil and always in the axils of both of a given pair of leaves; sepals various, not scabrous; corolla various but if connate then not with the above floral characters, white, lavender, rose, pink, purple, or yellow | 25 |
| 25 | Leaves connected by interposed stipules or foliaceous stipules, if foliaceous, then indistinguishable from the leaves, thus the leaves appearing whorled; corollas white, connate basally to form a tube, or separated into 3–4 distinct petals | Rubiaceae [in part] |
| – | Leaves not connected by interposed or foliaceous stipules, not appearing whorled; corolla yellow, purple, pink, rose, or lavendar, never connate basally to form a tube, always separated into 4−5 distinct petals | 26 |
| 26 | Leaves glabrous, punctate-dotted, entire, not decussate; petals 4−5, pink (flesh-colored) or yellow; stamens sometimes grouped into fascicles, staminodia sometimes present; ovary superior, fruit a septicidal capsule not enclosed within a hypanthium | Hypericaceae |
| – | Leaves often pubescent or sparingly pubescent, not punctate-dotted, usually serrated or coarsely toothed, decussate; petals 4, pink, rose, or lavendar; stamens never grouped into fascicles, staminodia lacking; ovary inferior; fruit a loculicidal capsuse enclosed within an urceolate-shaped hypanthium | Melastomataceae |
|
Anacardiaceae Key adapted from |
||
| 1 | Leaves imparipinnate, leaflets ≥ 7, rachis winged; fruits red, glandular pubescent; plant lacking contact poisons; inflorescences terminal |
Rhus copallinum var. copallinum Fig. |
| – | Leaves pinnately trifoliolate; fruits white to yellow, glabrous or puberulant, hairs eglandular; plant containing contact poisons; inflorescences axillary |
Toxicodendron radicans var. radicans Fig. |
|
Apiaceae Key adapted from |
||
| 1 | Stems elongate-rhizomatous, horizontal, low-growing; leaves simple, blades ovate to oblong, 1.5–5 (−10) × 1.5 −3.5 (−8) cm, apices rounded, base cordate to truncate, margins denticulate; umbels simple, 1−4 (−9) flowers per umbel, pedicels 0.5−3 mm long; fruit strongly flattened laterally, prominently nerved with raised reticulate venation between nerves, corky ribs lacking |
Centella asiatica Fig. |
| – | Stems erect, not horizontal or low-growing; leaves 1–3 times pinnately compound, blades to 30 × 25 cm, leaflets lanceolate to lance-oblong, 4−7 (−14) cm ×0.6−3 (−5) cm, apices acute, bases cuneate to rounded, frequently asymmetrically so, margins serrate; umbels compound, > 9 flowers per umbel, pedicels 2−10 mm long; fruit somewhat flattened laterally with strong, flattish corky ribs |
Cicuta maculata Fig. |
|
Aquifoliaceae Key adapted from |
||
| 1 | Leaves 1.5−3× as long as wide, ca. 2−3 cm wide, with a few, irregularly spaced, marginal spinose teeth, if present, spreading away from the leaf apex |
Ilex coriacea Fig. |
| – | Leaves 3−4× as long as wide, ca. 1 cm wide (almost never > 2 cm wide), crenate in the apical 1/2−1/3 of the leaf, marginal teeth pointing toward the leaf apex |
Ilex glabra Fig. |
|
Araliaceae Key adapted from Note: The North Carolina Natural Heritage Program ( |
||
| 1 | Leaves not peltate | Hydrocotyle ranunculoides |
| – | Leaves peltate | 2 |
| 2 | Inflorescence umbellate, leaf blades 1–4 (–7) cm wide |
Hydrocotyle umbellata Fig. |
| – | Inflorescence verticillate, all flowers borne sessile to subsessile on the unbranched inflorescence axis; leaf blades 1–6 cm wide |
Hydrocotyle verticillata Fig. |
|
Asteraceae Key adapted from |
||
| 1 | Plant a woody shrub, with obvious woody growth well above ground level |
Baccharis halimifolia Fig. |
| – | Plant an herb or twining vine, lacking obvious woody growth above ground level | 2 |
| 2 | Plant a twining vine; leaves opposite, bases cordate, margins coarsely toothed |
Mikania scandens Fig. |
| – | Plant an herb; leaves opposite, alternate, whorled, or basally disposed, bases and margins various | 3 |
| 3 | Plant exuding milky sap when cut or damaged; heads ligulate (containing only ligulate [ray] flowers) | 4 |
| – | Plant exuding clear sap when cut or damaged; heads discoid (only containing disc flowers) or radiate (with both ligulate [ray] and disc flowers in the same head) | 5 |
| 4 | Leaves completely basally disposed in a rosette, the flowering stem therefore being scapose (lacking leaves); involucre of 2 or more series of bracts; rays 1–1.5 cm long; cypselas beaked; pappus composed strictly of bristles |
Hypochaeris radicata Fig. |
| – | Leaves primarily basally disposed, sometimes a few leaves extending up the stem, these alternate; involucre of 1 series of bracts; rays 0.6–1 cm long; cypselas beakless, pappus composed of 5 bristles and 5 scales | |
| 5 | Leaves opposite or whorled (at least on the lower stem nodes) | 6 |
| – | Leaves alternate | 8 |
| 6 | Leaves whorled, 8−20 × 0.3−2 mm; inflorescence composed of a single, terminal, pink, discoid head; plants no more than 45 cm tall, mat-forming |
Sclerolepis uniflora Fig. |
| – | Leaves opposite (at least on lower stem nodes, sometimes becoming alternate distally), leaves > 20 × > 2 mm; inflorescence composed of more than one head, heads discoid or radiate, not pink; mature plants > 45 cm tall, erect, not mat-forming | 7 |
| 7 | Plant an annual; heads radiate, borne singly or in ± corymbiform arrays, rays yellow; leaves simple, (20−) 50−100 (−160+) × (5−) 10−25 (−40+) mm, sessile; phyllaries 8−12, ovate to obovate to lance-oblong, (4−) 6−8 (−10+) mm, tips orange to purplish; disc florets (25−) 60−100 (−150+); cypselae 6−10 mm, pappi of 2−4 retrorsely barbed awns, 3−5 mm long |
Bidens laevis Fig. |
| – | Plant a perennial; heads discoid, corymbiform or paniculiform arrays, corollas white; leaves simple or pinnate/pinnatifid, 5−100 × 0.2−10 (−15) mm, sessile; phyllaries 8−10, narrowly elliptic, 0.5−8 × 0.2−1.2 mm, tips green; disc florets 5; cypselae 1−3 mm, pappi of 20−40 antrorsely barbed bristles, 2−5 mm long | Eupatorium |
| 8 | Heads discoid, phyllaries pink, disc corollas rose-pink; stems, leaves, and phyllaries stipitate to sessile glandular (sometimes viscid) |
Pluchea baccharis Fig. |
| – | Heads radiate, phyllaries green, disc corollas yellow; stems, leaves, and phyllaries eglandular | 9 |
| 9 | Ray florets yellow | 10 |
| – | Ray florets white | 11 |
| 10 | Leaves on the middle to distal portions of the stem linear, 24−70 × 1−3 mm, bases attenuate, if sessile, not clasping the stem, abundantly gland-dotted, scabro-villous on mid-nerves; heads corymbose, ray florets 7−17 (−25), disc florets 3−22, corollas 3.3−4.8 mm long; stems sparsely pubescent, 2.5−10 dm tall |
Euthamia caroliniana Fig. |
| – | Leaves on the middle to distal portions of the stem lanceolate-ovate to ovate-oblong, larger leaves 35−120 × 8−35 mm, bases auriculate, broad and more or less clasping, hirsuto-villous on the midnerves, not gland-dotted; heads paniculate, ray florets (2−) 4−10, disc florets (2−) 4−7, corollas 4−5 mm long; stems conspicuously hirsute, 5−15 dm tall |
Solidago fistulosa Fig. |
| 11 | Leaves cauline, linear to lanceolate, 2−22 × 0.2−3 cm, not fleshy thickened; heads 50−100; ray florets 8−20 mm long; involucres 2.4−3.8 × 3.7−8.7 mm; cypselae obovoid, 1−3 mm, pappi comprised of 9 or 18 awns, (0−) 0.4−1.2 mm long; plants 3−20 dm tall, stoloniferous |
Boltonia asteroides var. glastifolia Fig. |
| – | Leaves mostly basal, narrowly to broadly oblanceolate to spatulate, 2−10 (−15+) × 0.4−2.5 cm, more or less fleshy thickened; heads (1−) 4−20 (−25); ray florets 5−10 mm long; involucres 3−4 ×5−11 mm; cypselae subterete, 1.2−1.6 mm, pappi comprised of setae (outer) and 16−25 bristles (inner), bristles 2.5−3.3 mm long; plants 1.5−5 dm tall, rhizomatous or fibrous-rooted |
Erigeron vernus Fig. |
|
Betulaceae Key adapted from |
||
| 1 | Buds stalked; pistillate catkins becoming hard and woody, forming a persisting cone-like catkin that persists through the winter and into the next growing season; plant a shrub, < 4 m tall; bark tight, not sloughing away from trunk; leaves 3-ranked, blades 5−10 cm × 2.5−5 cm, obovate, elliptic, or oblong, margins entire to serrulate |
Alnus serrulata Fig. |
| – | Buds sessile; pistillate catkins not becoming woody or hard and not persisting through the winter and into the next season; plant a tree, > 10 m tall; bark loose, sloughing away from trunk, usually with the consistency of paper; leaves 2-ranked, blades 3−10 cm × 1.5−3 cm, ovate-triangular or sub-rhombic, margins coarsely doubly serrate to dentate |
Betula nigra Fig. |
|
Cabombaceae Key adapted from |
||
| 1 | Leaves floating only, blades elliptic, 3.5–11 × 2–6.5 cm, peltate; submersed plant parts coated with a layer of transparent mucilage |
Brasenia schreberi Fig. |
| – | Leaves floating and submersed, blades of floating leaves elliptic, 0.6–3 × 0.1–0.4 cm, peltate, blades of submersed leaves dichotomously divided into linear segments; submersed plant parts not coated with mucilage |
Cabomba caroliniana Fig. |
|
Campanulaceae Key adapted from |
||
| 1 | Plant perennial; stems 7−10 dm tall; stem leaves linear to narrowly oblanceolate, 8−15 × 0.5−0.8 cm, margins callose glandular (sometimes not), often with short translucent trichomes on or near the margins; subtending bracts shorter than or exceeding the pedicels in length; corollas (including hypanthium) 18−33 mm long, fenestrate (with a slit or window on each side of the corolla tube at the base); plant of seasonally wet to inundated soils | Lobelia glandulosa |
| – | Plant annual; stems 2−7.5 dm tall, stem leaves lanceolate to linear, 1−3.5 × 0.1−0.4 cm, margins callose (not glandular), lacking short transluscent trichomes on or near the margins; subtending bracts shorter than or rarely equaling the pedicels in length; corollas (including the hypanthium) 8−14 mm long, not fenestrate; plant of various savnna-like habitats, and occassionally in wetter soils, but never found in inundated soils or wetlands |
Lobelia nuttallii Fig. |
|
Ericaceae Key adapted from |
||
| 1 | Ovary inferior; fruit a berry | Vaccinium |
| – | Ovary superior; fruit a capsule | 2 |
| 2 | Leaves evergreen, blades coriaceous, adaxial surface either dark green and shiny or dull olive green and lepidote (covered with small, white or yellowish scurfy scales) | 3 |
| – | Leaves deciduous, blades membranous or subcoriaceous, deciduous, adaxial surface light to dark green, dull, not lepidote | 4 |
| 3 | Twig and leaf blade surfaces prominently lepidote, adaxial leaf surface dull olive green, lacking a prominent perimarginal vein |
Chamaedaphne calyculata Fig. |
| – | Twig and adaxial leaf blade surfaces glabrous, not lepidote, adaxial leaf surface dark green and shiny, larger leaves with a prominent perimarginal vein ca. 1 mm from blade margin |
Lyonia [Lyonia lucida Fig. |
| 4 | Leaves predominantly obovate or oblanceolate, margins distinctly long-ciliate; corolla funnelform, lobes > 10 mm long; capsule elongate, > 2 × as long as broad, 7–23 mm long |
Rhododendron viscosum var. serrulatum Fig. |
| – | Leaves various, margins not long-ciliate; corolla urceolate, campanulate, or globose, lobes < 5 mm long, capsule oblate (spheroidal, but flattened apically and basally), ovoid, globose, or subglobose, nearly as broad as long or broader, 2–6.5 mm long | 5 |
| 5 | Leaf margins crenate; corolla campanulate; capsule oblate |
Zenobia pulverulenta Fig. |
| – | Leaf margins spinulose-serrate, serrulate, or entire; corolla urceolate or globose; capsule ovoid, globose, or subglobose | 6 |
| 6 | Leaf margins spinulose-serrate; inflorescence of racemes produced along stems of previous year; capsules not thickened and whitish along sutures; seeds 5–10 per capsule |
Eubotrys racemosa Fig. |
| – | Leaf margins entire to minutely serrulate; inflorescence of terminal panicles produced on stems of current year, proximal inflorescences often with conspicuous leaf-like bracts; capsules thickened and whitish along sutures; seeds 100–300+ per capsule |
Lyonia ligustrina Fig. |
|
Lyonia Nutt. Key adapted from |
||
| 1 | Leaves deciduous, blades subcoriaceous, dull, lacking a prominent perimarginal vein, margins serrulate; corollas urceolate 2–4(−4.5) mm long; calyx lobes 0.5–1.5 mm long |
Lyonia ligustrina var. foliosiflora Fig. |
| – | Leaves evergreen, blades coriaceous, shiny, with a prominent perimarginal vein, leaf margins entire; corollas cylindric 5–14 mm long; calyx lobes 2–9.5 mm long |
Lyonia lucida Fig. |
|
Vaccinium L. Key adapted from |
||
| 1 | Twigs of the year glabrous; leaves glabrous below, margins eciliate; berries blue |
Vaccinium formosum Fig. |
| – | Twigs of the year pubescent; leaves pubescent below, margins ciliate; berries black |
Vaccinium fuscatum Fig. |
|
Hypericaceae Key adapted from |
||
| 1 | Petals flesh-colored to pink; stamens in 3 fascicles, each fascicle containing 3 stamen; 3 orange staminodial glands alternating with the 3 fascicles of stamen | 2 |
| – | Petals yellow, stamens few, not in fascicles; orange staminodial glands lacking | 3 |
| 2 | Leaves sessile, clasping the stem, cordate or subcordate at the base, mostly 2−7 × 1−3 cm; sepals 5−8 mm long at maturity, acute to acuminate; filaments united basally; styles 1.8−3 mm long |
Hypericum virginicum Fig. |
| – | Leaves petiolate (at least the lower), not clasping the stem, cuneate at the base, up to 15 × 3.5 cm; sepals 3−5 mm long at maturity, apices obtuse; filaments united to above the middle; styles 1.5−3 mm long |
Hypericum walteri Fig. |
| 3 | Leaf blades lanceolate to linear, 1−3-nerved, 6−30 mm long, bases attenuate to cuneate, not clasping, apices blunt to acute; petals 5, 6−8 mm long; capsules purplish, slightly exceeding the calyx |
Hypericum canadense Fig. |
| – | Leaf blades ovate, elliptic, lanceolate, 5-nerved, 10−50 mm long, bases broad, sometimes clasping, apices rounded to blunt; petals 5, 2−3 mm long; capsules not purplish, equaling the calyx |
Hypericum mutilum var. mutilum Fig. |
|
Lentibulariaceae Key adapted from Note: Traditionally, in the southeastern United States, U. biflora and U. gibba have been recognized as distinct species ( |
||
| 1 | Plants aquatic, floating unattached in water (sometimes stranded on top of soil by receding water levels); bladders 0.7–5 mm long, mostly > than 1.0 mm long; seeds 0.5–2 mm long | 2 |
| – | Plants terrestrial, attached to soil (principal branches within the soil); bladders 0.2–1.1 mm long, mostly < 1.0 mm long; seeds 0.2−0.25 mm long | 5 |
| 2 | Flowers purple; leaves divided into verticillate segments with terminal traps |
Utricularia purpurea Fig. |
| – | Flowers yellow; leaves divided into alternate segments with lateral traps; upper corolla lip larger than the lower, obscurely 3–lobed | 3 |
| 3 | Plant exhibiting vegetative shoots of two types, some bearing leafy segments with few or no traps, others bearing reduced segments and many traps; seeds 1.0–2.5 mm long, with an irregularly deeply lobed or partial wing (plant of shallow water or left stranded on soil surface after receding water levels) |
Utricularia striata Fig. |
| – | Plant exhibiting uniform vegetative shoots, all bearing sparsely divided leaf segments with traps; seeds 0.8–1.1 mm long, with a continuous, circumferential wing, slightly to irregularly lobed | 4 |
| 4 | Lower corolla lip 8–10 mm long, equaling or slightly shorter than the conical, 5–9 mm long spur; leaves usually forked twice |
[Utricularia biflora Fig. |
| – | Lower corolla lip 5–6 mm long exceeding the blunt 3.5–4.5 mm long spur; leaves usually forked once |
Utricularia gibba Fig. |
| 5 | Corolla rose pink; inflorescence 1 (−2)-flowered; bract at base of pedicel tubular, attached circumferentially around stem; aerial leaves (when present) terete, septate |
Utricularia resupinata Fig. |
| – | Corolla yellow (sometimes fading white); inflorescence (1−) 2−15-flowered; bract at base of the pedicel peltate or ovate, attached on one side of the stem; aerial leaves (when present) flattened, not septate | 6 |
| 6 | Pedicels subtended by a single ovate (attached at base) bract; pair of bracteoles present, bracteoles linear to lanceolate, a little longer than the bract; corolla spur oriented downward or backward, at right angle to lower corolla lip |
Utricularia cornuta Fig. |
| – | Pedicels subtended by a single peltate (attached in middle) bract, unattached at either end; pair of bracteoles absent; corolla spur oriented forward, essentially appressed to lower corolla lip; aerial leaves (when present) with subacute or obtuse apices |
Utricularia subulata Fig. |
|
Melastomataceae Key adapted from |
||
| 1 | Sepal lobes aristate, awn tip 0.5–1.5 mm long, hairs 3–5 mm long, yellow, stiff |
Rhexia aristosa Fig. |
| – | Sepal lobes obtuse to acuminate, not aristate, hairs < 3 mm long, neither yellow nor stiff | 2 |
| 2 | Leaves linear or linear-elliptic, 1– 5(−8) mm wide | 3 |
| – | Leaves lanceolate, elliptic, or ovate, (5−) 7–20 (−35) mm wide | 4 |
| 3 | Petals lavender–rose, (1−) 1.5−2 (−2.5) cm long; mature hypanthium 10−14 mm long, hairs glandular; marginal nerves of leaf abaxial surface absent or obscure and discontinuous; anthers 7−10 mm long |
Rhexia cubensis Fig. |
| – | Petals white to pink, (0.7−) 0.9−1.4 cm long; mature hypanthium 6−10 mm long, glabrous or hairs glandular; marginal nerves of leaf abaxial surface prominent; anthers 5−8 mm long |
Rhexia mariana var. exalbida Fig. |
| 4 | Four stem faces at mid–stem noticeably unequal, one pair of opposite faces broader, convex, darker green, the narrower pair concave or flat, pale, arrangement of broader and narrower faces alternating at each internode up the stem, angles at midstem not winged |
Rhexia nashii Fig. |
| – | Four stem faces at mid–stem about equal, almost flat, angles at midstem conspicuously winged |
Rhexia virginica Fig. |
|
Nymphaeaceae Key adapted from |
||
| 1 | Perianth globose at anthesis, 2–5 cm diam.; margin of stigmatic disk crenate to dentate; leaves linear to lanceolate 15−30 (−50) × 5−10 (−11.5) cm, green both adaxially and abaxially, venation essentially pinnate, often of two types, submersed leaves (when present) thinner in texture than floating or emersed leaves, 60–90% of surface area of floating or emersed leaves with vasculature derived from the midrib; sepals 6, green to yellow, petaloid; petals inconspicuous, yellow, stamen-like, shorter than the sepals; rhizome with triangular or winged leaf scars |
Nuphar sagittifolia Fig. |
| – | Perianth spreading at anthesis, 4–20 cm diam.; margin of stigmatic disk with prominent, distinct, upwardly incurved appendages; leaves ovate to orbiculate (5−) 10−40 × (5−) 10−40 cm, green adaxially and deep reddish-purple abaxially, venation essentially palmate, of one type, floating, 25–40% of surface area with vasculature derived from the midrib; sepals 4, greenish or reddish tinged, not petaloid; petals showy, white to pink, distinctly longer than the sepals; rhizomes with circular leaf scars |
Nymphaea odorata var. odorata Fig. |
|
Nyssaceae Key adapted from |
||
| 1 | Petioles of mature leaves 3−6 cm long; mature leaf blades exceeding 10 cm long, margins with a few irregular teeth; drupes ≥ 20 mm long |
Nyssa aquatica Fig. |
| – | Petioles of mature leaves < 3 cm long; mature leaves ≤ 10 cm long, margins lacking irregular teeth; drupes 10−15 mm long |
Nyssa biflora Fig. |
|
Onagraceae Key adapted from |
||
| 1 | Leaves opposite, oblanceolate to elliptic, 8−20 mm; pedicels conspicuous, 5−16 mm long; petals 4, 4−5 mm long, slightly larger than the calyx segments; capsule obconical, 6−8 mm long, slightly quadrangular in cross-section, curved; seed coat with rectangular reticulations; plants pubescent with short hooked hairs; plant creeping and rooting at the nodes |
Ludwigia brevipes Fig. |
| – | Leaves alternate, lanceolate to linear-lanceolate, 3−10 cm; pedicels 0−1 mm long; petals lacking; capsules subglobose, 2.5−4.5 mm long, terete in cross-section or with broadly rounded lobes; seed coat with square reticulations, pentagonal or circular; plants glabrous to slightly pubescent, if pubescent, then hairs not hooked; plant erect and ascending, not rooting at the nodes |
Ludwigia sphaerocarpa Fig. |
|
Plantaginaceae Key adapted from |
||
| 1 | Plant a true aquatic, forming extensive mats in shallow water; plant parts spicy aromatic when crushed; stems lax, fleshy, semi-succulent, pubescent; leaves of the flowering stem 55−28 × 7–15 mm, opposite, ovate to widely elliptic, with 3−7 palmate veins; inflorescence composed of a single axillary flower; corolla bluish-purple, 9–11 mm long, not spurred, orifice distinct |
Bacopa caroliniana Fig. |
| – | Plant terrestrial, usually found at the upper margins of the high water mark in moist sandy soil; plant parts not aromatic when crushed; stems not lax, fleshy or semi-succulent, nor pubescent; leaves of the flowering stem 5–20 × 1−3.5 mm, alternate, linear < 3 veins; inflorescence a terminal raceme; corolla bluish-purple and white, 5–15 mm long (including the spur), spurred, orifice obscured |
Nuttallanthus canadensis Fig. |
|
Rosaceae Key adapted from |
||
| 1 | Leaves simple; fruit a pome | 2 |
| – | Leaves compound; fruit achenes enclosed within a hip or an aggregate of drupelets | 4 |
| 2 | Inflorescence corymbose; adaxial surface of leaves with dark glandular trichomes along the midrib, leaf margins finely serrate, teeth tipped with red glands; mature fruit red |
Aronia arbutifolia Fig. |
| – | Inflorescence racemose; adaxial surface of leaves lacking glandular trichomes, leaf margins serrate, teeth tips lacking red glands; mature fruit blue to purple [Amelanchier] | 3 |
| 3 | Plant a small shrub or tree, 8–20 m tall, not rhizomatous; pedicels of varying lengths, the longest > 1 cm long; petals 6–12 mm long |
Amelanchier canadensis Fig. |
| – | Plant a small shrub, 0.2–2.5 m tall, rhizomatous; pedicels nearly uniform in length, usually < 1 cm long; petals 5.9–7.7 mm long |
Amelanchier obovalis Fig. |
| 4 | Leaves odd-pinnately compound, leaflet margins usually crenulate to serrulate; fruit a hip, developing from an urceolate hypanthium, enclosing the ovaries and achenes except for the apical orifice |
Rosa palustris Fig. |
| – | Leaves palmately compound, leaflet margins usually serrate to doubly serrate; fruit an aggregate of drupelets, developing from a flatish to hemispheric hypanthium, ovaries and druplets exposed, not borne inside a hypanthium |
Rubus pensilvanicus Fig. |
|
Rubiaceae Key adapted from |
||
| 1 | Plant woody, a shrub or small tree; inflorescence in dense globose heads; corolla narrowly infundibuliform, white, lobes 4, shorter than the tube; length of exerted style ca. 3× or more the length of a corolla lobe |
Cephalanthus occidentalis Fig. |
| – | Plant herbaceous; inflorescence of solitary or few-flowered, axillary cymes; corolla salverform to subcampanulate, white, lobes 3−4; length of exerted style ca. 1× the length of a corolla lobe or less | 2 |
| 2 | Plant pubescent to glabrous, erect or spreading; stem often with a reddish tinge; leaves opposite, elliptic-lanceolate to oblanceolate, 2−7 cm × 4−12 mm; flowers sessile, borne in leaf axils, 1 (−2) per axil; corolla salverform, 7−9 mm long, lobes 4, 3−4 mm long, inner surface pubescent; fruit oblong-ellipsoid, pubescent, 3−5 mm wide, prominently ridged |
Diodia virginiana Fig. |
| – | Plant glabrous, erect or spreading; stem lacking a reddish tinge; leaves whorled, 4 per node, linear-obovate, 8−20 mm × 1.5−4 mm; flowers in branched terminal and axillary cymes, 1−3-flowered; corolla subcampanulate, corolla lobes 3−4, < 3 mm long, inner surface glabrous; fruit orbicular, glabrous, 2.5−4 mm wide, smooth, not ridged |
Galium obtusum var. obtusum Fig. |
|
Salicaceae Key adapted from |
||
| 1 | Buds scales imbricate; leaf blades ovate, < 3 × as long as broad, bases truncate to broadly rounded, slightly cordate; inflorescences pendulous; stamens 5−80 |
Populus heterophylla Fig. |
| – | Bud scales 1; leaf blades lanceolate, > 3 × as long as broad, bases cuneate, not cordate; inflorescences erect or spreading; stamens 1−9 | 2 |
| 2 | Mature leaf undersides glaucous, glabrous to sparsely pubescent, blades (4−) average 7.5 (−13) × as long as wide; stipules usually prominent and persisting, to 15 mm long |
Salix caroliniana Fig. |
| – | Mature leaf undersides green, not glaucous, glabrous, blades (4−) average 9 (−16) × as long as wide; stipules not persisting, to 12 mm long |
Salix nigra Fig. |
|
Sapindaceae Key adapted from |
||
| 1 | Leaves palmately compound; fruit a capsule |
Aesculus pavia var. pavia Fig. |
| – | Leaves simple; fruit a schizocarp composed of two 1-seeded samaras | 2 |
| 2 | Leaves (3−) 5 (−9) lobed, central lobe 4−8 cm long, upper two lateral lobes 2−5 cm long, bases generally cordate |
Acer rubrum var. rubrum Fig. |
| – | Leaves (0−) 3 (−5) lobed, central lobe 1−5 cm long, upper two lateral lobes (if leaves more than 3-lobed) 0.5−2 (−3) cm long, bases cuneate to rounded to subcordate |
Acer rubrum var. trilobum Fig. |
|
Vitaceae Key adapted from |
||
| 1 | Leaves simple, leaf margins prominently dentate-serrate throughout, bases cordate; tendrils unbranched, lacking adhesive pads |
Muscadinia rotundifolia Fig. |
| – | Leaves palmately compound, leaflet margins coarsely serrate above the middle, entire below middle, bases cuneate; tendrils branched, bearing adhesive pads at the tips |
Parthenocissus quinquefolia Fig. |
Acknowledgements
NH, AK, RB: We thank D. Cicuzza, W. Hoffmann, and T.R. Wentworth for their critical review of a previous version of this manuscript. We also thank D. Gamble (UNCW) for permission to re-use Fig. 1 from T.E. Ross's article "Pocosins and Carolina Bays Compared" (The North Carolina Geographer 11: 22−32, 2003) and J. Mickel (NY) for permission to re-use an illustration of Dryopteris ludoviciana.
NH: I thank the individuals and organizations that directly had a hand in the completion of this project; without their expertise, opinions, edits, financial assistance, and permission to access private property, this work would not have been possible.
The North Carolina State Parks graciously allowed me to collect plants from the shorelines of five Carolina bay lakes in Bladen County, North Carolina. The North Carolina Wildlife Resources Commission was kind enough to grant access to Horseshoe Lake and Little Singletary Lake. I thank Glenn and Carol Lewis for offering their land as an easement to Little Singletary Lake; they were very gracious, and I thoroughly enjoyed listening to Glenn’s stories of Native American artifacts, lake history, black bears, and wildfires.
Dr. Clemuel Johnson graciously gave permission to survey Bakers Lake Natural Area and I am very thankful for his generosity. Stephen Clark, son-in-law of Dr. Clemuel Johnson, also provided valuable information regarding wildlife use of Bakers Lake and surrounding natural areas. “Chick” Gaddy provided valuable information concerning Carolina bays and associated South Carolina natural community types. Mr. Gaddy’s enthusiastic disposition and knowledge of South Carolina ecosystems was very beneficial to this study and I am truly grateful for his time. Garrett German provided a wealth of information concerning waterfowl use of Carolina bay lakes. Rob Richardson and Justin Nawrocki of the NCSU Crop Science Department provided a list of several plant species found from Lake Waccamaw that added greatly to this work.
I am deeply indebted to the North Carolina Native Plant Society and the Society of Herbarium Curators. These two organizations were kind enough to provide funding for this research. Without their financial assistance, my wallet would surely be a little lighter. Ed Corey has helped me immeasurably through the years and I am deeply indebted to him. Dr. Jon Stucky has been a true pal and never once hesitated to reply to my numerous – sometimes assuredly annoying − emails concerning plant identifications. My girlfriend Morgan Kirby has stuck by my side through this project and on several occassions has been swindled into mounting plant specimens; for that, she deserves an award for her patience and understanding.
Colter Chitwood has been a loyal friend, editor, and dog sitter. I don’t know what I would have done without him. I wish him the very best in his future travels and research. Maybe we can meet on the Madison one of these days. I would also like to thank past and present floristics students at North Carolina State University. Robert Thornhill captivated me with his exuberant passion for North Carolina’s diverse Coastal Plain flora and encouraged me to pursue a flora of my own. To the kind gentleman who gave me a ride to Bay Tree Resorts after my boat was taken by devilish winds of Bay Tree Lake on the morning of July 9, 2014, THANK YOU! Drs. Layne Huiet and Bob Wilbur of DUKE and CarolAnn McCormick of NCU helped tremendously with herbarium crawls. Those herbaria can get quite lonely, and having a conversation with someone is worth its weight in gold.
References
- Adanson N, Rafinesque U, Rafinesque U (2002) Pontederia. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Suggs Mill Pond Game Land Management Plan.North Carolina Wildlife Resources Commission,Raleigh,255pp.
- Andrews EG, Windham MD (1993) Pleopeltis. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Argus GW, Eckenwalder JE, Kiger RW (2010) Salicaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Species and area.Journal of Ecology9(1):95‑99. https://doi.org/10.2307/2255763
- Ball PW, Reznicek AA (2002) Carex. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Ball PW, Reznicek AA, Murray DF (2002) Cyperaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Ball PW, Reznicek AA, Murray DF (2002) Salicaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Barkley TM, Brouillet L, Strother JL (2006) Key to Asteraceae tribes. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Barkworth ME (2003) Paniceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Barkworth ME (2003) Andropogoneae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Barkworth ME (2007) Poaceae: Key to tribes. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Diary of a journey through the Carolinas, Georgia, and Florida from July 1, 1765, to April 10, 1766.Transactions of the American Philosophical Society33(1):1‑120. https://doi.org/10.2307/1005551
- Distribution and status of Carolina Bays in South Carolina.South Carolina Wildlife and Marine Resources Department, Nongame and Heritage Trust Publications.,Columbia,98pp.
- 15 years of pond assessment in Britain: results and lessons learned from the work of pond conservation.Aquatic Conservation: Marine and Freshwater Ecosystems15(6):693‑714. https://doi.org/10.1002/aqc.745
- Bogler D (2006) Hypochaeris. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Bornstein A (1997) Myricaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Landscape properties of pocosins and associated wetlands.Wetlands11(1):441‑465. https://doi.org/10.1007/bf03160761
- An illustrated flora of the northern United States, Canada, and the British Possessions, 3 vols.Scribner,New York,1726pp.
- The biology of lakes and ponds.Oxford University Press,New York,304pp.
- Brooks R, Clemants S (2000) Juncus. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Use of shoreline timber reserves by moose.Journal of Wildlife Management47(3):673‑685. https://doi.org/10.2307/3808603
- Campbell C (2003) Andropogon. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Algal and physiochemical indicators of eutrophication in a lake harboring endemic species: Lake Waccamaw, North Carolina.Journal of the Elisha Mitchell Scientific Society100:83‑103.
- Line drawings. http://plants.ifas.ufl.edu/linedrawings
- Chambers K, O'Kennon R (2006) Krigia. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Christensen N (1999) Vegetation of the southeastern Coastal Plain. In: Barbour M, Billings W (Eds) North American terrestrial vegetation.Cambridge University Press,New York.
- Clark L, Triplett J (2007) Arundinaria. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Clemants S (2003) Amaranthaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- The statistics and biology of the species-area relationship.American Midland Naturalist113(6):791‑833. https://doi.org/10.1086/283438
- Definition of pH scales, standard reference values, measurement of pH and related terminology (Recommendations 1984).Pure and Applied Chemistry57(3):531‑542. https://doi.org/10.1351/pac198557030531
- Classification of wetlands and deepwater habitats of the United States.US Department of the Interior, Fish and Wildlife Service,Washington, DC,90pp.
- Cranfill R (1993) Woodwardia. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Species diversity in aquatic angiosperms: Latitudinal patterns.Aquatic Botany44:229‑258. https://doi.org/10.1016/0304-3770(93)90072-5
- Soil systems in North Carolina. Technical Bulletin 314.North Carolina Agricultural Research Service,Raleigh,118pp.
- Daniel T (2007) Sphenopholis. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Denny P (1984) Permanent swamp vegetation of the Upper Nile. In: Dumont H, Moghraby Ae, Desougi L (Eds) Limnology and Marine Biology in the Sudan.Dr W Junk Publishers,The Hague.
- A bibliography of North Carolina local floras.Castanea75(4):475‑483. https://doi.org/10.2179/10-015.1
- The relationship in lake communities between primary productivity and species richness.Ecology81(10):2662‑2679. https://doi.org/10.1890/0012-9658(2000)081[2662:trilcb]2.0.co;2
- Dorr LJ (2009) Zenobia. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Freshwater Biodiversity: importance, threats, status and conservation challenges.Biological Reviews81(2):163‑182. https://doi.org/10.1017/s1464793105006950
- Durand T (2000) Sagittaria. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Eckenwalder JE (2009) Diospyros. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Eckenwalder JE, Thieret JW (1993) Key to families of Gymnosperms. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Oxford atlas of exploration.Oxford University Press,New York,248pp.
- ArcGis Desktop: Release 10.2.2.Release date:2014-2-27.
- Distribution of migratory landbirds along the northern Lake Huron shoreline.Wilson Journal of Ornithology123(3):536‑547. https://doi.org/10.1676/09-122.1
- A phytosociological study of the Castalia-Myriophyllum community of Georgia Coastal Plain Boggy Ponds.American Midland Naturalist26(2):421‑438. https://doi.org/10.2307/2420968
- Fabijan DM (2009) Chamaedaphne. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Faden RB (2000) Mayacaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Anthropogenic correlates of biodiversity in southeastern Ontario wetlands.Conservation Biology11(4):1000‑1009.
- Scale-dependent response by breeding songbirds to residential development along Lake Superior.Wilson Journal of Ornithology122(2):296‑306.
- Freckmann RW, Lelong MG (2003) Panicum. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Freckmann RW, Lelong MG (2003) Dichanthelium. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Morphometry and hydrography of some natural lakes of the North Carolina coastal plain: the bay lake as a morphometric type.Journal of the Elisha Mitchell Scientific Society65(1):1‑37.
- Pollen succession in the sediments of Singletary Lake, North Carolina.Ecology32(3):518‑533.
- The fishes of North Carolina’s bay lakes and their intraspecific variation.Journal of the Elisha Mitchell Scientific Society67(1):1‑44.
- Evidence for the recent enlargement of the" Bay" lakes of North Carolina.Ecology35(1):78‑88. https://doi.org/10.2307/1931408
- Human shoreline development and the nutrient stoichiometry of aquatic plant communities in Canadian shield lakes.Journal of Fisheries and Aquatic Sciences69(10):1642‑1650.
- Furlow JJ (1997) Betulaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Effects of fire on vegetation of the southeastern United States.The Botanical Review9(9):617‑654. https://doi.org/10.1007/bf02872506
- A new voyage to Georgia: By a young gentleman. Giving an account of his travels to South Carolina, and part of North Carolina. To which is added, a curious account of the Indians. By an honourable person. And a poem to James Oglethorpe, Esq; on his arrival from Georgia.Printed for J. Wilford, at the Three Flower de Luces, behind the Chapter-House, in St. Paul's Church-Yard,London,62pp.
- The response of vegetation to chemical and hydrological gradients in the Lost River Peatland, Northern Minnesota.Journal of Ecology78(4):1021‑1048. https://doi.org/10.2307/2260950
- Some notes on Darlington (SC), 'Bays'.Science2(41):472.
- Aquatic and wetland plants of southeastern United States. Monocotyledons.Vol. 1.University of Georgia Press,Athens,712pp.
- Aquatic and wetland plants of southeastern United States. Dicotyledons.Vol. 2.University of Georgia Press,Athens,933pp.
- Goldman DH, Magrath LK, Catling PM (2002) Calopogon. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Hágsater E, Salisbury A, Rafinesque L, Rafinesque N, Small S, Rafinesque T (2002) Epidendrum. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Haines A (2006) Euthamia. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Harvey MJ (2007) Agrostis. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Haynes RR (2000) Najadaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Haynes RR (2000) Hydrocharitaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Hydrological and limnological aspects of lake monitoring.15.John Wiley & Sons,New York,392pp.
- The wetland vascular flora of four seeps in McDonough County, Illinois.Phytologia56(1):1‑15.
- Herndon A (2002) Hypoxis. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Manual of the grasses of the United States.United State Department of Agriculture,Washington,1051pp.
- Holmes WC (2002) Smilax. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Holmes WC (2006) Mikania. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Endemic fish fauna of Lake Waccamaw, North Carolina.University of Michigan Press,Ann Arbor,30pp.
- Mats on a southeastern Coastal Plain reservoir.Bulletin of the Torrey Botanical Club70(5):481‑488. https://doi.org/10.2307/2481394
- Jensen RJ (1997) Quercus. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- The transparency, the color and the specific conductance of the lake waters of northeastern Wisconsin.Transactions of the Wisconsin Academy of Sciences, Arts, and Letters28:205‑259.
- Judd WS (2009) Lyonia. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Judd WS, Kron KA (2009) Rhododendron. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Karaman-Castro V, Urbatsch LE (2006) Boltonia. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Kaul RB (1997) Platanaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Wetland Ecology: principles and conservation.Cambridge University Press,New York,614pp.
- Four general principles for the management and conservation of wetlands in large lakes: the role of water levels, nutrients, competitive hierarchies and centrifugal organization.Lakes & Reservoirs: Research & Management5(3):177‑185.
- An ecological study of the land plants and cold- blooded vertebrates of the Savannah River project area. PartVI. Conspicuous vegetational zonation in a “Carolina bay”.University of South Carolina Publication Series III. Biology1:244‑248.
- Vegetation changes and land-use legacies of depression wetlands of the western coastal plain of South Carolina: 1951–1992.Wetlands16(4):564‑576. https://doi.org/10.1007/bf03161347
- Kral R (1993) Pinus. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Kral R (2000) Xyridaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Kral R (2000) Eriocaulon. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Kral R (2002) Rhynchospora. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Kral R (2002) Fimbristylis. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Kral R, Persoon V (2002) Fuirena. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Environmental influences on aquatic plants in freshwater ecosystems.Environmental Reviews14(2):89‑136. https://doi.org/10.1139/a06-001
- Lamont EE (2006) Sclerolepis. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Soil survey of Bladen County, North Carolina.United States Soil Conservation Service,Washington, DC,179pp.
- Inventory of the natural areas and rare species of Columbus County, North Carolina.North Carolina Department of Environment, Health, and Natural Resources, Division of Parks and Recreation, Natural Heritage Program,Raleigh,162pp.
- Natural area inventory of Bladen County, North Carolina.North Carolina Department of Environment and Natural Resources, Office of Conservation and Community Affairs, Natural Heritage Program,Raleigh,212pp.
- Natural heritage program list of the rare animal species of North Carolina.North Carolina Department of Environment and Natural Resources, Office of Land and Water Stewardship, Natural Heritage Program,Raleigh,172pp.
- Lemke DE (2009) Cyrilla. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Lewis DQ (2002) Burmanniaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- An annotated check list of the North Carolina bay lakes fishes.Journal of the Elisha Mitchell Scientific Society78:68‑73.
- Luther HE, Brown GK (2000) Tillandsia. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Natural areas inventory of Washington County, North Carolina.North Carolina Department of Natural Resources and Community Development, Office of Coastal Management, Coastal Energy Impact Program,Raleigh,173pp.
- A Taxonomic Revision of Luziola (Poaceae: Oryzeae).Systematic Botany33(4):702‑718. https://doi.org/10.1600/036364408786500226
- Mastrogiuseppe J (2002) Dulichium. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Mastrogiuseppe J, Rothrock PE, Dibble AC, Reznicek AA (2002) Carex sect. Ovales. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Mellichamp TL, Chase FW (2009) Sarracenia. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- The Carolina" Bays": Are they meteorite scars?Journal of Geology41(1):52‑66.
- Meyer FG (1997) Hamamelidaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Meyer FG (1997) Magnoliaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Michener DC (1993) Chamaecyparis. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- How to know the ferns and fern allies.William C. Brown Co.,Dubuque,229pp.
- Wetlands, 2nd ed.Van Nostrand Reinhold and John Wiley & Sons,New York,722pp.
- Morin NR (2009) Iteaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- The monitoring of ecological quality and the classification of standing waters in temperate regions: A review and proposal based on a worked scheme for British waters.Biological Reviews71(2):301‑339. https://doi.org/10.1111/j.1469-185x.1996.tb00750.x
- Mosyaking SL (2005) Rumex. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- NatureServe Explorer: An online encyclopedia of life. http://www.natureserve.org
- Nesom GL (2006) Erigeron. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Nesom GL (2006) Pluchea. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Natural communities of New Hampshire – Photo guide. floating marshy peat mat. http://www.nhdfl.org/about-forests-and-lands/bureaus/natural-heritage-bureau/photo-index/floating-marshy-peat-mat.aspx. Accessed on: 2015-7-10.
- The water chemistry of Carolina bays: A regional survey.Archiv für Hydrobiologie118(2):147‑168.
- The “clay subsoil” bays of North Carolina.Report submitted to the North Carolina Nature Conservancy,Chapel Hill,90pp.
- Nifong TD (1998) An ecosystematic analysis of Carolina bays in the coastal plain of the Carolinas.University of North Carolina,Chapel Hill,794pp.
- Eighth biennial report of the department of conservation and development of the state of North Carolina. Biennium ending June 30, 1940.North Carolina Department of Conservation and Development,Raleigh,172pp.
- Water quality progress in North Carolina: 1992−1993 305(b) report. Report Number 94-06.North Carolina Department of Environment, Health, and Natural Resources,Raleigh,variouspp.
- A field guide to North Carolina wetlands. Report Number 96-01.North Carolina Department of Environment, Health, and Natural Resources,Raleigh,130pp.
- Singletary Lake State Park General Management Plan.North Carolina Department of Environment and Natural Resources,Raleigh,69pp.
- Bay Tree Lake State Park General Management Plan.North Carolina Department of Environment, Health, and Natural Resources,Raleigh,53pp.
- Jones Lake State Park general management plan.North Carolina Department of Environment and Natural Resources,Raleigh,55pp.
- Lake Waccamaw State Park general management plan.North Carolina Department of Environment and Natural Resources,Raleigh,61pp.
- Lake and reservoir assessments Cape Fear River basin: 4 June 2009.North Carolina Department of Environment, Health, and Natural Resources,Raleigh,45pp.
- Lake and Reservoir Assessments Lumber River Basin: 13 March 2012.North Carolina Department of Environment, Health, and Natural Resources,Raleigh,15pp.
- Element occurrence records.North Carolina Department of Environment and Natural Resources,Raleigh,variouspp.
- Protected plant species list. http://www.ncagr.gov/plantindustry/plant/plantconserve/plist.htm
- The demise of fire and the “mesophication” of forests in the eastern United States.Bioscience58(2):123‑138.
- Standards for the writing of floras.Bioscience45(5):339‑345. https://doi.org/10.2307/1312495
- Changes in plant species richness following reduced fire frequency in one of the most species-rich savannas in North America.Journal of Vegetation Science25(6):1426‑1437. https://doi.org/10.1111/jvs.12186
- The Longleaf Pine Ecosystem: ecology, restoration, and management.18th Tall Timbers Fire Ecology Conference.,Tall Timbers Research Station.Tallahassee
- VegBank: The vegetation plot archive of the Ecological Society of America. http://vegbank.org. Accessed on: 2015-6-25.
- Carolina Vegetation Survey database. Version 3.0.http://cvs.bio.unc.edu/
- Peterson PM (2003) Eragrostis. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Pieczynska E (1990) The lentic aquatic-terrestrial ecotones: Their structure, function, and importance. In: Decamps H, Naiman RJ (Eds) The ecology and management of aquatic-terrestrial ecotones.United Nations Educational, Scientific, and Cultural Organization,Paris.
- Update on the environmental and economic costs associated with alien-invasive species in the United States.Ecological Economics52(3):273‑288. https://doi.org/10.1016/j.ecolecon.2004.10.002
- Prince LM (2009) Theaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Pringle JS (1997) Clematis. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- "Carolina bays" and elliptical lake basins.Journal of Geology43(2):200‑207. https://doi.org/10.1086/624288
- Carolina bays and their origin.Geological Society of America Bulletin63(2):167‑224. https://doi.org/10.1130/0016-7606(1952)63[167:cbato]2.0.co;2
- Manual of the vascular flora of the Carolinas.University of North Carolina Press,Chapel Hill,1183pp.
- Consequences of human lakeshore development on emergent and floating-leaf aquatic vegetation abundance.North American Journal of Fisheries Management21(1):46‑61.
- Hydrilla verticillata. http://plants.ifas.ufl.edu/183
- The humic content of lake water and its relationship to watershed and lake morphometry.Limnology and Oceanography34(7):1336‑1343. https://doi.org/10.4319/lo.1989.34.7.1336
- Reznicek AA (2002) Carex sect. Lupulinae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Reznicek AA, Catling PA (2002) Carex sect. Paludosae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Pocosin wetlands: An integrated analysis of Coastal Plain freshwater bogs in North Carolina.Hutchinson Ross Publication Company,Stroudsburg,364pp.
- Pocosins: Vanishing wastelands or valuable wetlands?Bioscience33(10):626‑633.
- Richardson CJ, Gibbons JW (1993) Pocosins, Carolina bays, and mountain bogs. In: Martin WH, Boyce SG, Echternacht AC (Eds) Biodiversity of the southeastern United States/lowland terrestrial communities.John Wiley & Sons,New York,502pp.
- The submersed macrophyte communities of Adirondack lakes (New York, USA) of varying degrees of acidity.Aquatic Botany21(3):219‑235.
- Robertson KR (2002) Lachnanthes. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Natural heritage program list of rare plant species of North Carolina.North Carolina Department of Environment and Natural Resources, Office of Land and Water Stewardship, Natural Heritage Program,Raleigh,138pp.
- Impact of acidification and eutrophication on macrophyte communities in soft waters in the Nehterlands 1. Field observations.Aquatic Botany17(2):139‑155.
- Romero-Gonzáles GA, Fernández-Concha GC, Dressler RL, McGrath LK, Argus GW (2002) Orchidaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Principal determinants of aquatic macrophyte richness in northern European lakes.Aquatic Botany39(1):173‑193. https://doi.org/10.1016/0304-3770(91)90031-y
- Species diversity in space and time.Cambridge University Press,New York,436pp.
- Carolina Bays: An annotated and comprehensive bibliography, 1844-2000.Carolinas Press,Southern Pines,113pp.
- Pocosins and Carolina bays compared.The North Carolina Geographer11:22‑32.
- The vascular flora and plant communities of the Bennett Wetland Complex in Henry County, Indiana.Proceedings of the Indiana Academy of Science118(1):39‑54.
- The mysterious Carolina bays.University of South Carolina Press,Columbia,121pp.
- Guide to the natural communities of North Carolina. Fourth approximation.North Carolina Department of Environment and Natural Resources, Division of Parks and Recreation, Natural Heritage Program,Raleigh,217pp.
- Effects of acid rain on freshwater ecosystems.Science239(4836):149‑157. https://doi.org/10.1126/science.239.4836.149
- Biology of aquatic vascular plants.Edward Arnold Publishing,London,610pp.
- Semple JC, Cook RE (2006) Solidago. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Carolina bay wetlands: Unique habitats of the southeastern United States.Wetlands23(3):550‑562. https://doi.org/10.1672/0277-5212(2003)023[0550:cbwuho]2.0.co;2
- Ecology of southeastern shrub bogs (pocosins) and Carolina bays: A community profile.U.S. Fish and Wildlife Service,Washington, DC,93pp.
- Sherman-Broyles SL (1997) Ulmus. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Sheviak CJ (2002) Habenaria. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Sheviak CJ, Brown PM (2002) Spiranthes. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Sheviak CJ, Catling PM (2002) Pogonia. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- The flora of limesink depressions in Carolina Beach State Park (North Carolina).Rhodora94(878):156‑166.
- Siripun KC, Schilling EE (2006) Eupatorium. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Smith AR (1993) Key to pteridophyte families. In: Flora of North America FoNAEC( Flora of North America.Oxford University Press,New York.
- Smith AR (1993) Dryopteridaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- The potential effects of global climate change on the United States. Report to Congress.US Environmental Protection Agency, Office of Planning, Policy, and Evaluation, Office of Research and Development,Washington, DC,471pp.
- Smith SG, Bruhl JJ, Gonzáles-Elizondo MS, Menapace FJ (2002) Eleocharis. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Geology and tectonic history of the lower Cape Fear River valley, southeastern North Carolina. U.S. Geological Survey Professional Paper 1466-A.United States Government Printing Office,Washington, DC,60pp.
- Pond or lake: does it make any difference?Archiv für Hydrobiologie162(2):143‑165.
- Zonation of plants in freshwater lakes. Vol. 12.Advances in ecological research.Academic Press,New York,37-126pp.
- Soil survey of Columbus County, North Carolina.United States Soil Conservation Service,Washington, DC,138pp.
- The age and trophic history of Lake Waccamaw, North Carolina.Journal of the Elisha Mitchell Scientific Society103(1):1‑13.
- 1971-Climate Normals. http://www.nc-climate.ncsu.edu/cronos/normals.php. Accessed on: 2014-7-23.
- Stone DE (1997) Carya. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Strother JL, Weedon RR (2006) Bidens. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- A floristic analysis of the vascular plants of a marsh at Perry’s Victory Monument, Lake Erie.Michigan Botanist14:144‑166.
- Sundberg SD, Bogler DJ (2006) Baccharis. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- The ecology, status, and conservation of two non-alluvial wetland communities in the south Atlantic and eastern Gulf coastal plain, USA.Biological Conservation68(3):235‑243.
- Swanson A, Rabeler RK (2005) Stipulicida. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- The genus Utricularia: a taxonomic monograph. Volume 14. Kew Bulletin Additional Series.Royal Botanic Gardens,Kew,724pp.
- The southern megapolis: Using the past to predict the future of urban sprawl in the southeast US.PLOS ONE9(7):e102261.
- Terrell EE (2007) Luziola. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Carolina Bays in Horry and Marion Counties, South Carolina.Geological Society of America Bulletin81(3):783‑814. https://doi.org/10.1130/0016-7606(1970)81[783:cbiham]2.0.co;2
- Thompson SA (2000) Araceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Guide to the vascular flora of the savannas and flatwoods of Shaken Creek Preserve and vicinity (Pender & Onslow counties, North Carolina, U.S.A.).Biodiversity Data Journal2:1‑422. https://doi.org/10.3897/bdj.2.e1099
- Agricultural sustainability and intensive production practices.Nature418(6898):671‑677. https://doi.org/10.1038/nature01014
- Geographically isolated wetlands of the United States.Wetlands23(3):494‑516. https://doi.org/10.1672/0277-5212(2003)023[0494:giwotu]2.0.co;2
- Tucker GC (2002) Cladium. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Tucker GC (2009) Ericaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Tucker GC (2009) Eubotrys. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Tucker GC, Jones SC (2009) Clethra. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Tucker GC, Marcks BG, Carter JR (2002) Cyperus. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Report on the geology of South Carolina.AS Johnston,Columbia,293pp.
- Wetland flora: Field office illustrated guide to plant species. http://plants.usda.gov. Accessed on: 2015-6-02.
- The PLANTS database. http://plants.usda.gov. Accessed on: 2015-6-02.
- Vander Kloet SP (2009) Vaccinium. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Wetland soils: genesis, hydrology, landscapes, and classification.CRC Press,Boca Raton,417pp.
- Aquatic macrophyte richness in Danish lakes in relation to alkalinity, transparency, and lake area.Canadian Journal of Fisheries and Aquatic Sciences57(10):2022‑2031. https://doi.org/10.1139/f00-156
- The relationships of vegetation to surface water chemistry and peat chemistry in fens of Alberta, Canada.Vegetatio89(2):87‑106.
- Wagner WH, Beitel JM (1993) Lycopodiella. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Wagner WH, Montgomery JD (1993) Dryopteris. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Vascular flora of a southern Appalachain fen and floodplain complex.Castanea69(2):116‑124.
- Watson FD (1993) Taxodium. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Watson FD, Eckenwalder JE (1993) Cupressaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Flora of the southern and mid-Atlantic states.Courtesy of the author, University of North Carolina,Chapel Hill,1225pp.
- The trophic status of North Carolina lakes. Rpt. 119.Water Resources Research Institute, Department of Environmental Sciences and Engineering, School of Public Health, University of North Carolina,Chapel Hill,224pp.
- Carolina bays: additional data on their origin, age and history.Journal of the Elisha Mitchell Scientific Society69(2):119‑141.
- Limnology: Lake and River Ecosystems.3.Academic Press,San Diego,1006pp.
- Whetstone DR, Atkinson TA (1993) Osmundaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Developmental and environmental history of the Dismal Swamp.Ecological Monographs42:301‑315. https://doi.org/10.2307/1942212
- Late Pleistocene vegetational changes in northeastern North Carolina.Ecological Monographs51:451‑471. https://doi.org/10.2307/2937324
- Wiersema JH (1997) Nelumbonaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Wiersema JH (1997) Cabombaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Wiersema JH, Hellquist CH (1997) Nymphaeaceae. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Patterns in the balance of nature.Academic Press,London,324pp.
- Comparative biodiversity of rivers, streams, ditches and ponds in an agricultural landscape in Southern England.Biological Conservation115(2):329‑341. https://doi.org/10.1016/s0006-3207(03)00153-8
- North Carolina wetlands, their distribution and management. Federal aid in wildlife restoration project W-6-R.North Carolina Wildlife Resources Commission,Raleigh,169pp.
- Wipff JK (2003) Sacciolepis. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
- Wofford BE (1993) Persea. In: (Eds) FoNAEC Flora of North America.Oxford University Press,New York.
Supplementary materials
List of citations regarding Carolina bay lakes
List of some floras, manuals, guides, and broader floristic works aquatic/wetland habitats of the eastern United States that may be of interest to readers.
Taxa are organized by major plant groups (i.e., pteridophytes, gymnosperms, basal angiosperms, magnoliids, monocotyledons, and eudicotyledons), then alphabetically by family, genus, and species. Parentheses around a taxon indicate that it is not vouchered (i.e., it has been reported by state agencies or has been observed by the first author, but has not been collected as a voucher specimen; see text for details). For taxa collected from Carolina bay lake littoral zones by the present author, abundance estimates sensu Palmer et al. (1995) are provided. Abundance estimates in this checklist reflect the abundance in which the taxa occur within each lake. Status and rank designations are also provided for rare taxa monitored by the NC Natural Heritage Program (Robinson and Finnegan 2014). The term “restricted” is used here only to indicate the presence of a taxon within a particular lake among all those surveyed and not in a global sense (e.g., a taxon here considered restricted to Lake Waccamaw has not been found in the other lakes surveyed, but may exist in other localities in the state or country). A = Abundant; F= Frequent; I=Infrequent; O = Occasional; R = Rare; = þ restricted to lake indicated; () = not vouchered (i.e., reported by state agencies or observed by the present author, but not collected as a voucher specimen; see text for details); H = taxon has been collected and vouchered in the past but not by the present author. BALA = Bakers Lake; BATR = Bay Tree Lake; HOLA = Horseshoe Lake; JOLA = Jones Lake; LAWA = Lake Waccamaw; LISI = Little Singletary; SALA = Salters Lake; SILA = Singletary Lake.
This checklist does not represent a complete inventory of this locality, but rather serves as a baseline for future research. Taxa are arranged by major groups (i.e., gymnosperms, magnoliids, monocotyledons, and eudicotyledons), then alphabetically by family, genus, and species. Basal angiosperms and pteridophytes were not represented by vouchers, observations, or reports and are therefore not included in the following checklist. Brackets around a taxon indicate that it is unvouchered (i.e., it has been reported by outside agencies or has been observed by the present author, but has not been collected). Status and rank designations are also provided for rare taxa monitored by the NC Natural Heritage Program (Robinson and Finnegan 2014).
Monthly mean temperature and precipitation data for Bladen and Columbus County.
Counts of the number of taxa in the categories of herb, tree/shrub, and vine for each Carolina bay lake flora.
Counts of the number of taxa in each of the thirteen most species-rich vascular plant families in each Carolina bay lake flora.
Comma delimited file of occurrence data (DwC) for the specimens collected by the first author from Carolina Bay Lakes. Precise locality data has been redacted for species of conservation concern. Specimens are deposited at NCSC. Images are available through http://sernecportal.org.