|
Biodiversity Data Journal :
Research Article
|
|
Corresponding author: Christopher T. Martine (ctm015@bucknell.edu)
Academic editor: Quentin Groom
Received: 24 Aug 2017 | Accepted: 09 Nov 2017 | Published: 13 Nov 2017
© 2017 Matthew Wilson, Anna Freundlich, Christopher Martine
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Wilson M, Freundlich A, Martine C (2017) Understory dominance and the new climax: Impacts of Japanese knotweed (Fallopia japonica) invasion on native plant diversity and recruitment in a riparian woodland. Biodiversity Data Journal 5: e20577. https://doi.org/10.3897/BDJ.5.e20577
|
|
Abstract
Riparian forests exhibit levels of ecological disturbance that leave them especially prone to biological invasions. Japanese knotweed (Fallopia japonica) is particularly suited to these habitats and is an aggressive invader along watercourses throughout its now-global range as an exotic invader. Using one of the few Silver Maple Floodplain Forest communities that has not been invaded by F. japonica in the West Branch Susquehanna River valley (Pennsylvania, USA) as a baseline, this study examines whether and how this primarily intact riparian forest community differs from nearby invaded communities in terms of 1) native species richness, 2) native species density, and 3) riparian forest tree recruitment. Defining a baseline (intact) community composition will inform restoration plans for local riparian forests where knotweed might be eradicated or reduced. Invaded and non-invaded sites differed statistically across species richness, species density, and tree recruitment. Our results suggest that F. japonica has reduced the diversity and abundance of native understory riparian plant species. The species also appears to have suppressed long-term tree recruitment, setting up a trajectory whereby the eventual decline of trees currently in the canopy could shift this community from a tree-dominated riparian forest to a knotweed-dominated herbaceous shrubland.
Keywords
biodiversity, Polygonum cuspidatum, recruitment, Reynoutria japonica, riparian, undergraduate research
Introduction
Invasive species are known to cause community level effects that include reductions in richness, density, and recruitment of native flora and fauna. This can be especially true for habitats in which dynamism and disturbance, characteristics invasive taxa are often adapted to, are natural elements of ecological cycles (e.g.
Although some sexual reproduction occurs in invasive populations of F. japonica (
We conducted this study along the West Branch of the Susquehanna River (Lewisburg, Pennsylvania, USA), where non-native Japanese knotweed (Fallopia japonica (Houtt.) Ronse Decr.; Synonym: Polygonum cuspidatum, Reynoutria japonica) is the dominant understory component in the majority of associated Silver Maple Floodplain Forests (SMFF). While this forest type is not rare in Pennsylvania, it has been identified as a community of conservation concern because large contiguous SMFFs are uncommon and increasingly lost to development and agriculture, impacted by changing flood regimes, or invaded by aggressive exotic plants that displace native ones (
Methods
Study Area
This study was conducted in the riparian zone of the West Branch Susquehanna River in Lewisburg, PA (
Vegetation Sampling
Each study area was divided into a set of 30x15 m (=450 m2) rectangular plots run along 400 m transects (separated along transects by 30 m lengths), with the long sides of the plot parallel to the riverbank and the shorter sides running perpendicular to the riverbank. Forty-four 450 m2 ‘large plots’ were sampled between the two bank communities. Within each large plot, vegetation was sampled across the herb and canopy layers during the 2013 field season (May-July). At the bisector of each large plot, running perpendicular to the bank, stem counts were conducted within 0.25 m2 square plots at the 2 m, 7 m, and 12 m marks (where 0m occurred closest to the water’s edge). Plants were considered to be within the herb-layer if they were less than 0.5 m tall. Follow-up observations were conducted in the subsequent fall and spring seasons to establish presence/absence for all understory species encountered within the 30x15 large plots, including taxa that were not captured in the 0.25 m2 plots.
The canopy layer was surveyed from the midpoint of each 30x15m large plot within a circle plot of 5 m radius (80 m2 survey area). Plants were counted as canopy trees if they were measured to have a diameter at breast height (dbh) of at least 10 cm and had any portion of stem within the circle plot. For each of these trees, dbh was recorded and used as a rough proxy for age as tree cores proved problematic because silver maples typically hollowed with age. All individuals across vegetation layers were identified to species using
Data Analysis
To investigate the effects of F. japonica on overall patterns in community composition between 0.25 m2 herbaceous samples we used Principal Component Analysis (PCA) in the R package vegan (version 2.0-10;
Results
Overall, our 0.25 m2 plots captured 32 (6 non-native and 26 native) of 80 herb-layer species observed by presence/absence, with only 12 species occurring in both non-invaded and invaded plots (Table 1). Canopy surveys (80 m2) captured all eight observed tree species by presence/absence. In the herbaceous layer stem densities ranged widely from 576 stems/m2 to a minimum of 8 stems/m2. Non-invaded communities were dominated by native Impatiens pallida Nutt. (yellow touch-me-not, Balsaminaceae, 15/m2), Toxicodendron radicans (L.) Kuntze (poison-ivy, Anacardiaceae, 13/m2), and Pilea pumila (L.) A. Gray (clearweed, Urticaceae, 12/m2). After F. japonica (12/m2) the most common species in invaded communities were T. radicans (2.7/m2) and I. pallida (2.5/m2). In addition, the only native species with higher densities in invaded communities were Arisaema dracontium (L.) Schott (green dragon, Araceae, 0.10 versus 2.1/m2), Polygonatum biflorum (Walter) Elliott (Solomon’s-seal, Ruscaceae, 1.3 versus 1.8/m2), and Matteuccia struthiopteris (L.) Tod. (ostrich fern, Polypodiaceae, 0 versus 0.099/m2). Overstory surveys were dominated by Acer saccharinum (0.017/m2) in both non-invaded and invaded plots (Table
Densities of stems in 0.25 m2 herbaceous-layer plots by species and river bank. (I) denotes invasive or non-native species and (N) denotes native. Bolded lines indicate species with higher densities in the presence of Japanese knotweed. Significant differences are noted as: *** = p < 0.001, ** = p < .01, * = p < 0.05.
|
Species Name |
Non-invaded Bank |
Invaded Bank |
|
Alliaria petiolata (I) *** |
4.7 |
0.049 |
|
Cerastium fontanum (I) |
0.81 |
0 |
|
Hesperis matronalis (I) |
0.15 |
0 |
|
Microstegium vimineum (I) |
9.0 |
1.7 |
|
Persicaria perfoliata (I) |
0.10 |
0 |
|
Fallopia japonica (I) *** |
1.7 |
12 |
|
Acer saccharinum (N) |
0.050 |
0.049 |
|
Arisaema dracontium (N) |
0.10 |
2.1 |
|
Arisaema triphyllum (N) |
0.51 |
0 |
|
Asclepias incarnata (N) |
0.25 |
0 |
|
Asclepias syriaca (N) |
0.20 |
0 |
|
Boehmeria cylindrica (N) |
0.15 |
0.049 |
|
Cryptotaenia canadensis (N) |
0.10 |
0 |
|
Eupatorium fistulosum (N) |
0.051 |
0 |
|
Galium asprellum (N) |
0.46 |
0 |
|
Geum canadense (N) |
0.20 |
0 |
|
Impatiens pallida (N) *** |
15 |
2.5 |
|
Laportea canadensis (N) |
0.96 |
0 |
|
Lilium superbum (N) |
0.20 |
0.049 |
|
Matteuccia struthiopteris (N) |
0 |
0.099 |
|
Onoclea sensibilis (N) |
1.1 |
0 |
|
Parthenocissus quinquefolia (N) ** |
3.6 |
0 |
|
Persicaria virginiana (N) *** |
4.6 |
0.25 |
|
Pilea pumila (N) |
12 |
0.25 |
|
Polygonatum biflorum (N) |
1.3 |
1.8 |
|
Sicyos angulatus (N) |
0.051 |
0 |
|
Thalictrum pubescens (N) |
0.10 |
0 |
|
Toxicodendron radicans (N) *** |
13 |
2.7 |
|
Ulmus americana (N) |
0.051 |
0 |
|
Verbesina alternifolia (N) * |
1.1 |
0 |
|
Viola cucullata (N) *** |
7.6 |
0.74 |
|
Vitis spp. (N) |
0.20 |
0 |
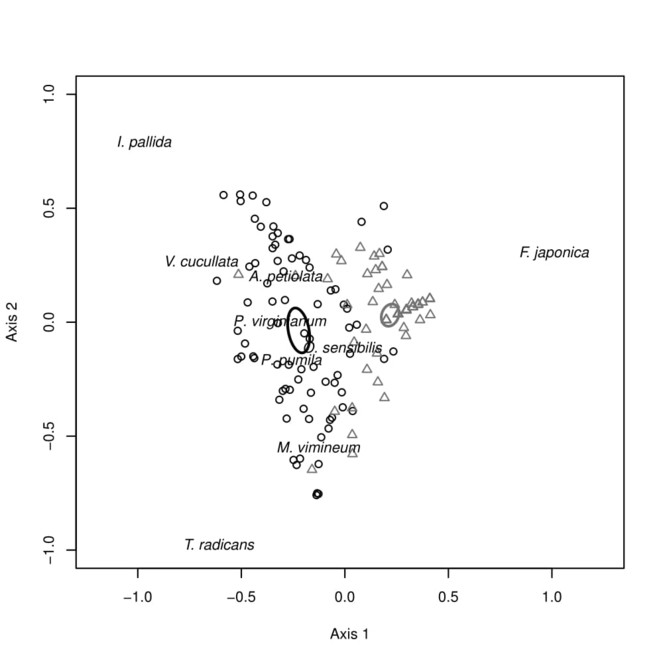
Our PCA results showed sampled communities cluster tightly by invaded and non-invaded river banks, with clear separation of 95% confidence ellipses for the centroid of each bank (Fig.
Principal Component Analysis (PCA) of herbaceous samples with 95% CI ellipses surrounding the centroid of each site type (invaded or non-invaded). Circles represent samples collected from the non-invaded Silver Maple Floodplain Forest site and triangles represent the site invaded by F. japonica, with strong species drivers of Axis 1 and 2 overlaid.
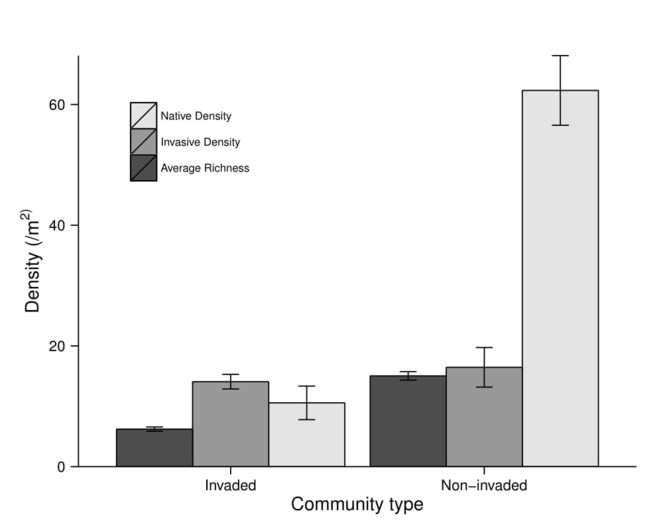
For the non-invaded bank community, we found significantly higher native plant density (p < 0.0001), higher average richness per square meter (p < 0.0001), and higher exotic/invasive plant density (p = 0.015; Fig.
Discussion
Our results showed significantly lower native plant density, species diversity, and tree recruitment in the presence of F. japonica. Although we do not know what pre-invasion conditions were like in our study sites, we can infer the three differences noted above may be connected to the life history of F. japonica given the stark contrast in the abundance of this aggressive invader. Whether or not F. japonica is less of a driver of species diversity than a passenger (i.e.
Local decreases in native species density and richness between invaded and non-invaded SMFFs translated in our study to a loss of beta diversity in the herbaceous layer at the between-patch scale. The homogenization of local communities reduced overall densities of native species and may translate into localized extirpations. This homogenization is also evident in our PCA results, as the spread of samples collected from the non-invaded community is larger than that of the invaded community. While not a significant finding in itself, it is noteworthy that this increased spread (i.e. beta diversity) is visible primarily along Axis 2, the axis which was not strongly influenced by whether samples were collected in the invaded or non-invaded community type. Instead, our data suggest this high heterogeneity is at least partly driven by a tradeoff in dominance between Impatiens pallida and Toxicodendron radicans in non-invaded communities. In these samples, when I. pallida was present (59% of samples) it had a density of 26/m2 while the density of T. radicans in these samples was 9.4/m2. Conversely, in plots where T. radicans was present (48% of samples) it had a density of 26/m2 while the density of I. pallida was 11/m2. In addition, non-invaded sites exhibited patchy distributions of other abundant species such as Viola cucullata, Pilea pumila, and Persicaria virginiana (L.) Gaertn. contributing to the higher beta diversity between samples in the non-invaded community type.
While nearly all of the native understory species observed on both banks had lower densities in invaded plots (Table 1), three native herbaceous species appeared to do just as well on either side. The apparent success of Arisaema dracontium, Matteucia struthiopteris, and Polygonatum biflorum likely has less to do with ecological tolerance than it does with life history strategy. As long-lived species re-growing annually from perennial rootstocks (
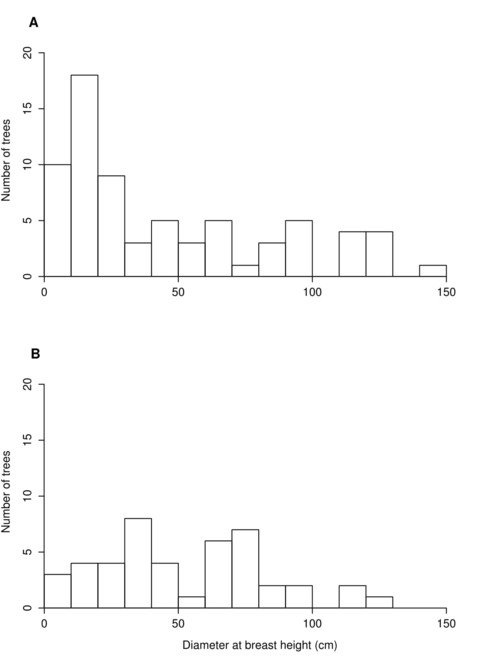
Similarly, our finding that younger age classes of trees are significantly less prominent in invaded sites allows us to infer that Japanese knotweed dominance might suppress tree recruitment in our study area, a potential threat previously hypothesized by PA Natural Heritage Program botanists for the SMFF community type (Zimmerman 2011). This is further supported by a general dearth of saplings and small trees in the invaded sites. Anecdotal reports from volunteers in nearby Milton State Park, a riparian island, claim little to no F. japonica occurred in local riparian forest until the early 1960s – an observation that corresponds temporally with the expansion of the species throughout northeastern North America noted by
The combination of limited tree and native herbaceous species recruitment in local habitats dominated by F. japonica suggests a potential shift in the ecological trajectory of local SMFF sites and a pathway to a new climax. If the observed patterns and the decline of the relict overstory continue in the coming decades, trees will not be replaced – eventually resulting in a climax community in which the canopy might consist of 2-3 meter tall Japanese knotweed thickets functioning as an herbaceous shrubland. As our data are limited to a small section of the Susquehanna Valley, more work is needed to investigate the patterns documented here and the hypothesized mechanisms behind them.
We suggest the maintenance of SMFF diversity in the study region is now likely reliant on conservation management practices, including removal of Japanese knotweed. Ongoing studies on the management of F. japonica in Pennsylvania (
One potential solution to diversity decline in regional SMFF communities is to encourage the growth of native woody cover in riparian zones. This may include Toxicodendron radicans (poison-ivy), a native (though often maligned) species that trades dominance (along with Impatiens pallida) with F. japonica in our sites. Even when dominant, T. radicans tends not to choke out native herbaceous species and appears to act as a nursery species for other native riparian forest species.
Our results suggest F. japonica has reduced the diversity and abundance of native understory plant species in one of the few intact Silver Maple Floodplain Forests on the West Branch Susquehanna River. The invader also appears to have suppressed long-term tree recruitment, with the potential to shift this SMFF community from a tree-dominated riparian forest to a knotweed-dominated herbaceous shrubland. While the findings of this study apply most directly to our local stretch of the Susquehanna River, they are generally translatable across SMFF communities in Pennsylvania and, given conservation concerns about this community type (Zimmerman 2011), may serve as a model and comparison for additional surveys in other parts of the state and beyond. Likewise, diversity survey results from our intact study site will now provide pertinent ecoregional data for a redefinition of the SMFF community type as part of current Natural Heritage Program efforts to revise statewide plant community designations (Ephraim Zimmerman, pers. comm.). Any future attempts at SMFF community restoration will depend on a clear definition of what pre-invasion communities may have looked like.
Acknowledgements
The authors thank Ray Schlitt for assistance with data/specimen collection and Jason Cantley for providing helpful comments during manuscript preparation. Funding was provided through Bucknell University via the David Burpee Endowment and the Wayne E. Manning Internship Fund (to AF) and the Botanical Society of America Undergraduate Research Award (to AF).
References
- The biology of invasive alien plants in Canada. 5. Polygonum cuspidatum Sieb. & Zucc. [=Fallopia japonica (Houtt.) Ronse Decr.].Canadian Journal of Plant Science86(3):887‑906. https://doi.org/10.4141/p05-170
- Life histories and demography of shade-tolerant temperate forest herbs.New Phytologist90(4):757‑776. https://doi.org/10.1111/j.1469-8137.1982.tb03285.x
- Seed germinability and its seasonal onset of Japanese knotweed (Polygonum cuspidatum).Weed Science52(5):759‑767. https://doi.org/10.1614/p2002-053
- In situ growth and rapid response management of flood-dispersed Japanese knotweed (Fallopia japonica).Invasive Plant Science and Management7(01):84‑92. https://doi.org/10.1614/ipsm-d-13-00027.1
- Extending the timeframe for rapid response and best management practices of flood-dispersed Japanese knotweed (Fallopia japonica).Invasive Plant Science and Management8(2):250‑253. https://doi.org/10.1614/ipsm-d-14-00046.1
- Invasion by Fallopia japonica increases topsoil mineral nutrient concentrations.Ecoscience14(2):230‑240. https://doi.org/10.2980/1195-6860(2007)14[230:ibfjit]2.0.co;2
- Fike J (1999) Terrestrial and Palustrine Plant Communities of Pennsylvania.Pennsylvania Department of Conservation and Natural Resources,Harrisburg, Pennsylvania, USA.
- Sexual reproduction in the invasive species Fallopia japonica (Polygonaceae).American Journal of Botany90(4):586‑592. https://doi.org/10.3732/ajb.90.4.586
- Exotic invasive knotweeds (Fallopia spp.) negatively affect native plant and invertebrate assemblages in European riparian habitats.Biological Conservation141(3):646‑654. https://doi.org/10.1016/j.biocon.2007.12.009
- Gover A, Johnson J, Kuhns L (2005) Managing Japanese knotweed and giant knotweed on roadsides. Roadside Vegetation Management Factsheet 5.Pennsylvania State University,State College, Pennsylvania, USA.
- Gover A, Johnson J, Lloyd K, Sellmer L (2008) Invasive Plant Species Management QuickSheet 1.Pennsylvania State University,State College, Pennsylvania, USA.
- Genetic diversity and clonal vs. sexual reproduction in Fallopia spp. (Polygonaceae).American Journal of Botany94(6):957‑964. https://doi.org/10.3732/ajb.94.6.957
- Reduction of riparian arthropod abundance and diversity as a consequence of giant reed (Arundo donax) invasion.Biological Invasions5(3):167‑177. https://doi.org/10.1023/a:1026190115521
- Vulnerability of riparian zones to invasion by exotic vascular plants.Plant Ecology148(1):105‑114. https://doi.org/10.1023/a:1009800327334
- Changes in different trophic levels of litter-dwelling macrofauna associated with giant knotweed invasion.Ecosystems10(5):734‑744. https://doi.org/10.1007/s10021-007-9052-9
- Kenkel N (1997) Demography of clonal Ostrich fern (Matteucia struthiopteris): a five year summary.UFS (Delta Marsh) Annual Report, Vol. 32
- Stream ecosystems respond to riparian invasion by Japanese knotweed (Fallopia japonica).Canadian Journal of Fisheries and Aquatic Sciences64(9):1273‑1283. https://doi.org/10.1139/f07-092
- Effects of forest age and disturbance on population persistence in the understory herb, Arisaema triphyllum (Araceae).Plant Ecology (formerly Vegetatio)172(1):73‑82. https://doi.org/10.1023/b:vege.0000026036.32013.7a
- Locandro R (1973) Reproduction ecology of Fallopia japonica.Rutgers University,New Brunswick, New Jersey, USA.
- Are invasive species the drivers or passengers of change in degraded ecosystems?Ecology86(1):42‑55. https://doi.org/10.1890/04-0669
- Green frogs show reduced foraging success in habitats invaded by Japanese knotweed.Biodiversity and Conservation14(12):2901‑2911. https://doi.org/10.1007/s10531-004-0223-0
- ellipse: Functions for drawing ellipses and ellipse-like confidence regions.0.3-8.CRAN. URL: http://CRAN.R-project.org/package=ellipse
- Invasive knotweed affects native plants through allelopathy.American Journal of Botany98(1):38‑43. https://doi.org/10.3732/ajb.1000135
- The ecology of interfaces: riparian zones.Annual Review of Ecology and Systematics28(1):621‑658. https://doi.org/10.1146/annurev.ecolsys.28.1.621
- vegan: Community Ecology Package.2.0-10.CRAN. URL: http://CRAN.R-project.org/package=vegan
- Invasibility of species-rich communities in riparian zones.Conservation Biology10(2):598‑607. https://doi.org/10.1046/j.1523-1739.1996.10020598.x
- Potential phytotoxic and shading effects of invasive Fallopia (Polygonaceae) taxa on the germination of native dominant species.NeoBiota9:31‑47. https://doi.org/10.3897/neobiota.9.1266
- Vegetative regeneration in invasive Reynoutria (Polygonaceae) taxa: the determinant of invasibility at the genotype level.American Journal of Botany90(10):1487‑1495. https://doi.org/10.3732/ajb.90.10.1487
- R: A language and environment for statistical computing.R Foundation for Statistical Computing, Vienna, Austria. URL: http://www.R-project.org
- What attributes make some plant species more invasive?Ecology77(6):1655‑1661. https://doi.org/10.2307/2265768
- Plants of Pennsylvania, An Illustrated Manual.2nd.University of Pennsylvania Press,Philadelphia, Pennsylvania, USA.
- An evaluation of mechanisms preventing growth and survival of two native species in invasive Bohemian knotweed (Fallopia xbohemica, Polygonaceae).American Journal of Botany94(5):776‑783. https://doi.org/10.3732/ajb.94.5.776
- Response of ground-dwelling beetle (Coleoptera) assemblages to giant knotweed (Reynoutria spp.) invasion.Biological Invasions10(4):381‑390. https://doi.org/10.1007/s10530-007-9137-6
- Community and ecosystem consequences of giant knotweed (Polygonum sachalinense) invasion into riparian forests of western Washington, USA.Biological Conservation142(7):1536‑1541. https://doi.org/10.1016/j.biocon.2009.02.023
- Habitat factors and species' traits influence riparian community recovery following removal of Bohemian knotweed (Polygonum x bohemicum).Northwest Science88(3):246‑260. https://doi.org/10.3955/046.088.0307
- USGS current water data for Pennsylvania.US Department of the Interior and US Geological Survey. Release date:2016-1-04. URL: http://waterdata.usgs.gov/pa/nwis/uv?site_no=01553500
- ggplot2: elegant graphics for data analysis.CRAN. URL: https://cran.r-project.org/web/packages/ggplot2
- Data files for: Understory dominance and the new climax: Impacts of Japanese knotweed (Fallopia japonica) invasion on native plant diversity and recruitment in a riparian woodland.Knowledge Network for Biocomplexity. URL: https://knb.ecoinformatics.org/#view/doi: 10.5063/F19Z9310
- Polygonum cuspidatum (Polygonaceae) genetic diversity in a small region of Eastern Kentucky.Journal of the Kentucky Academy of Science68(1):89‑95. https://doi.org/10.3101/1098-7096(2007)68[89:pcpgdi]2.0.co;2
- Zimmerman E (2011) Silver Maple Floodplain Forest Factsheet.Pennsylvania Natural Heritage ProgramURL: http://www.naturalheritage.state.pa.us/Community.aspx?=16026