|
Biodiversity Data Journal :
Short Communications
|
|
Corresponding author: Daniel Whitmore (whitmore.daniel@gmail.com)
Academic editor: Vladimir Blagoderov
Received: 25 Jun 2018 | Accepted: 06 Sep 2018 | Published: 10 Sep 2018
© 2018 Ananda Banerjee, Knut Rognes, Daniel Whitmore
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Banerjee A, Rognes K, Whitmore D (2018) Two species of Caiusa Surcouf (Diptera: Calliphoridae) new to India, with data on larval behaviour and morphology. Biodiversity Data Journal 6: e27736. https://doi.org/10.3897/BDJ.6.e27736
|
|
Abstract
Caiusa Surcouf (Diptera: Calliphoridae) is an Old World genus of blow flies, the larvae of which feed on egg masses in the foam nests of various species of rhacophorid tree frogs. Here, we provide the first records for India (West Bengal, Eastern India) of Caiusa coomani Séguy, 1948 and C. karrakerae Rognes, 2015, together with new information on the behaviour and morphology of their larvae. Active surface swimming to disperse from infested nests is documented in blow fly larvae for the first time, as is the presence of a large internal air sac presumably acting as a floating aid. Chiromantis simus (Annandale, 1915) (Anura: Rhacophoridae) egg masses are first recorded as a feeding substrate of Caiusa larvae.
Keywords
blow flies, Oriental Region, Phumosiinae, Rhacophoridae, tree frogs, West Bengal
Introduction
The blow fly genus Caiusa Surcouf (Diptera: Calliphoridae: Phumosiinae) was recently revised and contains eight valid species described to date, distributed in the Oriental, Australasian and Oceanian regions (
Prior to this study, two species of Caiusa were known to occur in India: the type species C. indica Surcouf, 1920 and C. testacea Senior-White, 1923, both recorded from South India (
Materials and methods
Study area. Observations of live adults and larvae of Caiusa and rearings of adult flies from the foam nests (containing egg masses) of two tree frog species, Chiromantis simus (Fig.
Frog biology and nest rearing. Many frogs in the family Rhacophoridae lay egg masses in foam nests over puddles and abandoned ponds (Fig.
Preservation and identification of Caiusa specimens. Adult Caiusa specimens reared in captivity (Fig.
Images and videos. Figures 1, 3, 4b, 5, 6a and 7 were captured using a Panasonic Lumix FZ50 camera. Figures 2, 6b, 8, 9 and 10 were captured using a Samsung On8 smartphone. Figure 4a was captured with an Olympus E-420 digital camera with a 10 Mpx MOS sensor (17.3 x 13 mm), mounted by means of an LM-Scope photo adapter on a Wild M8 stereomicroscope equipped with a phototube (38 mm inner diameter).
New records
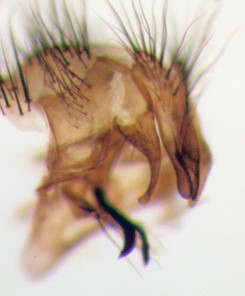
The adult flies reared from Chiromantis simus egg masses at Shyamkhola More were identified as belonging to two species: Caiusa coomani (Fig.
Biology, larval behaviour and larval morphology
Oviposition on Chiromantis simus nests. Adult females of Caiusa sp. were seen ovipositing on Chiromantis simus nests at Shyamkhola More, and a maximum of three specimens were observed at any given time (Fig.
Caiusa adults and larvae on Chiromantis simus (Annandale) foam nests at Shyamkhola More (West Bengal, India).
b: Remains of an infested nest containing seven Caiusa larvae. All the frog's eggs and foam were consumed by the larvae.
Abundance of Caiusa larvae in C. simus and P. leucomystax nests. The number of Caiusa larvae found in infested Chiromantis simus foam nests varied between one and eight, most commonly six to seven. Besides the eggs, the larvae generally consumed the entire nest before pupariation, including the protective foam (Fig.
Behaviour of third instar Caiusa larvae. Because the foam nests of rhacophorid frogs are usually hanging from vegetation above the water, the question of how mature Caiusa larvae reach dry land to pupariate has remained open. Observations of C. simus and P. leucomystax nests during this study have provided new information on the dispersal of mature Caiusa larvae from their feeding sites. At Shyamkhola More in September 2011, one larva was observed at night while descending from an outlier C. simus nest (suspended about 1 m above land at a short distance from the pond) by using a thread of unknown nature seemingly secreted by its posterior end (Fig.
Morphology of third instar Caiusa larvae. The swimming behaviour observed in Caiusa larvae would appear to be enabled by at least one conspicuous morphological adaptation, in the form of a large internal air sac located in the abdominal segments of the larva (Fig.
Acknowledgements
Many thanks to S.K. Dutta (Indian Institute of Science, Bangalore, India), I. Das (University of Sarawak, Sarawak, Malaysia), K. Deuti (Zoological Survey of India, Kolkata, India), P. Choudhury (University of Calcutta, Kolkata, India), S.N. Ghosh (West Bengal Biodiversity Board, Kolkata, India) and A. Roy (West Bengal Biodiversity Board, Kolkata, India) for their help and encouragement. Many thanks to Nazma Banerjee (Shyamkhola More, West Bengal, India) for all types of help whenever required. The study was self funded by the first author.
References
- Breeding record of fourlined treefrog Polypedates leucomystax (Gravenhorst, 1829) at Rajpur South 24 Paganas District, West Bengal.Cobra64:16‑17.
- An ecological study on Chiromantis simus (Anura: Rhacophoridae), with special reference to breeding behavior at Rajpur, eastern India.Hamadryad35(1):73‑83.
- Jelly secretion by a foam nesting tree frog Chiromantis simus (Anura: Rhacophoridae) an unreported behavior.Alytes31:77‑82. URL: http://www.amphibians.org/wp-content/uploads/2014/12/Alytes_20141220_banerjee.pdf
- Breeding ecology of Annandale's tree frog Chirixalus simus (Anura: Rhacophoridae) near Kolkata, West Bengal.Journal of the Bombay Natural History Society98(3):341‑346. URL: https://www.biodiversitylibrary.org/page/48583566#page/367/mode/1up
- First record of Polypedates leucomystax (Gravenhorst 1829) (Anura: Rhacophoridae) from southern West Bengal.Journal of the Bombay Natural History Society102(1):123. URL: https://www.biodiversitylibrary.org/page/48378806#page/129/mode/1up
- The sources of amphibian embryo mortality.Biological Bulletin of National Taiwan Normal University35:1‑11. [InChinese, with English summary].
- Oviposition behavior and host selection of the frog fly, Caiusa coomani (Diptera: Calliphoridae).Chinese Journal of Entomology20:281‑292. [InChinese, with English summary]. URL: http://140.112.100.36/old/chinese/publication/journal/pdf/c2004/c200403.pdf
- Investigation of foam nests (Rhacophoridae) infested by frogflies (Diptera) in Taiwan.Chinese Journal of Entomology20:267‑280. [InChinese, with English summary]. URL: http://140.112.100.36/old/chinese/publication/journal/pdf/c2004/c200402.pdf
- Predation on foam nests of leptodactyline frogs (Anura: Leptodactylidae) by larvae of Beckeriella niger (Diptera: Ephydridae).Journal of Zoology261:239‑243. https://doi.org/10.1017/S0952836903004138
- Revision of the frog fly genus Caiusa Surcouf, 1920 (Diptera, Calliphoridae), with a note on the identity of Plinthomyia emimelania Rondani, 1875.Zootaxa3952(1):1‑80. https://doi.org/10.11646/zootaxa.3952.1.1
- The Sciomyzidae (Diptera) of Fennoscandia and Denmark.Fauna Entomologica Scandinavica14:1‑224.
- The fauna of British India, including the remainder of the Oriental Region. VI Calliphoridae.Taylor & Francis,London,288pp.
- Révision des Muscidae testaceae.Nouvelles Archives du Muséum d'Histoire Naturelle de Paris5(6):27‑124.