|
Biodiversity Data Journal :
Single Taxon Treatment
|
|
Corresponding author: David J Merritt (d.merritt@uq.edu.au)
Academic editor: Yasen Mutafchiev
Received: 26 Sep 2017 | Accepted: 16 Nov 2017 | Published: 22 Nov 2017
© 2017 James Dorey, David Merritt
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Dorey JB, Merritt DJ (2017) First observations on the life cycle and mass eclosion events in a mantis fly (Family Mantispidae) in the subfamily Drepanicinae. Biodiversity Data Journal 5: e21206. https://doi.org/10.3897/BDJ.5.e21206
|
|
Abstract
Background
The Mantispidae are a distinctive group of Neuroptera known for the adults’ possession of raptorial forelegs. There are four recognised, extant subfamilies of Mantispidae: the Mantispinae, Symphrasinae, Calomantispinae and Drepanicinae. The life history and larval behaviour of the subfamily Mantispinae is best known: the immatures are spider egg predators. Among the three remaining subfamilies, larval Symphrasinae and Calomantispinae most likely predate on other small arthropods, while the immature life history of Drepanicinae, until now, remained completely unknown.
New information
Here we provide observations of annual, near-synchronised, mass emergences of adults of the drepanicine, Ditaxis biseriata (Westwood), within a well-established Macadamia orchard in northern New South Wales, Australia. A female deposited fertile eggs, allowing this first report of egg batch and first instar morphology. The mass emergence of mobile pharate adults from the ground was observed in the same month in two consecutive years. The pharates climbed tree-trunks for a distance before undergoing eclosion. The newly-hatched first instar larvae are campodeiform and prognathous; a typical morphology among Mantispidae. After hatching, they drop to the ground and burrow into soil. They are unpigmented and appear to lack stemmata. Together, the observations infer that the immature component of the life cycle takes place underground in forested habitats. If this feature is common among the Drepanicinae, it might explain why so little is known of the biology of the immature stages.
Keywords
mantis flies, campodeiform larvae, Mantispidae, Drepanicinae
Introduction
The Neuroptera is one of the most ancient orders of insects that show complete metamorphosis. Larvae are typically predaceous with elongate, slender mouthparts that are adapted for piercing and sucking (
Compared to Mantispinae, knowledge of the morphology, biology and ecology of immatures of the other subfamilies of Mantispidae is “sketchy and fragmentary” (
This report provides information on the motility of pharate adults and adult eclosion behaviour of the Australian drepanicine mantispid, Ditaxis biseriata and the morphology of first instar larvae that emerged from a batch of deposited eggs is described. The observations were made possible by mass adult eclosion events occurring in a Macadamia orchard in northern New South Wales, Australia, at the same time of year over two consecutive years.
Materials and methods
Location
The study site was a Macadamia orchard, planted in 1988, located near Newrybar, NSW, Australia. It is located in a region of New South Wales colloquially known as “the Big Scrub”. Prior to European settlement, the region encompassed the largest area of subtropical rainforest in Australia. In the 19th century, extensive timber-getting and clearing for agriculture took place; consequently, remnant rainforest is patchy and a number of rainforest regeneration projects are underway. The region is recognised as a biodiversity hotspot by the Australian Government Department of Environment and Energy. Parts of the orchard have a closed canopy and moist soil with abundant moss and other epiphytes on the macadamia trees. The northwest corner of the orchard has the most complete canopy, most epiphytes and dampest soil. The orchard where D. biseriata were observed was sprayed with pesticide (dipterex 500 sl) between 11th and 16th September 2015. This appeared to have no effect on subsequent emergences of pharate adults.
Observations of adult eclosion were made between 5th to 22nd September 2015 and from 17th to 24th September 2016. Mature adults and pharate adults were collected on and after 5th September 2015.
Methods
Some adults were kept in captivity (in petri dishes or insect containers) while others were killed and pinned. Pharate adults were preserved in ethanol.
Both field and studio photographs were taken, with studio images being of both live and preserved specimens. To record larval morphology, ethanol-preserved first instars were washed in water and cleared in KOH before slide-mounting and photomicroscopy.
For the timelapse observations of adult eclosion, a Canon EOS 60D dSLR camera with 100 mm macro lens was mounted on a tripod and focused on a pharate adult and 759 images were taken over a period of 43 minutes. The camera was controlled by an external remote control and a canon speedlite 580EX II was used to light the specimen. A video was produced comprising of all 759 images played at 25 frames per second.
Taxon treatment
Ditaxis biseriata
-
scientificName: Ditaxis biseriata; taxonID:urn:lsid:biosci.ohio-state.edu:osuc_names:275502; kingdom:Animalia; phylum:Arthropoda; class:Insecta; order:Neuroptera; family:Mantispidae; genus:Ditaxis; country:Australia; stateProvince:New South Wales; locality:Newrybar; verbatimElevation:11 m; locationRemarks:label transliteration: "Newrybar, NSW, 28˚43'52.0"S 153˚33'18.8"E, J.B.Dorey, 05/09/2015, JD1DB"; verbatimCoordinates:28˚43'52.0"S 153˚33'18.8"E; decimalLatitude:-28.731111; decimalLongitude:153.555222; georeferenceProtocol:label; samplingProtocol:sweeping; individualCount:1; lifeStage:adult; catalogNumber:JD1DB; recordedBy:James B Dorey; associatedMedia:http://www.jamesdoreyphotography.com.au/Nonpublic-galleries/Mantispids/n-s3NpJH/; identifiedBy:Kevin Lambkin; dateIdentified:2017; language:en; collectionID:JD1DB; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Ditaxis biseriata; taxonID:urn:lsid:biosci.ohio-state.edu:osuc_names:275502; kingdom:Animalia; phylum:Arthropoda; class:Insecta; order:Neuroptera; family:Mantispidae; genus:Ditaxis; country:Australia; stateProvince:New South Wales; locality:Newrybar; verbatimElevation:11 m; locationRemarks:label transliteration: "Newrybar, NSW, 28˚43'52.0"S 153˚33'18.8"E, J.B.Dorey, 05/09/2015, JD2DB"; verbatimCoordinates:28˚43'52.0"S 153˚33'18.8"E; decimalLatitude:-28.731111; decimalLongitude:153.555222; georeferenceProtocol:label; samplingProtocol:sweeping; individualCount:1; lifeStage:adult; catalogNumber:JD2DB; recordedBy:James B Dorey; associatedMedia:http://www.jamesdoreyphotography.com.au/Nonpublic-galleries/Mantispids/n-s3NpJH/; identifiedBy:Kevin Lambkin; dateIdentified:2017; language:en; collectionID:JD2DB; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Ditaxis biseriata; taxonID:urn:lsid:biosci.ohio-state.edu:osuc_names:275502; kingdom:Animalia; phylum:Arthropoda; class:Insecta; order:Neuroptera; family:Mantispidae; genus:Ditaxis; country:Australia; stateProvince:New South Wales; locality:Newrybar; verbatimElevation:11 m; locationRemarks:label transliteration: "Newrybar, NSW, 28˚43'52.0"S 153˚33'18.8"E, J.B.Dorey, 05/09/2015, JD3DB"; decimalLatitude:-28.731111; decimalLongitude:153.555222; georeferenceProtocol:label; samplingProtocol:As pharat adult; individualCount:1; lifeStage:adult; catalogNumber:JD3DB; recordedBy:James B Dorey; associatedMedia:http://www.jamesdoreyphotography.com.au/Nonpublic-galleries/Mantispids/n-s3NpJH/; identifiedBy:Kevin Lambkin; dateIdentified:2017; language:en; collectionID:JD3DB; collectionCode:Insects; basisOfRecord:PreservedSpecimen
Description
A description of the subject of this study, Ditaxis biseriata, is provided by
Diagnosis
Ditaxis biseriata was distinguished from its sister species, Ditaxis meridiei, by characters of the adult vertex and colouration of the sclerites (
Distribution
The observation and collection site used in this study is within the distribution area described by
Biology
Pharate adults and eclosion behavior
The location within the orchard with the highest density of D. biseriata is wetter and has a more complete canopy then the rest of the orchard and is close to a 30-year old regenerated rainforest patch. Evening observations in early September found between one and five D. biseriata eclosing on most trees, while later in September very few were found, indicating that the mass eclosion is probably restricted to a few weeks of the year. Upon searching, eclosing adults were found in the regenerated rainforest patch near the Macadamia orchard, but at much lower densities.
Pharate adults (Fig.
Four steps in the process of eclosion are depicted in Fig.
Eclosion of Ditaxis biseriata. The images, from bottom to top, show a pharate stage when it becomes stationary before eclosion begins (bottom), an adult in the process of extracting the wings and legs from the pupal case, an adult with its legs free of the pupal case and (top) an adult with its wings in the splayed condition progressively becoming transparent.
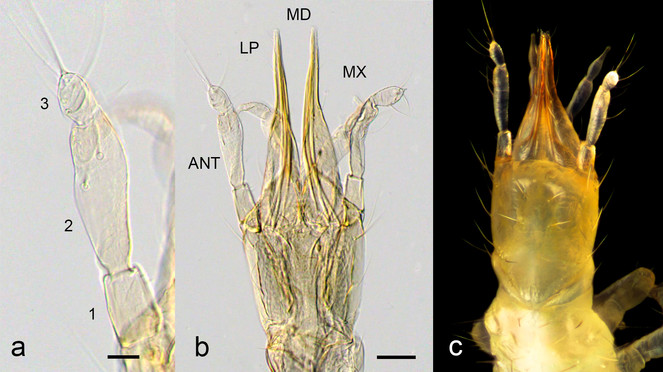
Larval head of Ditaxis biseriata. a and b. Cleared whole-mount of first instar larval antenna (a) and head (b). The antennal segments are numbered from proximal to distal. The head structures depicted in (b) include the antennae (ANT), labial palps (LP), mandibles (MD) and maxillae (MX). c. Dorsal view of the head of a preserved first instar larva. Scale bar in (a) is 20 microns and in (b) 50 microns.
Eggs and first instar larvae
An adult female collected on 5th September 2015 laid c. 120 eggs attached to the lid of a petri dish two days after collection. The lime-green eggs, each c. 0.83 mm long, were deposited in a cluster suspended on a cord of diffuse silken threads (Fig.
The larvae are campodeiform, approximately 2 mm long, generally unsclerotised and creamy-white to light brown in colour. The mouthparts and parts of the head capsule are lightly sclerotized. The head capsule is longer than it is broad with distinctive prognathous mouthparts and anteriorly-directed antennae and labial palps (Fig.
The larval antennae are 3-segmented with a lobed terminal segment (Fig.
Discussion
Mass eclosion
As far as the authors are aware, mass, near-synchronous emergence of mantispids is a previously unreported phenomenon. It is uncertain if the emergence in such large numbers is a natural occurrence or an artefact of monoculture farming. Adults and exuviae were observed in other local Macadamia orchards at around the same time as the emergence near Newrybar (pers. comm. Jarah Coates and Ken Dorey) and emergence was observed simultaneously in nearby regenerated rainforest, suggesting that emergence is highly seasonal over the local area, perhaps through detection of photoperiod or some attribute of their yet-unknown food source, and that the life cycle covers a single year. Further, the presence in rainforest as well as in a location within the orchard with high soil moisture, dense canopy cover and numerous epiphytes hints at a rainforest association. The much higher prevalence of emergence in the Macadamia orchard compared to nearby regenerating rainforest could be due to more favourable conditions in the Macadamia orchard. It is possible that the coordinated emergence of D. biseriata facilitates mating because at least one of the females collected at the site produced fertile eggs. The species might not just be associated with rainforest; reports suggest that it is also common at the same time of year in open eucalyptus forest.
Pharate adult behaviour
Pharate adults are remarkably mobile. They use negative geotaxis, possibly combined with visual orientation, to detect and move upward on a tree-trunk or other vertical object before becoming immobile and initiating eclosion behaviour. In Mantispidae, pupation generally takes place within a silken cocoon in a concealed location and the pharate adults leave the cocoon and walk some distance away before eclosing (
Eggs and larvae
The cluster of eggs suspended on silken threads is similar to the egg batch deposited by Lomamyia lattipennis (Berothidae) (
Together, the behaviour and morphology of larvae, including their lack of stemmata, their tendency to drop from the egg and burrow into soil, and the subterranean origin of the pharate adults, support the premise that the larval and pupal stages of D. biseriata are subterranean. If this life history was common to all or most Drepanicinae, it could explain the lack of historical knowledge about the immature stages of the subfamily. The larval diet remains unknown; they could be subterranean arthropod predators like the subfamilies Symphrasinae and Calomantispinae or spider-egg predators like their sister sub-family, Mantispinae. A clue, at least in this species, might lie in the apparent preference for moist soil and the rainforest association. Future studies could focus on taking soil samples in the orchard to determine what potential prey or host species are present and possibly to detect larvae or pupae in situ.
Acknowledgements
The authors would like to thank Kevin Lambkin for help in identification and alerting us to the significance of the findings. Further thanks goes to Ken Dorey and Jarrah Coates for sharing their knowledge and sightings from the region as well as Christina Elmer, Amelia Carlson and Matthew Elmer for aiding in the search for mantipsids and early discussion of their biology.
Author contributions
James Dorey observed, collected and photographed specimens and carried out timelapse photography. David Merritt cleared and whole-mounted larval bodies and took photomicrographs. Both authors contributed to writing the text and producing the figures.
References
- The phylogeny of the Neuropterida: long lasting and current controversies and challenges (Insecta: Endopterygota).Arthropod Systematics & Phylogeny70(2):119‑129.
- Bionomics of Lomamyia hamata (Neuroptera: Berothidae).Annals of the Entomological Society of America80(5):671‑679. https://doi.org/10.1093/aesa/80.5.671
- Observations on the biology of Anchieta fumosella (Westwood 1867)(Neuroptera Mantispidae) from Brazil.Tropical Zoology21(1):91‑95.
- Reproductive behaviour of Trichoscelia santareni (Navas) (Neuroptera: Mantispidae) and parasitization of the colonies of Polybia diguetana R. du Buysson (Hymenoptera: Vespidae).Neuroptera International6(1):19‑26.
- Notes on Dilaridae and Berothidae, with special reference to the immature stages of the Nearctic genera (Neuroptera).Psyche54(3):145‑169. https://doi.org/10.1155/1947/78317
- Descriptions of the larvae and pupae of some North American Mantispinae (Neuroptera: Mantispidae) and development of a system of larval chaetotaxy for Neuroptera.Transactions of the American Entomological Society11:159‑196.
- First fossil larvae of Berothidae (Neuroptera) from Baltic amber, with notes on the biology and termitophily of the family.Zootaxa3716(2):236‑258. https://doi.org/10.11646/zootaxa.3716.2.6
- New fossil mantidflies (Insecta: Neuroptera: Mantispidae) from the Mesozoic of north‐eastern China.Palaeontology56(3):603‑613. https://doi.org/10.1111/pala.12005
- Development and behavior of a snakefly, Raphidia bicolor Albarda (Neuroptera: Raphidiidae).Southwestern Entomologist16:353‑364.
- Phylogeny of endopteryygote insects, the most successful lineage of living organisms.European Journal of Entomology96:237‑254.
- A revision of the Australian Mantispidae (Insecta: Neuroptera) with a contribution to the classification of the family. I. General and Drepanicinae.Australian Journal of Zoology Supplementary Series34(116):1‑142. https://doi.org/10.1071/AJZS116
- A new genus of mantidflies discovered in the Oriental region, with a higher‐level phylogeny of Mantispidae (Neuroptera) using DNA sequences and morphology.Systematic Entomology40(1):183‑206. https://doi.org/10.1111/syen.12096
- Larval platymantispine mantispids (Neuroptera: Planipennia): possibly a subfamily of generalist predators.Neuroptera International2:37‑41.
- Out with the garbage: the parasitic strategy of the mantisfly Plega hagenella mass-infesting colonies of the eusocial bee Melipona subnitida in northeastern Brazil.Naturwissenschaften100(1):101‑105. https://doi.org/10.1007/s00114-012-0994-1
- Annotated catalog of the Mantispidae of the world (Neuroptera).Contributions on Entomology, International5(3):129‑264.
- Systematic and biological notes on the tribe Platymantispini (Neuroptera: Mantispidae) and the description of a new species of Plega from Mexico.The Canadian Entomologist97(6):604‑612. https://doi.org/10.4039/Ent97604-6
- Biology of the Mantispidae.Annual Review of Entomology43(1):175‑194. https://doi.org/10.1146/annurev.ento.43.1.175
- The developmental ecology of Mantispa uhleri Banks (Neuroptera: Mantispidae).University of Illinois Press,Champaign, Illinois. [ISBN025201085X]
- Lomamyia latipennis (Neuroptera: Berothidae) life history and larval descriptions.The Canadian Entomologist100(6):623‑629. https://doi.org/10.4039/Ent100623-6
- Tauber CA, Tauber MJ, Albuquerque GS (2003) Neuroptera (Lacewings, Antlions). In: Resh V, Carde R (Eds) Encyclopedia of Insects.Academic Press,13pp. https://doi.org/10.1016/B978-0-12-374144-8.00190-9
- Observations on Lomamyia latipennis, with a description of the first instar larva.Pan-Pacific Entomologist40:21‑26.
- First fossil larvae of Berothidae (Neuroptera) from Baltic amber, with notes on the biology and termitophily of the family.Zootaxa3716(2):236‑258. https://doi.org/10.11646/zootaxa.3716.2.6
- On wings of lace: phylogeny and Bayesian divergence time estimates of Neuropterida (Insecta) based on morphological and molecular data.Systematic Entomology35(3):349‑378. https://doi.org/10.1111/j.1365-3113.2010.00521.x