|
Biodiversity Data Journal :
Single Taxon Treatment
|
|
Corresponding author: Rachael M Wade (rmwade@hawaii.edu)
Academic editor: Andreas Beck
Received: 12 Oct 2017 | Accepted: 02 Oct 2018 | Published: 05 Oct 2018
© 2018 Rachael Wade, Heather Spalding, Kimberly Peyton, Kevin Foster, Thomas Sauvage, Matthew Ross, Alison Sherwood
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Wade R, Spalding H, Peyton K, Foster K, Sauvage T, Ross M, Sherwood A (2018) A new record of Avrainvillea cf. erecta (Berkeley) A. Gepp & E. S. Gepp (Bryopsidales, Chlorophyta) from urbanized estuaries in the Hawaiian Islands. Biodiversity Data Journal 6: e21617. https://doi.org/10.3897/BDJ.6.e21617
|
|
Abstract
Background
A second species in the siphonous green algal genus Avrainvillea was recently discovered off the island of O‘ahu in the Main Hawaiian Islands. Specimens were collected from Honolulu Harbor, including its entrance channel, and near Ke‘ehi Harbor. These locations are both in Mālama Bay on O‘ahu’s south shore in or adjacent to urbanized estuaries, respectively. In situ observations, morphological and molecular assessments were conducted to examine the alga’s habit and distribution, as well as to assess its putative species identification.
New information
The alga occurred in sand as single individuals or in clusters of several individuals at both sites, and near or within seagrass beds (Halophila decipiens) and algal meadows composed of the green alga Halimeda kanaloana and an unidentified Udotea species at the Ke‘ehi Harbor site. All analyses supported both populations as representative of the same taxa, reported until further investigation in the broad Pacific as Avrainvillea cf. erecta based on morphological and molecular analyses. This record of a second Avrainvillea species in Hawai'i is of particular concern considering that an alga recognized as A. amadelpha, first observed in 1981 from two locales on O‘ahu’s south shore, has become invasive in Hawai‘i’s intertidal to mesophotic environments.
Keywords
Avrainvillea, Bryopsidales, Chlorophyta, estuary, Hawai‘i, invasive, rbcL, seagrass, tufA
Introduction
The siphonous green algal order Bryopsidales includes over 500 extant species (
An unknown species of Avrainvillea was first documented on the western shore of O‘ahu in 1981. By 1985, the alga had spread to the inter- and subtidal environments of O‘ahu’s south shores. This distribution was documented by
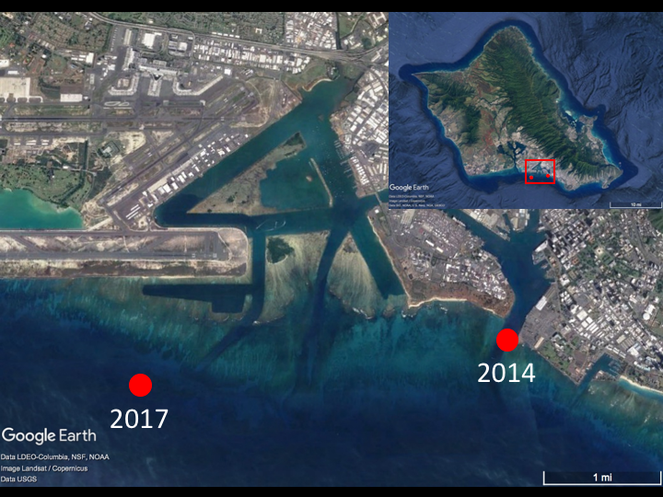
More recently, a population of a second Avrainvillea species, distinct in habit from "A. amadelpha", was discovered on October 14-16, 2014 in Honolulu Harbor, including its entrance channel and turning basin, from 12-15 m depths (Fig.
Avrainvillea cf. erecta occurrence map. Specimens were collected from the Honolulu Harbor entrance channel in 2014 during a regular seagrass survey by the U.S. Fish and Wildlife Service and the Hawai‘i Department of Land and Natural Resources, Division of Aquatic Resources and again near Ke‘ehi Harbor in 2017 during a non-research dive by M. Ross.
Materials and methods
In situ observations
A quantitative seagrass community survey using SCUBA from 12-18m depth was conducted jointly by the U.S. Fish and Wildlife Service and State of Hawai‘i Department of Land and Natural Resources - Division of Aquatic Resources in Honolulu Harbor from October 14-16, 2014 as part of regular benthic surveys in preparation of scheduled dredging. Field data were collected in the planned dredge footprint at eight locations within the turning basin and entrance channel, locations which are referred to as "Impact Sites" (Suppl. material
Specimens were also discovered on April 22, 2017 offshore of Ke‘ehi Lagoon, south O‘ahu using SCUBA at 25-40 m depths. Subsequent qualitative SCUBA surveys were conducted at 20-30 m depths at three sites near the original collection site on May 18, 2017 to make qualitative observations regarding its habitat and associated organisms.
Morphological characterization
Two specimens collected in 2014 (BISH768338-9) and six collected in 2017 (BISH768278-83) that included what appeared to be mature and juvenile forms or possibly ecotypes were selected for morphological and molecular characterization (Suppl. material
Molecular assessment
In addition to the specimens used for morphological assessment, two type specimens of heterotypic synonyms of Avrainvillea erecta (Chloroplegma papuanum Zanardini and Rhipilia andersonii G. Murray) were borrowed from the Natural History Museum of London and included in our molecular assessment (morphological assessment, and therefore additional destructive sampling, was not permitted). DNA extraction was completed using the OMEGA E.Z.N.A® Plant DNA Kit (OMEGA bio-tek, Norcross, GA U.SA.). For the two type specimens, the protocol developed by
Taxon treatment
Avrainvillea erecta
-
scientificName: Avrainvillea cf. erecta; kingdom:Plantae; phylum:Chlorophyta; class:Ulvophyceae; order:Bryopsidales; family:Dichotomosiphonaceae; genus:Avrainvillea; specificEpithet:erecta; scientificNameAuthorship:(Berkeley) A. Gepp & E.S. Gepp; country:USA; municipality:Honolulu; locality:Mālama Bay, seaward of Ke‘ehi Lagoon; minimumDepthInMeters:25; maximumDepthInMeters:40; decimalLatitude:21.29; decimalLongitude:157.9205; georeferenceProtocol:GPS; eventDate:Apr-22-2017; individualCount:6; catalogNumber:BISH768278-83; recordedBy:Matthew Ross; otherCatalogNumbers:ARS09414,-09417,-09418, -09429,-09431,-09432; associatedSequences:MF872080-85, MF872105-110; identifiedBy:Rachael M. Wade; dateIdentified:May-2017; identificationReferences:Olsen-Stojkovich 1985; language:en; basisOfRecord:PreservedSpecimen
-
scientificName: Avrainvillea cf. erecta; kingdom:Plantae; phylum:Chlorophyta; class:Ulvophyceae; order:Bryopsidales; family:Dichotomosiphonaceae; genus:Avrainvillea; specificEpithet:erecta; scientificNameAuthorship:(Berkeley) A. Gepp & E.S. Gepp; country:USA; municipality:Honolulu; locality:Mālama Bay, Honolulu Harbor; minimumDepthInMeters:12; maximumDepthInMeters:15; decimalLatitude:21.30; decimalLongitude:157.8689; georeferenceProtocol:GPS; eventDate:Oct-15-2015; individualCount:2; catalogNumber:BISH768338-9; recordedBy:Kimberly Peyton, Kevin Foster, Paul Murakawa; otherCatalogNumbers:ARS09436-7; associatedSequences:MF969093-6; identifiedBy:Rachael M. Wade; dateIdentified:Aug-2017; identificationReferences:Olsen-Stojkovich 1985; language:en; basisOfRecord:PreservedSpecimen
-
scientificName: Avrainvillea erecta; originalNameUsage:Chloroplegma papuanum Zanaradini 1878; acceptedNameUsage:Avrainvillea erecta Gepp & Gepp 1911; taxonomicStatus:heterotypic synonym; kingdom:Plantae; phylum:Chlorophyta; class:Ulvophyceae; order:Bryopsidales; family:Dichotomosiphonaceae; genus:Avrainvillea; specificEpithet:erecta; waterBody:Pacific Ocean; country:Indonesia; stateProvince:Papua; year:1872; month:May; fieldNotes:Collected by Odoardo Beccari; associatedSequences:MH938452; identifiedBy:Zanardini; dateIdentified:1878; institutionID:BM000561613; basisOfRecord:PreservedSpecimen
During the 2014 seagrass community survey, the newly discovered Avrainvillea sp. was observed at six of 16 survey sites in the Honolulu Harbor entrance channel from 12-15m depths (four "Control Sites", two "Impact Sites"; Suppl. material
In 2017, the newly recorded Avrainvillea sp. was observed as single individuals or in patches with 10-20 individuals per m2 (estimated visually) in areas with deep sand (Fig.
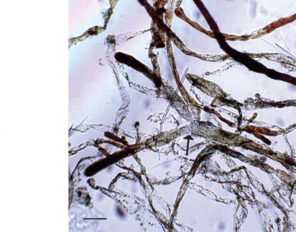
A-E: Avrainvillea cf. erecta habit and diagnostic features from O‘ahu in the Main Hawaiian Islands; F: Chloroplegma papuanum.
b: (indicated by arrow) amongst other psammophytic macroalgae, including the green algae Udotea sp. and Halimeda kanaloana.
c: Fresh specimen of (BISH768278) exhibiting the slightly eroded margin of loose siphons, sub-reniform blade, and well-developed holdfast. Scale = 2 cm.
d: Dried specimen of (BISH768278) exhibiting the fulvous coloration and light radial zonation. Scale = 2 cm.
e: Siphons showing cylindrical to slightly torulose shape, acute constriction above the dichotomy (arrow), and fulvous coloration, particularly towards the apices. Scale = 100 micrometers.
f: Press of the Chloroplegma papuanum type specimen (BM000561613), a heterotypic synonym of Avrainvillea erecta. Scale = 2 cm.
The specimens were olive-green upon collection and dried to a darker green with fulvous, or tawny, coloration (Fig.
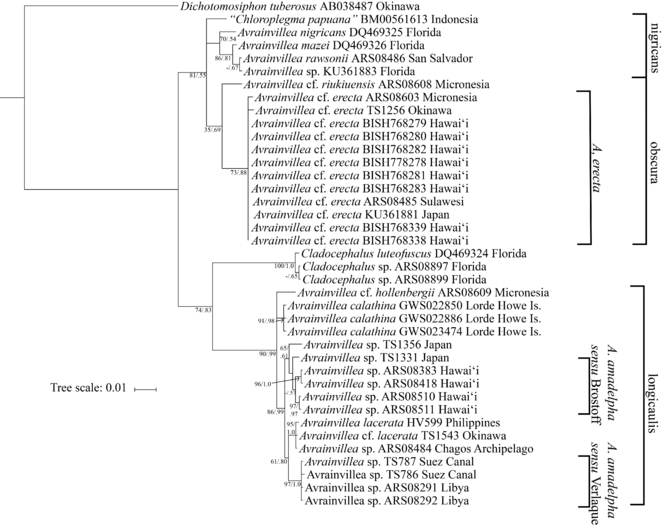
The majority of examined specimens were sequenced for both rbcL and tufA, however, molecular characterization of historical material was only successful for rbcL for one of the heterotypic synonym type specimens - Chloroplegma papuanum BM000561613. The concatenated alignment of the two gene regions yielded a dataset of 1,360 bp. Both the Maximum Likelihood and Bayesian inference phylogenetic reconstructions strongly supported that the newly recorded Avrainvillea species, A. cf. erecta, was clearly distinct from Hawai‘i specimens identified as “A. amadelpha” (
Bayesian inferred phylogeny from the rbcL and tufA concatenated alignment. The leftmost clade notations specify the two Avrainvillea spp. found in Hawai‘i, the righmost clade notations specify the Avrainvillea groups described by
Discussion
The morphological and molecular characterization of the newly recorded Avrainvillea species showed most affinities to the description of A. erecta based on stipe length, blade habit, siphon width and morphology (constriction at dichotomy); however considering that we could not obtain material from type locality or the basionym type specimen (Dichonema erectum Berkeley 1842), we temporarily consider the newly recorded species as A. cf. erecta until further research can be conducted (Suppl. material
Avrainvillea cf. erecta was not observed at the Honolulu Harbor sites when it was resurveyed in March 2016, and reduced Halophila decipiens cover was found as compared to 2014. This is most likely due to scheduled dredging that occurs approximately every 15 years. Interestingly, Halimeda kanaloana was observed at one site where it was absent two years previously. Soft bottom assemblages in the urbanized estuary are subject to disturbance, including naturally occurring factors like storms as well as anthropogenic forces like dredging that result in light attenuation (
The site examined near Ke‘ehi Lagoon has historically been dominated by H. decipiens (M. Ross, unpublished data). However, during the past two years, H. kanaloana has begun to appear, and in many places, is now one of the dominant species. Similarly, Udotea sp. was only observed for the first time in the area earlier in 2017. Based on these observations, this habitat may be undergoing significant shifts in species composition, in which A. cf. erecta is playing a part (M. Ross, unpublished data).
The morphological record for A. erecta (which may include genetically divergent cryptic diversity and is thus to be considered carefully) encompasses the East coast of Africa and the Red Sea to as far as the western Pacific in the waters of New Zealand and several Pacific Islands (
Alternatively, the alga could have arrived as a result of Pacific currents; the Pacific Gyre carries water from Southeast Asia and Japan through the Pacific Ocean north of the Hawaiian Islands to California, and returns to the East Pacific south of the Hawaiian Islands. Additionally, the Equatorial Countercurrent feeds into the gyre, supplying it with water from Australia, New Zealand, and the Pacific Islands (
Given that A. erecta was originally described from specimens collected from 15-36 m (
Acknowledgements
We thank Drs. Gerald Kraft, Daryl Lam, Chris Lane, Gary Saunders, and Tom Schils for provision of both specimens and taxonomic expertise, as well as the Natural History Museum of London for their generous loan of type specimens. We also thank Yue Tang for her early work on “A. amadelpha” and Paul Murakawa for diving and field collection support. Additional thanks to Drs. Anthony Amend, Patrick Krug, Daniel Rubinoff, and Celia Smith for their continued support and advice.
Author contributions
RW conceptualized the study and drafted the manuscript. HS, KP and KF and MR contributed field collections and ecological observations via scuba. RW and TS contributed shallow field collections and molecular sequencing. All authors provided critical revisions and approved the final manuscript.
References
- Till algernes systematik.[To the algal systematics].5,23.Lunds Univ. Arsskr.,174pp.
- Feeding preferences and host associations of specialist marine herbivores align with quantitative variation in seaweed secondary metabolites.Marine Ecology Progress Series396:1‑12. https://doi.org/10.3354/meps08359
- Chemical defenses of the sacoglossan mollusk Elysia rufescens and its host alga Bryopsis sp.Journal of Chemical Ecology27(11):2287‑99. https://doi.org/10.1023/A:1012287105923
- Avrainvillea amadelpha (Codiales, Chlorophyta) from O‘ahu, Hawai‘i.Pacific Science43:166‑169.
- Tsunami-driven rafting: Transoceanic species dispersal and implications for marine biogeography.Science357(6358):1402‑1406. https://doi.org/10.1126/science.aao1498
- Ecological and biological studies of ocean rafting: Japanese tsunami marine debris in North America and the Hawaiian Islands.Aquatic Invasions13(1):1‑9. https://doi.org/10.3391/ai.2018.13.1.01
- Patterns of transoceanic marine biological invasions in the Pacific Ocean.Bulletin of Marine Science41:452‑465.
- Spatial and temporal variation of diverse inter-tidal algal assemblages in Southwest O‘ahu.Marine Ecology38(3):e12429. https://doi.org/10.1111/maec.12429
- Phylogenetic analysis of the large subunit Rubisco gene supports the exclusion of Avrainvillea and Cladocephalus from the Udoteaceae (Bryopsidales, Chlorophyta).Journal of Phycology44:761‑767. https://doi.org/10.1111/j.1529-8817.2008.00519.x
- Essai sur une classification des algues et des polypier calciferes de Lamouroux.Annales des Sciences Naturelles, Botanique, Séries 217:297‑380.
- Biomechanical properties of coenocytic algae (Chlorophyta, Caulerpales).ScienceAsia1:57‑62. https://doi.org/10.2306/scienceasia1513-1874.2006.32(s1).057
- Marine algae (Chlorophyceae and Phaeophyceae) and marine phanerograms of the 'Sealark' expedition, collected by J. Stanley Gardiner, M.A., F.R.S., F.Z.S.Transactions of the Linnean Society of London7(10):178‑180.
- The Codiaceae of the Siboga Expedition including a monograph of Flabellarieae and Udoteae.Siboga-Expeditie Monographie LXII,150pp.
- AlgaeBase. http://www.algaebase.org. Accessed on: 2017-5-30.
- Genetic identification of macroalgal species on Japanese tsunami marine debris and genetic comparisons with their wild populations.Marine Pollution Bulletin132:74‑81. https://doi.org/10.1016/j.marpolbul.2017.06.053
- Chemical Defense Against Different Marine Herbivores: Are Amphipods Insect Equivalents?Ecology68(6):1567‑1580. https://doi.org/10.2307/1939849
- Tropical marine algae: growth in laboratory culture.Journal of Phycology1(2):69‑78. https://doi.org/10.1111/j.1529-8817.1965.tb04560.x
- MRBAYES: Bayesian inference of phylogenetic trees.Bioinformatics17(8):754‑755. https://doi.org/10.1093/bioinformatics/17.8.754
- Solving taxonomic and nomenclatural problems in Pacific Gigartinaceae (Rhodophyta) using DNA from type material.Journal of Phycology37(6):1091‑1109. https://doi.org/10.1046/j.1529-8817.2001.01048.x
- PartitionFinder: Combined Selection of Partitioning Schemes and Substitution Models for Phylogenetic Analyses.Molecular Biology and Evolution29(6):1695‑1701. https://doi.org/10.1093/molbev/mss020
- DNA-based species delimitation in algae.European Journal of Phycology49(2):179‑196. https://doi.org/10.1080/09670262.2014.904524
- Systematics of Avrainvillea (Bryopsidales, Chlorophyta) in the tropical western Atlantic.Phycologia31(5):375‑418. https://doi.org/10.2216/i0031-8884-31-5-375.1
- Seaweed ecology and physiology.Cambridge University Press,Cambridge. https://doi.org/10.1017/cbo9780511626210
- Nitrogen load and irradiance affect morphology, photosynthesis and growth of Caulerpa prolifera (Bryopsidales: Chlorophyta).Marine Ecology Progress Series298:101‑114. https://doi.org/10.3354/meps298101
- Fifth Education and Science Forum Session 4A, Coastal Areas, Wetlands and Oceans.American Meteorological Society,Phoenix, AZ,13/11/2009.
- The seed reserve of Halophila decipiens Ostenfeld (Hydrocharitaceae) in Panama.Aquatic Botany31:177‑182. https://doi.org/10.1016/0304-3770(88)90047-2
- The Introduced Green Alga Caulerpa taxifolia Continues to Spread in the Mediterranean.Biological Invasions3(2):201‑210. https://doi.org/10.1023/a:1014549500678
- Creating the CIPRES Science Gateway for inference of large phylogenetic trees.Proceedings of the Gateway Computing Environments Workshop.2010 Gateway Computing Environments Workshop (GCE),New Orleanshttps://doi.org/10.1109/gce.2010.5676129
- Natural History Museum Occurrence Dataset. https://doi.org/10.5519/0002965. Accessed on: 2017-7-03.
- A systematic study of the genus Avrainvillea Decaisne (Chlorophyta, Udoteaceae).Nova Hedwigia41:1‑68.
- Peyton K (2009) Aquatic invasive species impacts in Hawaiian soft sediment habitats.UMI Dissertation Publishing.
- A morphological and molecular comparison between Elysia crispata and a new species of kleptoplastic sea slug (Gastropoda:Opisthobranchia) from the Florida Keys, USA.Molluscan Research26(1):23‑38. [InEnglish].
- Tracking the invasive history of the green alga Codium fragile ssp. tomentosoides.Molecular Ecology14(1):189‑194. https://doi.org/10.1111/j.1365-294x.2004.02384.x
- MrBayes 3: Bayesian phylogenetic inference under mixed models.Bioinformatics19(12):1572‑1574. https://doi.org/10.1093/bioinformatics/btg180
- The Persistence of Anthropogenic Turbidity Plumes in a Shallow Water Estuary.Estuarine, Coastal and Shelf Science47(5):579‑592. https://doi.org/10.1006/ecss.1998.0366
- Morphological and molecular clarification of the enigmatic Caulerpa floridana W.R. Taylor (Chlorophyta, Bryopsidales) from the Dry Tortugas, Florida.European Journal of Phycology49(3):370‑383. https://doi.org/10.1080/09670262.2014.947330
- Nutrient and growth dynamics of Halimeda tuna on Conch Reef, Florida Keys: Possible influence of internal tides on nutrient status and physiology.Limnology and Oceanography49(6):1923‑1936. https://doi.org/10.4319/lo.2004.49.6.1923
- Spalding H (2012) Ecology of mesophotic macroalgae and Halimeda kanaloana meadows in the Main Hawaiian Islands.UMI Dissertation Publishing.
- RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies.Bioinformatics30(9):1312‑1313. https://doi.org/10.1093/bioinformatics/btu033
- Regional oceanography: an introduction.Elsevier Science Inc.,Tarrytown. https://doi.org/10.1016/B978-0-08-041021-0.50007-2
- Phylogenetic analysis of Pseudochlorodesmis strains reveals cryptic diversity above the family level in the siphonous green algae (Bryopsidales, Chlorophyta).Journal of Phycology45(3):726‑31. https://doi.org/10.1111/j.1529-8817.2009.00690.x
- A multi-locus time-calibrated phylogeny of the siphonous green algae.Molecular Phylogenetics and Evolution50(3):642‑53. https://doi.org/10.1016/j.ympev.2008.12.018
- Introduction of a New Potential Invader into the Mediterranean Sea: The Indo-Pacific Avrainvillea amadelpha (Montagne) A. Gepp & E.S. Gepp (Dichotomosiphonaceae, Ulvophyceae).Cryptogamie, Algologie38(3):267‑281. https://doi.org/10.7872/crya/v38.iss3.2017.267
- The Challenge of Siphonous Green Algae.American Scientist89(6):524. https://doi.org/10.1511/2001.40.748
- Field biology of Halimeda tuna (Bryopsidales, Chlorophyta) across a depth gradient: comparative growth, survivorship, recruitment, and reproduction.Hydrobiologia501:149‑166. https://doi.org/10.1023/A:1026287816324
- Morphological and molecular assessment of Avrainvillea (Ulvophyceae, Chlorophyta) and implications for invasive species identification.Annual Phycological Society of America Meeting,Philadelphia, PA.
- Molecular determination of kleptoplast origins from the sea slug Plakobranchus ocellatus (Sacoglossa, Gastropoda) reveals cryptic bryopsidalean (Chlorophyta) diversity in the Hawaiian Islands.Journal of Phycology53(3):467‑475. https://doi.org/10.1111/jpy.12503
- Updating Plakobranchus cf. ianthobapsus (Gastropoda, Sacoglossa) host use: Diverse algal-animal interactions revealed by NGS with implications for invasive species management.Molecular Phylogenetics and Evolution128:172‑181. https://doi.org/10.1016/j.ympev.2018.07.010
- Rapid rhizoid production in Halimeda discoidea Decaisne (Chlorophyta, Caulerpales) fragments: a mechanism for survival after separation from adult thalli.Journal of Experimental Marine Biology and Ecology175(1):105‑120. https://doi.org/10.1016/0022-0981(94)90178-3
- Flora of drift plastics: a new red algal genus, Tsunamia transpacifica (Stylonematophyceae) from Japanese tsunami debris in the northeast Pacific Ocean.Algae31(4):289‑301. https://doi.org/10.4490/algae.2016.31.10.20
- Demographic feedback between clonal growth and fragmentation in an invasive seaweed.Ecology87(7):1744‑1754. https://doi.org/10.1890/0012-9658(2006)87[1744:dfbcga]2.0.co;2
Supplementary materials
ARS = Sherwood Lab accession number; BISH = Bernice Pauahi Bishop Museum accession number; BM = Natural History Museum of London accession numbers TS = accession numbers of specimens provided by Thomas Sauvage; GWS = accession numbers of specimens provided by Gary Saunders.
Reference species characters retrieved from the descriptions provided by Olsen-Stojkovich (1985). Bolded character text represent character congruence with the newly discovered species from Hawai‘i.