|
Biodiversity Data Journal :
Research Article
|
|
Corresponding author: Véronique Forbes (veroforbes@gmail.com)
Academic editor: David Bilton
Received: 05 Dec 2017 | Accepted: 23 Feb 2018 | Published: 02 Mar 2018
© 2018 Véronique Forbes, Derek Sikes
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Forbes V, Sikes D (2018) A survey of beetles (Coleoptera) from the tundra surrounding the Nunalleq archaeological site, Quinhagak, southwestern Alaska. Biodiversity Data Journal 6: e22788. https://doi.org/10.3897/BDJ.6.e22788
|
|
Abstract
This paper presents the results of a survey of beetles conducted in the vicinity of the archaeological site of Nunalleq, a pre-contact (16th-17th century AD) indigenous forager settlement located near the modern Yup’ik village of Quinhagak, in the Yukon-Kuskokwim delta, southwestern Alaska. Records and habitat data are reported for 74 beetle taxa collected in tundra, riparian, aquatic and anthropogenic environments from a region of Alaska that has been poorly studied by entomologists. This includes the first mainland Alaskan record for the byrrhid Simplocaria metallica (Sturm). Beyond improving our knowledge of the local beetle fauna’s diversity and ecology, this survey provides the basis for comparisons between modern and sub-fossil beetle assemblages from Nunalleq and Quinhagak.
Keywords
Coleoptera, Archaeology, Insect subfossils, Alaska, Yup’ik
Introduction
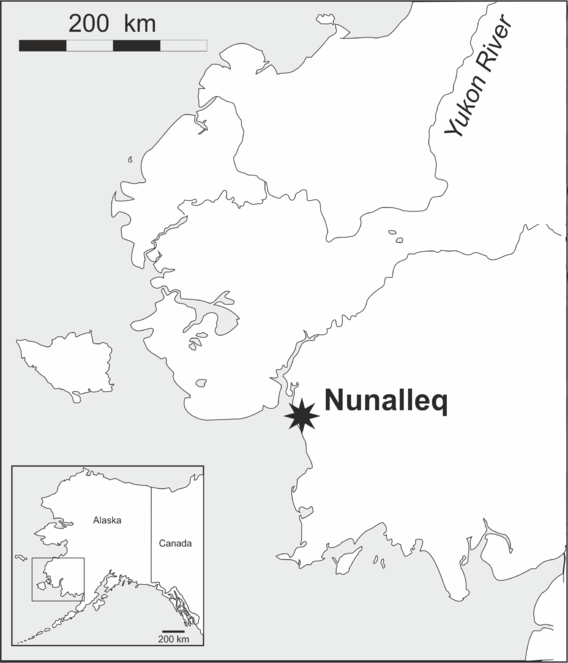
Until recently, the arthropod fauna of Alaska has been comparatively less well studied than that of other states and provinces of Canada and the USA. In part this is caused by the fact that some regions are particularly difficult to access due to their topography, hydrology or remoteness to urban agglomerations. This is the case of the Yukon-Kuskokwim (Y-K) delta, a flat, treeless area of south-western Alaska where the tundra environment is dissected by numerous rivers, streams, lakes and ponds and underlain by discontinuous permafrost. Travel by motorised vehicle is impractical over most of the delta’s expanse, making small boats and planes the most reliable transport means within the area. Karl Lindroth and Georges E. Ball are two of the few entomologists known to have visited the region, when, as part of Lindroth’s seminal study of the ground beetles of Canada and Alaska (
From 2013, one of the team (VF) became engaged in a scientific, community and heritage project involving the excavation of Nunalleq, a pre-contact Thule-era (16th-17th century AD) site located on the Bering Coast of the Yukon-Kuskokwim delta, approximately 20 km south of the Yup’ik village of Quinhagak. One of the objectives of the project was to reconstruct past climatic conditions and human environment-interactions on the basis of ecological information derived from beetle remains preserved in the archaeology (
Material and methods
Fieldwork was conducted during two consecutive field seasons within a 5 km radius of the Nunalleq archaeological site (
List and description of the different habitats sampled in the vicinity of the Nunalleq archaeological site.
|
Habitat |
Description |
Sampling techniques |
|
Open tundra |
Flat tundra with moist to wet ground and low-lying vegetation characterised by herbs (e.g. Eriophorum angustifolium), mosses (e.g. Sphagnum sp., Polytrichium sp.), lichen, heaths and dwarf shrubs (e.g. Ledum palustre, Rubus chamaemorus, Empetrum nigrum, Betula nana). |
Pitfall and interception traps, hand collection, sifting/mini-Winkler |
|
Scrub tundra |
Flat tundra with moist to dry ground and vegetation dominated by dwarf willow (Salix sp.) scrub heaths and shrub (e.g. Empetrum nigrum, Vaccinium vitis-idea, Betula nana, Rubus chamaemorus). |
Pitfall traps, beating vegetation |
|
Aquatic |
Small ponds of stagnant water with Sphagnum mosses and Eriophorum angustifolium at the water edge. |
Dipping net and hand collection |
|
Seashore |
Beach with sandy and clayey soil, some areas with sparse vegetation (e.g. Honckenya peploides, Senecio pseudoarnica, Mertensia maritima, Leymus arenarius). |
Pitfall traps, hand collection |
|
Disturbed/anthropogenic |
Habitats created through disturbance by human activity including spoil heaps, trampled areas and excavation trench of the Nunalleq archaeological site. Vegetation cover is typically sparse and characterised by, but not limited to, species such as Achillea millefolium, Matricaria matricarioides, Rumex graminifolius and Rorippa islandica. This category also includes the interior of modern buildings in Quinhagak. |
Pitfall traps, hand collection |
|
Riparian |
Gravelly bank of the Arolik river, with willow (Salix) trees a few metres away from the water. |
Beating vegetation, dipping net |
Photographs of some of the traps and sampling devices employed.
b: interception trap
c: mini-Winkler extractor (supplied by Sante Traps http://www.santetraps.com/).
Identifications of the beetle taxa were achieved through anatomical comparisons with specimens from the University of Alaska Museum Insect Collection (UAM) in Fairbanks, the Canadian Collection of Insects, Arachnids and Nematodes in Ottawa (CNC) and the Laurentian Forestry Centre’s René-Martineau Insectarium in Quebec City (LFC). For some specimens, such as those belonging to the Staphylinidae subfamilies Aleocharinae, Omaliinae and Staphyliniinae, as well as the Pterostichus subgenus Cryobius Chaudoir, this was facilitated by microdissections to allow observation of the genitalia. Identifications were aided by consultation of identification keys and descriptions in entomology publications (
Information regarding the ecology of individual taxa was compiled from habitat records and descriptions in the literature (
Data resources
Vouchers specimens were donated to UAM, CNC and LFC and the remaining specimens are currently in the care of the first author. Data for specimens that were donated to UAM (accession: UAM-2014.20-Forbes-Ento) can be accessed through the Arctos database using the following link http://arctos.database.museum/saved/QuinhagakColeoptera. The full dataset is archived online and can be accessed at: doi.org/10.6084/m9.figshare.5630296.v1.
Result & Discussion
This survey recovered a total of 500 beetle specimens belonging to 74 different taxa and spanning 15 families (Suppl. material
Thirty-four of the identified taxa may be first records for the Y-K delta region. Many of these were collected in regions adjacent to the Y-K delta (e.g. the Seward, Alaska and Kenai peninsulas as well as central Alaska). This applies to Notiophilus borealis Harris, Elaphrus lapponicus Gyllenhal, Hydroporus lapponum Gyllenhal, H. morio Aubé, H. striola (Gyllenhal), Acidota quadrata (Zetterstedt) and Eucnecosum cf. tenue LeConte). They have probably been established in the Y-K delta for a long time, but perhaps were never collected before simply due to geographical sampling bias.
This survey also produced several records of Amara alpina (Paykull). This species is generally considered an indicator of cold climates in palaeo-entomological studies (
Three dytiscid specimens were identified as Ilybius angustior (Gyllenhal) complex. These appear to be closely related to the species I. angustior, a Holarctic species occuring in still water with abundant vegetation (
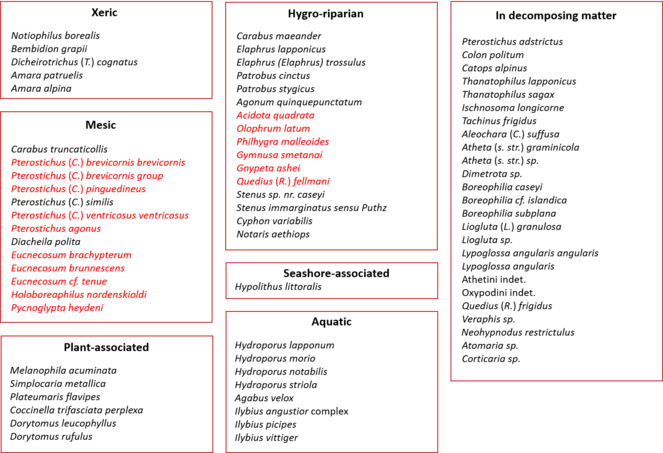
Ecological grouping of taxa
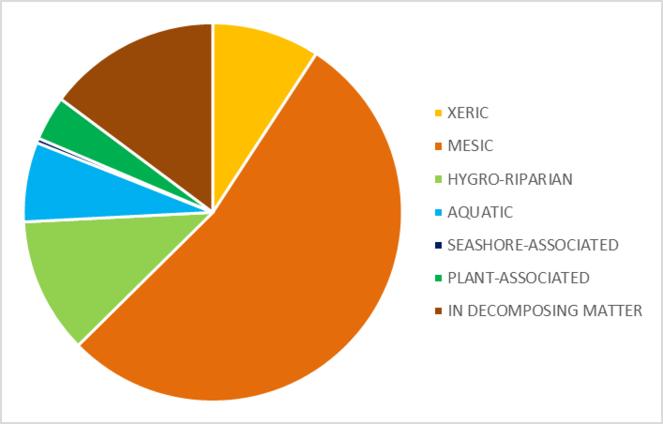
Each identified taxon has been classified into an ecological group (Fig.
Taxa that are typical of mesic to wet tundra habitats and which occur on both sides of the Bering and Chukchi Seas, are the most represented in this survey (Fig.
Many of the taxa included in the ‘Xeric’ and ‘Mesic’ groups are typical of tundra environments, but appear to exploit niches provided by decomposing organic matter, for example rotting wood, leaf litter and flood debris (Fig.
Acknowledgements
VF would like to thank Qanirtuuq inc. for hospitality in Quinhagak, logistical support and for authorising this survey on their land, as well as Paul Ledger, Roy Mark and Rick Knecht for assistance during fieldwork. Patrice Bouchard, Yves Bousquet, Anthony Davies, Hume Douglas, Jan Klimaszewski, Dave Larson, George Pelletier, Karine Savard, Aleš Smetana and Margaret K. Thayer provided invaluable help with identifications and Caroline Bourdon helped with microdissections.
Funding program
This project received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 703322. Fieldwork was funded through a grant from the Arts and Humanities Research Council of the United Kingdom (To R. Knecht, K. Britton & Charlotta Hillerdal, University of Aberdeen, AH/K006029/1).
References
- The Carrion Beetles of Canada and Alaska. Coleoptera: Silphidae and Agyrtidae.Agriculture Canada,Ottawa.
- American Beetles, Volume 1: Archostemata, Myxophaga, Adephaga, Polyphaga: Staphyliniformia.CRC Press,Boca Raton.
- American Beetles, Volume 2: Scarabaeoidea through Curculionoidea.CRC Press,Boca Raton.
- The Response of Arctic Carabidae (Coleoptera) to Climate Change Based on the Fossil Record of the Quaternary Period.Annales Zoologici Fennici33:125‑131.
- Classification, reconstructed phylogeny, and geographic history of the New World members of Plateumaris Thomson, 1859 (Coleoptera: Chrysomelidae: Donaciinae).Memoirs of the Entomological Society of Canada123(Supplement S157):5‑175. https://doi.org/10.4039/entm123157fv
- A Revision of the North American Species of the subgenus Cryobius Chaudoir (Pterostichus, Carabidae, Coleoptera).Entomologiska Sällskapet,Lund.
- Tundra and boreal forest of interior Alaska during terminal MIS 6 and MIS 5e.Vegetation History and Archaeobotany23:177‑193. https://doi.org/10.1007/s00334-013-0425-z
- Beetles Associated with Stored Products in Canada. An Identification Guide.Ministry of Supply and Services,Ottawa.
- Illustrated Identification Guide to Adults and Larvae of Northeastern North American Ground Beetles (Coleoptera: Carabidae).Pensoft,Sofia-Moscow.
- Checklist of Beetles (Coleoptera) of Canada and Alaska.Second Edition.Pensoft,Sofia-Moscow.
- The Metallic Wood-boring Beetles of Canada and Alaska. Coleoptera: Buprestidae.Agriculture Canada,Ottawa.
- A short review of Notaris (Coleoptera: Curculionidae).Bulletin of the Brooklyn Entomological Society12:36‑40.
- The Bugs Coleoptera Ecology Package (BugsCEP). The Development and Implementation of a Software for Palaeoenvironmental and palaeoclimatological Research.VDM Verlag Dr. Müller Aktiengesellschaft & Co,Saarbrücken.
- A revision of the genus Tachinus (Coleoptera: Staphylinidae) of North and Central America.Memoirs of the Entomological Society of Canada105(S90):7‑137. https://doi.org/10.4039/entm10590fv
- A revision of the North American Omaliinae (Coleoptera: Staphylinidae). 1. The genera Haida Keen, Pseudohaida Hatch, and Eudectoides new genus.Memoirs of the Entomological Society of Canada106:1‑20. https://doi.org/10.4039/entm111106fv
- A revision of the North American Omaliinae (Coleoptera: Staphylinidae). 3. The genus Acidota Stephens.The Canadian Entomologist114(11):1003‑1029. https://doi.org/10.4039/Ent1141003-11
- A revision of the North American Omaliinae (Coleoptera: Staphylinidae) The genus Olophrum Erichson.The Canadian Entomologist115(6):577‑622. https://doi.org/10.4039/Ent115577-6
- A revision of the North American Omaliinae (Coleoptera: Staphylinidae). The genera Arpedium Erichson and Eucnecosum Reitter.The Canadian Entomologist116:487‑527. https://doi.org/10.4039/Ent116487-4
- A revision of the genera Mycetoporus Mannerheim and Ischnosoma Stephens (Coleoptera: Staphylinidae: Tachyporinae) of North and Central America.Memoirs of the Entomological Society of Canada156.
- Revision of the Stenini of America north of Mexico insects of the family, Staphylinidae, order Coleoptera.Collins,Philadelphia.
- Insects of South-Central Alaska.Kenai Watershed Forum,Soldotna.
- The Beetles of Northeastern North America. Volume I: Introduction; Suborders Archaeostemata, Adephaga, and Polyphaga, through Superfamily Cantharoidea.Sandhill Crane Press,Gainesville.
- The Beetles of Northeastern North America. Volume II: Polyphaga: Series Bostrichiformia through Curculionoidea.Sandhill Crane Press,Gainesville.
- Late Quaternary beetle faunas of southwestern Alaska: evidence of a refugium for mesic and hygrophilous species.Arctic and Alpine Research24(2):133‑144. https://doi.org/10.2307/1551533
- Paleoecology of an interglacial peat deposit, Nuyakuk, Southwestern Alaska, U.S.A.Géographie Physique et Quaternaire46(1):85‑96. https://doi.org/10.7202/032890ar
- Paleoecology of late-glacial peats from the Bering Land Bridge, Chukchi Sea shelf region, Northwestern Alaska.Quaternary Research38:371‑378. https://doi.org/10.1016/0033-5894(92)90045-K
- Late Quaternary environments, Denali National Park and Preserve, Alaska.Arctic49(3):292‑305.
- Late Wisconsin environments of the Bering Land Bridge.Palaeogeography, Palaeoclimatology, Palaeoecology136:293‑308. https://doi.org/10.1016/S0031-0182(97)00038-2
- Insights on the climatic constraints on the beetle fauna of coastal Alaska, U.S.A., derived from the mutual climatic range method of paleoclimate reconstruction.Arctic, Antarctic, and Alpine Research31(1):94‑98. https://doi.org/10.2307/1552626
- Late Pleistocene climates of Beringia, based on analysis of fossil beetles.Quaternary Research53:229‑235. https://doi.org/10.1006/qres.1999.2093
- Late Pleistocene beetle faunas of Beringia: where east met west.Journal of Biogeography27:1349‑1363. https://doi.org/10.1046/j.1365-2699.2000.00503.x
- Mutual climatic range reconstructions of seasonal temperatures based on Late-Pleistocene fossil beetle assemblages in Eastern Beringia.Quaternary Science Reviews20:77‑91. https://doi.org/10.1016/S0277-3791(00)00130-X
- Arctic North American seasonal temperatures from the latest Miocene to the early Pleistocene, based on mutual climatic range analysis of fossil beetle assemblages.Canadian Journal of Earth Sciences39:911‑920. https://doi.org/10.1139/e01-096
- Advances in Quaternary Entomology.Elsevier,Amsterdam.
- A Treatise on the Western Hemisphere Caraboidea (Coleoptera). Their Classification, Distributions, and Ways of Life. Volume 1. Trachypachidae, Carabidae – Nebriiformes 1.Pensoft,Sofia-Moscow.
- Archaeoentomological Research in the North Atlantic: Past, Present, and Future.Journal of the North Atlantic26:1‑24.
- Preliminary archaeoentomological analyses of permafrost-preserved cultural layers from the pre-contact Yup’ik Eskimo site of Nunalleq, Alaska: Implications, potential and methodological considerations.Environmental Archaeology20(2):158‑167. https://doi.org/10.1179/1749631414Y.0000000037
- The Life and Death of Barn Beetles: Faunas from Manure and Stored Hay inside Farm Buildings in Northern Iceland.Ecological Entomology41:480‑499. https://doi.org/10.1111/een.12321
- Coléoptères, poux et puces subfossiles provenant d’habitats de chasseurs-cueilleurs: l’apport des recherches archéoentomologiques dans le nord circumpolaire.Recherches Amérindiennes au Québec.
- The Coccinellidae (Coleoptera) of America North of Mexico.Journal of New York Entomological Society93(1):1‑912.
- A revision of the genus Lypoglossa Fenyes, 1918 (Coleoptera: Staphylinidae: Aleocharinae).Zootaxa747:1‑36.
- The Beetles of the Pacific Northwest. Part V: Rhipiceroidea, Sternoxi, Phytophagci, Rhynchophora, and Lamellicornia.University of Washington Press,Seattle.
- Survey of Chironomidae (Insecta: Diptera) from the Kuskokwim River watershed in Western Alaska.Western North American Naturalist74(2):208‑215. https://doi.org/10.3398/064.074.0206
- Check-List and Bibliography on the Occurrence of Insects in Birds’ Nests.Iowa State College Press,Ames.
- Synopsis of the Dascyllidæ of the United States.Transactions of the American Entomological Society and Proceedings of the Entomological Section of the Academy of Natural Sciences8(1/2):76‑114. https://doi.org/10.2307/25076386
- Taxonomic notes, new records, and a key to the adults of North American Byrrhidae (Coleoptera).Proceedings of the Entomological Society of Washington93(2):322‑332.
- Northern Regional Review of Environmental Archaeology: Invertebrates in Archaeology in the North of England.English Heritage,Portsmouth.
- Kenward HK, Allison E (1994) Rural Origins of the Urban Insect Fauna. In: Kenward HK, Allison E (Eds) Urban-Rural Connexions: Perspectives from Environmental Archaeology.Oxbow Books,Oxford.
- Species review of the genus Gnypeta Thomson from Canada, Alaska and Greenland (Coleoptera, Staphylinidae, Aleocharinae): systematics, bionomics and distribution.ZooKeys2:11‑84. https://doi.org/10.3897/zookeys.2.4
- Aleocharine Beetles (Coleoptera, Staphylinidae) of the Province of Newfoundland and Labrador, Canada.Pensoft,Sofia-Moscow.
- A review of Canadian and Alaskan species of the genus Liogluta Thomson, and descriptions of three new species (Coleoptera, Staphylinidae, Aleocharinae).ZooKeys573:217‑256. https://doi.org/10.3897/zookeys.573.7878
- A Natural History of the Ground Beetles (Coleoptera: Carabidae) of America north of Mexico.Pensoft,Sofia-Moscow.
- Predaceous Diving Beetles (Coleoptera: Dytiscidae) of the Nearctic Region, with Emphasis on the Fauna of Canada and Alaska.NRN Research Press,Ottaw.
- The ground beetles (Carabidae, excl. Cicindellidae) of Canada and Alaska, Part 2.Opuscula Entomologica Supplementum20:1‑200.
- The faunal history of Newfoundland illustrated by carabid beetles.Opuscula Entomologica Supplementum23:1‑112.
- The ground Beetles (Carabidae, excl. Cicindellidae) of Canada and Alaska, Part 3.Opuscula Entomologica Supplementum24:201-408.
- The ground beetles (Carabidae, excl. Cicindellidae) of Canada and Alaska, Part 4.Opuscula Entomologica Supplementum29:409‑648.
- The ground beetles (Carabidae, excl. Cicindellidae) of Canada and Alaska, Part 5.Opuscula Entomologica Supplementum33:649‑944.
- The ground beetles (Carabidae, excl. Cicindellidae) of Canada and Alaska, Part 1.Opuscula Entomologica Supplementum35:1‑48.
- The ground beetles (Carabidae, excl. Cicindellidae) of Canada and Alaska, Part 6.Opuscula Entomologica Supplementum34:945‑1192.
- Revision of Arctic Aleocharinae of North America (Coleoptera: Staphylinidae).The Coleopterists Bulletin44(2):121‑202.
- The Leiodidae (Coleoptera) of Atlantic Canada: new records, faunal composition, and zoogeography.ZooKeys2:357‑402. https://doi.org/10.3897/zookeys.2.56
- The Byrrhidae (Coleoptera) of Atlantic Canada.Journal of the Acadian Entomological Society7:32‑43.
- A method for comparison of northern fossil insect assemblages.Géographie Physique et Quaternaire37(3):297‑306. https://doi.org/10.7202/032524ar
- Stratigraphy, fossils, and age of sediments at the upper pit of the Lost Chicken gold mine: new information on the late Pliocene environment of east central Alaska.Quaternary Research60:9‑18. https://doi.org/10.1016/S0033-5894(03)00087-5
- A Taxonomic Revision of the Weevil Genus Dorytomus in North America (Coleoptera: Curculionidae).University of Los Angeles California Press,Berkeley.
- The carrion beetles (Coleoptera: Silphidae) of Nebraska.Bulletin of the University of Nebraska State Museum,Lincoln.
- Molecular Genetic Evidence for the Post-Pleistocene Divergence of Populations of the Arctic-Alpine Ground Beetle Amara alpina (Paykull) (Coleoptera: Carabidae).Journal of Biogeography26(4):785‑794. https://doi.org/10.1046/j.1365-2699.1999.00321.x
- A late–Middle Pleistocene (Marine Isotope Stage 6) vegetated surface buried by Old Crow tephra at the Palisades, interior Alaska.Quaternary Science Reviews29:801‑811. https://doi.org/10.1016/j.quascirev.2009.12.003
- Identification of last interglacial deposits in eastern Beringia: a cautionary note from the Palisades, interior Alaska.Journal of Quaternary Science26(3):345‑353. https://doi.org/10.1002/jqs.1464
- Late Quaternary History of the Northern Beetle Fauna of North America: A Synthesis of Fossil and Distributional Evidence.Memoirs of the Entomological Society of Canada144:93‑107. https://doi.org/10.4039/entm120144093-1
- A review of the genus Pycnoglypta Thomson, 1858 (Staphylinidae, Omaliinae, Omaliini) with notes on related taxa.Zootaxa4077(1):1‑94. https://doi.org/10.11646/zootaxa.4077.1.1
- Non-marine invertebrates of the St. Matthew Islands, Bering Sea, Alaska.AKES Newsletter9(1):11‑20.
- Revision of the tribe Quediini of North America north of Mexico (Coleoptera: Staphylinidae).79.Memoirs of the Entomological Society of Canada,Ottawa.
- Two new species of Neohypdonus (Coleoptera: Elateridae) from North America with a key to Nearctic species.Entomological News102(2):73‑78.
- Paleoecological significance of Holocene insect fossil assemblages from the north coast of Alaska.Arctic39(2):150‑157.
- The detailed paleoecology of a mid-Wisconsinan interstadial (ca. 32 000 14C a BP) vegetation surface from interior Alaska.Journal of Quaternary Science26(7):746‑756. https://doi.org/10.1002/jqs.1497
Supplementary material
List of beetle taxa identified at Nunalleq, with a summary of their habitats/ecology. Ticked (✓) boxes indicate habitat records collected as part of this survey, those marked with an ‘x’ represent habitat records identified from the literature.
Ecology and habitat abbreviations: BG = bare ground; BP = bogs, peaty soils/wet meadows; CA = carrion; CG = coastal grassland; DS = disturbed/synanthropic habitats; DT = dry tundra/heath; DU = dung; DV = decaying vegetal matter; F = fungi; H = hygrophilous; MO = moss; MT = mesic tundra; NB = nests and burrows; O = open ground; R= riparian; SB= seashore, beach; SW= standing water; TV = thin/sparse vegetation; WB = wood/bark; WL = forest, woodland, scrubs; WT = wet tundra; X = xeric habitats.
The ‘ID’ column designates the determiner for each taxa (PB = Patrice Bouchard; YB = Yves Bousquet; AD = Anthony Davies; HD = Hume Douglas; VF = Véronique Forbes; JK = Jan Klimaszewski; DL = Dave Larson; DS = Derek Sikes; AS = Aleš Smetana; MKT = Margaret K. Thayer).
The ‘DIS’ column details each taxon’s global distribution (Ho = Holarctic; Ne = Nearctic; Ad = adventive to North America).