|
Biodiversity Data Journal :
Data Paper (Biosciences)
|
|
Corresponding author:
Academic editor: Torsten Dikow
Received: 22 Jul 2016 | Accepted: 25 Aug 2016 | Published: 07 Sep 2016
© 2016 Michael Skvarla, Jeffrey Barnes, Danielle Fisher, Ashley Dowling
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Skvarla M, Barnes J, Fisher D, Dowling A (2016) Terrestrial arthropods of Steel Creek, Buffalo National River, Arkansas. IV. Asilidae and other Diptera. Biodiversity Data Journal 4: e9977. https://doi.org/10.3897/BDJ.4.e9977
|
|
Abstract
Background
This is the fourth in a series of papers detailing the terrestrial arthropods collected during an intensive survey of a site near Steel Creek campground along the Buffalo National River in Arkansas. The survey was conducted over a period of eight and a half months in 2013 using twelve trap types, including Malaise and canopy traps, Lindgren multifunnel traps, and pan traps.
New information
We provide collection records for 38 species of Asilidae and other Diptera, 7 of which are new state records for Arkansas: (Asilidae) Lasiopogon opaculus Loew, 1874; (Lygistorrhinidae) Lygistorrhina sancthecatharinae Thompson, 1975; (Stratiomyidae) Cephalochrysa nigricornis (Loew, 1866), Gowdeyana punctifera (Malloch, 1915), Sargus decorus Say, 1824; (Ulidiidae) Callopistromyia annulipes Macquart, 1855; and (Xylophagidae) Rachicerus obscuripennis Loew, 1863.
Introduction
The Interior Highlands is mountainous region in the central United States. It has remained exposed for the last 270 million years and has historically acted as a refugium during times of inhospitable climate (
Because this paper covers species from a variety of fly families, we provide the following summaries of each family and lower taxon treated.
Anisopodidae comprises 154 species world wide, including nine that are present in North America, five of which belong to Sylvicola (
Asilidae, the members of which are commonly known as robber flies, are a diverse family of exclusively predatory flies. More than 7,500 species in approximately 550 genera are known worldwide, of which approximately 1,040 species in 100 genera occur in North America north of Mexico (
Stratiomyidae include approximately 2700 species arranged in more than 380 genera worldwide; more than 250 species in 41 genera are present in North America north of Mexico (
Lygistorrhinidae is an uncommonly collected family of Sciaroidea that is easily recognizable by the generally elongate proboscis and reduced wing venation (
Little is known about lygistorrhinids. Most specimens are collected using passive traps (i.e., Malaise traps) or by sweep netting vegetation and almost nothing is known about their behavior and biology, including the immature stages (
Ulidiidae, commonly known as picture-wing flies, are distinctive for the striking wing patterning and propensity of some species to wave or flash their wings. The peacock fly, Callopistromyia annulipes, is especially noticeable as it often holds its wings above the thorax.
Rachicerus Walker, 1854, the most speciose genus of Xylophagidae, contains approximately half of the known species worldwide, and is the only genus of Xylophagidae present in tropical forests (
Sampling methods
The sampling protocol was covered in detail by
The following traps were maintained within a 4 ha site at Steel Creek, Buffalo National River, Arkansas (see Geographic coverage for a description of the site): five Malaise traps, twenty-five pan traps (five of each color: blue, purple, red, yellow, white) which were randomly arranged under the Malaise traps (one of each color per Malaise trap); fifteen Lindgren multi-funnel traps (five of each color: black, green, purple); four SLAM (Sea, Land, and Air Malaise) traps with top and bottom collectors placed in the canopy; and seventeen pitfall trap sets. Additionally, ten leaf litter samples were collected for Berlese extraction when traps were serviced.
Trap placement began on 8 March 2013 and all traps were set by 13 March 2013, except Lindgren funnels, which were set on 1 April 2013. Traps set earlier than 13 March were reset on that date in order to standardize trap catch between traps. Traps were serviced approximately every two weeks. The last collection of pitfall traps and pan traps occurred on 6 November 2013; Malaise, SLAM, and Lindgren funnel traps were run for an additional month, with the final collection on 4 December 2013. In total, 1311 samples were collected.
RV and marine antifreeze, which contains both propylene glycol and ethanol, was used as the preservative in all traps as it is non-toxic, inexpensive, and preserves specimens reasonably well (
Samples were coarse-sorted using a Leica MZ16 stereomicroscope illuminated with a Leica KL1500 LCD light source and a Wild M38 stereomicroscope illuminated with an Applied Scientific Devices Corp. Eco-light 20 fiber optic light source. After sorting, specimens were stored individually or by family in 2 mL microtubes in 70% ethanol until they could be pinned or pointed.
Asilidae were identified by author Barnes, who is an internationally recognized robber fly expert. Specimens of other families were identified using published keys (Table
| Family | Genus | Reference |
| General identification |
|
|
| Anisopodidae | Sylvicola | |
| Drosophilidae | Drosophila | D. suzukii is a distinctive species, no key necessary |
| Drosophilidae | Zaprionus | Z. indianus is a distinctive non-native species, no key necessary |
| Lygistorrhinidae | Lygistorrhina |
|
| Mydidae | Mydas | M. clavatus is a distincitve species in Arkansas, no key necessary |
| Oestridae | Cephenemyia |
|
| Oestridae | Cuterebra |
|
| Ptychopteridae | Bittacomorpha | Distinctive genus represented by a single species in the Eastern United States, no key necessary. |
| Stratiomyiidae |
|
|
| Stratiomyiidae | Ptecticus |
|
| Xylophagidae | Rachicerus |
|
Asilidae were the focal group of this study; all specimens were removed when bulk samples were sorted so specimens reported here reflect the seasonality and relative abundance of the species sampled by the traps at the site. Specimens of other families were not consistentatly removed by everyone who processed samples, so specimens reported here are indicative of a species presence at the site but not other measurements such as seasonality and relative abundance.
All specimens are deposited in the University of Arkansas Arthropod Museum (UAAM).
Geographic coverage
The survey was conducted within a 4 hectare plot established at Steel Creek along the Buffalo National River in Newton County, Arkansas, centered at approximately N 36°02.269', W 93°20.434'. The site is primarily 80–100 year old mature second-growth Eastern mixed deciduous forest dominated by oak (Quercus) and hickory (Carya), though American beech (Fagus grandifolia) and eastern red cedar (Juniperus virginiana) are also abundant. A small (14 m x 30 m), fishless pond and glade (10 m x 30 m) with sparse grasses are present within the boundaries of the site. See
36.0367 and 36.0397 Latitude; -93.3917 and -93.3397 Longitude.
Taxonomic coverage
| Rank | Scientific Name |
|---|---|
| order | Diptera |
Usage rights
Data resources
| Column label | Column description |
|---|---|
| typeStatus | Nomenclatural type applied to the record |
| catalogNumber | Unique within-project and within-lab number applied to the record |
| recordedBy | Who recorded the record information |
| individualCount | The number of specimens contained within the record |
| lifeStage | Life stage of the specimens contained within the record |
| kingdom | Kingdom name |
| phylum | Phylum name |
| class | Class name |
| order | Order name |
| family | Family name |
| genus | Genus name |
| specificEpithet | Specific epithet |
| scientificNameAuthorship | Name of the author of the lowest taxon rank included in the record |
| scientificName | Complete scientific name including author and year |
| taxonRank | Lowest taxonomic rank of the record |
| country | Country in which the record was collected |
| countryCode | Two-letter country code |
| stateProvince | State in which the record was collected |
| county | County in which the record was collected |
| municipality | Closest municipality to where the record was collected |
| locality | Description of the specific locality where the record was collected |
| verbatimElevation | Average elevation of the field site in meters |
| verbatimCoordinates | Approximate center point coordinates of the field site in GPS coordinates |
| verbatiumLatitude | Approximate center point latitude of the field site in GPS coordinates |
| verbatimLongitude | Approximate center point longitude of the field site in GPS coordinate |
| decimalLatitude | Approximate center point latitude of the field site in decimal degrees |
| decimalLongitude | Approximate center point longitude of the field site in decimal degrees |
| georeferenceProtocol | Protocol by which the coordinates were taken |
| identifiedBy | Who identified the record |
| eventDate | Date or date range the record was collected |
| habitat | Description of the habitat |
| language | Two-letter abbreviation of the language in which the data and labels are recorded |
| institutionCode | Name of the institution where the specimens are deposited |
| basisofRecord | The specific nature of the record |
Additional information
Results
We collected and identified specimens representing 12 families, 27 genera, and 38 species during this study (Table
Species collected, including total number of specimens. New state records are indicated by an asterisk (*).
| Family | Genus | Species | Number of specimens |
| Anisopodidae | Sylvicola | Sylvicola fenestralis (Scopoli, 1763) | 3 |
| Asilidae | Diogmites | Diogmites misellus Loew, 1866 | 6 |
| Asilidae | Diogmites | Diogmites neoternatus (Bromley, 1951) | 1 |
| Asilidae | Efferia | Efferia aestuans (Linnaeus, 1763) | 3 |
| Asilidae | Holopogon | Holopogon phaeonotus Loew, 1874 | 3 |
| Asilidae | Laphria | Laphria divisor (Banks, 1917) | 2 |
| Asilidae | Laphria | Laphria flavicollis Say, 1824 | 15 |
| Asilidae | Laphria | Laphria index McAtee, 1919 | 4 |
| Asilidae | Laphria | Laphria sicula McAtee, 1919 | 11 |
| Asilidae | Lasiopogon | Lasiopogon opaculus Loew, 1874* | 2 |
| Asilidae | Leptogaster | Leptogaster aegra Martin, 1957 | 1 |
| Asilidae | Leptogaster | Leptogaster brevicornis Loew, 1872 | 10 |
| Asilidae | Leptogaster | Leptogaster flavipes Loew, 1862 | 1 |
| Asilidae | Leptogaster | Leptogaster virgata Coquillett, 1904 | 5 |
| Asilidae | Machimus | Machimus antimachus (Walker, 1849) | 22 |
| Asilidae | Machimus | Machimus sadyates (Walker, 1849) | 6 |
| Asilidae | Machimus | Machimus virginicus (Banks, 1920) | 3 |
| Asilidae | Neoitamus | Neoitamus flavofemoratus (Hine, 1909) | 33 |
| Asilidae | Ommatius | Ommatius gemma Brimley, 1928 | 2 |
| Asilidae | Ommatius | Ommatius ouachitensis Bullington and Lavigne, 1984 | 3 |
| Asilidae | Taracticus | Taracticus octopunctatus (Say, 1823) | 3 |
| Drosophilidae | Drosophila | Drosophila suzukii (Matsumura, 1931) | 9 |
| Drosophilidae | Zaprionus | Zaprionus indianus Gupta, 1970 | 1 |
| Limoniidae | Cladura | Cladura flavoferruginea Osten Sacken, 1859 | 26 |
| Lygistorrhinidae | Lygistorrhina | Lygistorrhina sanctaecatharinae Thompson, 1975* | 2 |
| Mydidae | Mydas | Mydas clavatus (Drury, 1773) | 3 |
| Oestridae | Cephenemyia | 2 | |
| Oestridae | Cuterebra | Cuterebra emasculator Fitch, 1856 | 1 |
| Oestridae | Cuterebra | Cuterebra f. fontinella Clark, 1827 | 4 |
| Ptychopteridae | Bittacomorpha | Bittacomorpha clavipes (Fabricius, 1781) | 1 |
| Stratiomyidae | Cephalochrysa | Cephalochrysa nigricornis (Loew, 1866)* | 1 |
| Stratiomyidae | Gowdeyana | Gowdeyana punctifera (Malloch, 1915)* | 1 |
| Stratiomyidae | Ptecticus | Ptecticus trivattus (Say, 1829) | 680 |
| Stratiomyidae | Sargus | Sargus decorus Say, 1824* | 2 |
| Tipulidae | Tanyptera | Tanyptera dorsalis (Osten Sacken, 1864) | 2 |
| Ulidiidae | Callopistromyia | Callopistromyia annulipes (Macquart, 1855)* | 1 |
| Ulidiidae | Idana | Idana marginata (Say, 1830) | 6 |
| Xylophagidae | Rachicerus | Rachicerus obscuripennis Loew, 1863* | 4 |
Notes on newly reported species
Lasiopogon opaculus Loew, 1874 (Asilidae) (Fig.
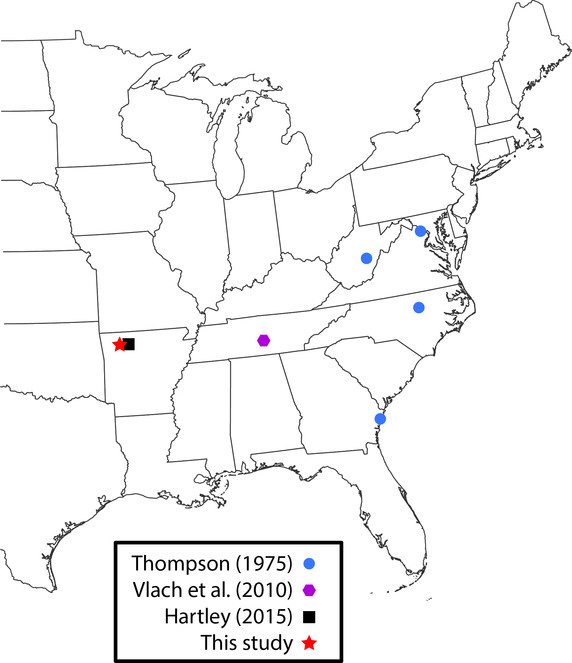
Lygistorrhina santaecatharinae (Lygistorrhinidae) has only been reported in the literature on two occasions and is known from localities in West Virginia, Virginia, Georgia, and Tennessee (Fig.
Lygistorrhina sanctaecatharinae feeding. Photographs by Chris Hartley, used and manipulated with permission.
b: Cropped detail of figure 2a.
c: The same two L. sanctaecatharinae as figure 2a at slightly different angles.
d: Detail of figure 2c, clearly showing nectivory.
Cephalochrysa nigricornis (Loew, 1866) (Stratiomyidae) (Fig.
Gowdeyana punctifera (Malloch, 1915) (Stratiomyidae) is widespread in eastern North America and occurs from Massachusetts south to Alabama, west to South Dakota, Wyoming, Utah, and Morelos and Sinaloa, Mexico (
Sargus decorus Say, 1824 (Stratiomyidae) (Fig.
Callopistromyia annulipes Macquart, 1855 (Ulidiidae) is widespread in North America and has been reported from Maine south to Louisiana, west to Washington (
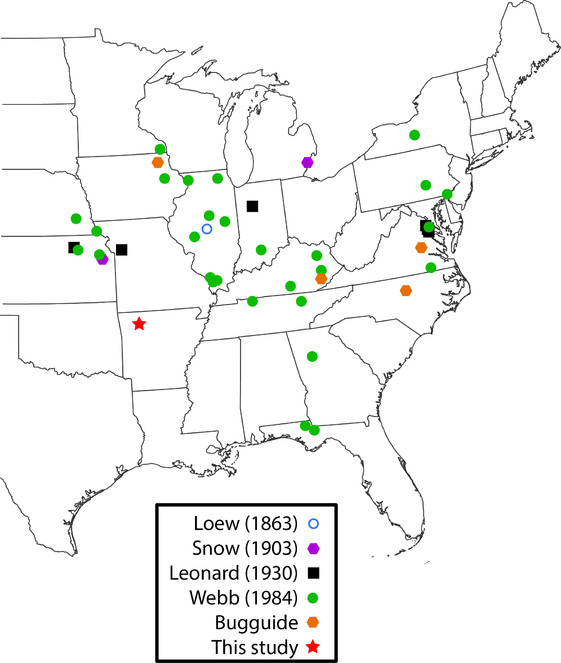
Rachicerus obscuripennis Loew, 1863 (Xylophagidae) (Fig.
Discussion
It is unsurprising that only one of the twenty species of Asilidae was newly recorded in Arkansas as author Barnes has been studying robber flies in the state for over a decade. However, that such a distinctive species as Rachicerus obscuripennis has been known from Missouri since 1901 but is just now reported from neighboring Arkansas illustrates how poorly surveyedsome groups are in the state. This is in line with previous publications in this series, which also reported species previously unrecorded in Arkansas, some of which are quite distinctive (
Previous publications have utilized social media and citizen science websites such as Facebook, Flickr, and Bugguide to discover new species (e.g.,
Acknowledgements
We thank Chris Haryley, Gayle and Jeanell Strickland, Steve Nanz, Phil Huntley-Franck, and Stephen A. Marshall for giving permission to use their photographs and Lisa Bentley, Patrick Coin, and M.J. Hatfield for posting their photographs of R. obscuripennis to Bugguide and granting permission to use their locality data. This project and the preparation of this publication were funded in part by the State Wildlife Grants Program (Grant # T-45) of the U.S. Fish and Wildlife Service through an agreement with the Arkansas Game and Fish Commission.
References
- Insect endemism in the Interior Highlands of North America.The Florida Entomologist73(4):539‑569. https://doi.org/10.2307/3495270
- The Nearctic species of the genus Cephenemyia (Diptera: Oestridae).Canada Journal of Zoology40:431‑448. https://doi.org/10.1139/z62-041
- Fly?http://bugguide.net/node/view/932941/bgimage. Accessed on: 2016-5-16.
- The Systematics of Lasiopogon (Diptera: Asilidae).Royal British Columbia Museum,Victoria, Canada,354pp.
- Xylophagid flies mating. http://bugguide.net/node/view/59245/bgimage. Accessed on: 2016-5-16.
- Description of the female and first-instar larva of a new bot fly species from Central Texas.Southwestern Entomologist32(1):37‑41. https://doi.org/10.3958/0147-1724-32.1.37
- Fungus gnats online. http://sciaroidea.info/. Accessed on: 2015-10-15.
- Robber flies (Asilidae). www.geller-grimm.de/asilidae.htm. Accessed on: 2016-5-06.
- Drosera magnifica (Droseraceae): the largest New World sundew, discovered on Facebook.Phytotaxa220(3):257‑267. https://doi.org/10.11646/phytotaxa.220.3.4
- Fly ID - Lygistorrhina sanctaecatharinae. http://bugguide.net/node/view/1109001. Accessed on: 2015-12-10.
- Xylophagidae: Rachicerus obscuripennis. http://bugguide.net/node/view/248345/bgimage. Accessed on: 2016-5-16.
- The family Lygistorrhinidae (Diptera: Sciaroidea) in Mexico and the description of two new species.Zootaxa1808:44‑52.
- Myennidini, a new tribe of the subfamily Otitinae (Diptera: Ulidiidae), with discussion of the suprageneric classification of the family.Israel Journal of Entomology35:497‑586.
- A revision of the dipterous family Rhagionidae (Leptidae) in the United States and Canada.Memoirs of the American Entomological Society7:1‑181.
- Diptera Americae septentrionalis indigena. Centuria tertia.Berliner Entomologische Zeitschrift1:1‑55. https://doi.org/10.1002/mmnd.18630070104
- Manual of Nearctic Diptera.1.Canadian Government Publishing Centre,Hull, Quebec, Canada,674pp. [ISBN0-660-10731-7]
- Manual of Nearctic Diptera.2.Canada Communication Group - Publishing,Ottawa, Ontario, Canada,1332pp. [ISBN0-660-12125-5]
- Two new species of Ptecticus with a key to species occurring in America north of Mexico (Diptera: Stratiomyiidae).Pan-Pacific Entomologist47(2):94‑100.
- The soldier flies of Canada and Alaska (Diptera: Stratiomyiidae): I. Beridinae, Sarginae, and Clitellariinae.The Canadian Entomologist104(4):531‑561. https://doi.org/10.4039/Ent104531-4
- Description of Dirrhagofarsus ernae n. sp. with a key to the known Dirrhagofarsus species (Coleoptera: Eucnemidae).Zootaxa3878(2):179‑1844. https://doi.org/10.11646/zootaxa.3878.2.4
- Family Lygistorrhinidae. A catalog of the Diptera of the Americas South of the United States.19D.Museo de Zoologia, University de São Paulo,São Paulo, Brazil,2pp.
- Pape T, Blagoderov V, Mostovski MB (2011) Order Diptera. In: Zhang Z- (Ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness.Magnolia Press,Auckland, New Zealand,237pp.
- First record of the adventive oriental aphid Schizaphis piricola (Matsumura, 1917) (Hemiptera, Aphididae) in Europe.ZooKeys89:49‑56. https://doi.org/10.3897/zookeys.89.903
- Notes on Nearctic Sylvicola (Diptera: Anisopodidae).Proceedings of the Entomological Society of Washington82:86‑98.
- A new species of Sabatia (Gentianaceae) from Saline County, Arkansas.SIDA21(3):1249‑1262.
- Only in Arkansas: A study of the endemic plants and animals of the state.University of Arkansas Press,Fayetteville, Arkansas,121pp. [ISBN1-55728-326-5]
- The Arkansas endemic biota: An update with additions and deletions.Journal of the Arkansas Academic of Science62:84‑96.
- North American species of Cuterebra, the rabbit and robent bot flies (Diptera: Cuterebridae).Entomological Society of America,College Park, Maryland,240pp.
- Terrestrial arthropods of Steel Creek, Buffalo National River, Arkansas. I. Select beetles (Coleoptera: Buprestidae, Carabidae, Cerambycidae, Curculionoidea excluding Scolytinae).Biodiversity Data Journal3:1‑42. https://doi.org/10.3897/bdj.3.e6832
- Arthropods of Steel Creek, Buffalo National River, Arkansas. III. Heteroptera (Insecta: Hemiptera).Biodiversity Data Journal4(7607):1‑13. https://doi.org/10.3897/BDJ.4.e7607
- Pitfalls and preservatives: A review.Journal of the Entomological Society of Ontario145:15‑43.
- Terrestrial arthropods of Steel Creek, Buffalo National River, Arkansas. II. Sawflies (Insecta:Hymenoptera: "Symphyta").Biodiversity Data Journal4(8830):1‑17. https://doi.org/10.3897/BDJ.4.e8830
- New records of Orussus minutus Middlekauff, 1983 (Hymenoptera: Orussidae) represent a significant western range expansion.Biodiversity Data Journal3(5793):1‑22. https://doi.org/10.3897/BDJ.3.e5793
- A preliminary list of the Diptera of Kansas.Kansas University Science Bulletin11:211‑223. https://doi.org/10.5962/bhl.part.26911
- A new bot fly species (Diptera: Oestridae) from Central Texas.The Great Lakes Entomologist37:76‑80.
- Ozarks ecoregional conservation assessment.The Nature Conservancy Midwestern Resource Office,Minneapolis, Minnesota,52pp.
- Notes on the genus Lygistorrhina Skuse with the description of the first Nearctic species (Diptera: Mycetophiloidea).Proceedings of the Washington Entomological Society77:434‑445.
- Diversity of the insect fauna within the unique sinking pond habitat in middle Tennessee.Journal of the Tennessee Academy of Science85:62‑86.
- Vockeroth JR (2009) Lygistorrhinidae (Long-beaked fungus gnats). In: Brown BV, Borkent A, Cumming JM, Wood DM, Woodley NE, Zumbado M (Eds) Manual of Central American Diptera.1.NRC Press,Ottawa, Canada,714pp.
- A revision of the Nearctic species of Rachicerus (Diptera: Rachiceridae).Journal of the Kansas Entomological Society57(2):298‑315.
- Notes and descriptions of North American Xylophagidae and Stratiomyiidae.The Canadian Entomologist17(7):121‑128. https://doi.org/10.4039/Ent17121-7
- A charismatic new species of green lacewing discovered in Malaysia (Neuroptera, Chrysopidae): the confluence of citizen scientist, online image database and cybertaxonomy.ZooKeys214:1‑11. https://doi.org/10.3897/zookeys.214.3220
- A World Catalog of the Stratiomyidae (Insecta: Diptera).Myia11:1‑473.
- A catalog of the world Xylophagidae (Insecta: Diptera).Myia12:455‑500.
- Endemic vascular plants of the Interior Highlands.SIDA21:1781‑1791.