|
Biodiversity Data Journal :
Research Article
|
|
Corresponding author: Zoya Tyabji (zoya.tyabji@gmail.com)
Academic editor: Yasen Mutafchiev
Received: 24 Jul 2018 | Accepted: 04 Sep 2018 | Published: 10 Sep 2018
© 2018 Zoya Tyabji, Rima Jabado, Dipani Sutaria
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Tyabji Z, Jabado R, Sutaria D (2018) New records of sharks (Elasmobranchii) from the Andaman and Nicobar Archipelago in India with notes on current checklists. Biodiversity Data Journal 6: e28593. https://doi.org/10.3897/BDJ.6.e28593
|
|
Abstract
The diversity of sharks occurring off the Andaman and Nicobar Archipelago in India has received increased attention in recent years. Yet, available checklists are out of date, often with inaccurate information and a number of commercially important species have not been documented through research and fish landing surveys. Here we report on shark species examined during fish landing surveys conducted from January 2017 to April 2018. Records of twelve previously unreported species from the archipelago are presented and include the bignose shark (Carcharhinus altimus), pigeye shark (Carcharhinus amboinensis), bull shark (Carcharhinus leucas), snaggletooth shark (Hemipristis elongata), slender weasel shark (Paragaleus randalli), Arabian smoothhound shark (Mustelus mosis), Indonesian houndshark (Hemitriakis indroyonoi), sand tiger shark (Carcharias taurus), Indonesian bambooshark (Chiloscyllium hasseltii), tawny nurse shark (Nebrius ferrugineus), dwarf gulper shark (Centrophorus atromarginatus), and the Indonesian shortsnout spurdog (Squalus hemipinnis). These records increase the reported shark species for the archipelago from 47 to 59 and for India from 114 to 116. Additionally, a size extension in the total length of C. hasseltii by 27 cm and of P. randalli by 8 cm is reported. Owing to the bio-geographical location of these islands, species diversity around the archipelago is unique and appears to overlap with that of southeast Asia. With increasing reports of over-exploitation and the operation of a targeted shark fishery by distant water fleets in these waters, the limited information on shark diversity from this region is of concern. Systematic and long-term monitoring of catches, combined with accurate species identification, is crucial to provide information on management measures.
Keywords
biodiversity, elasmobranchs, range extensions, fishery-dependent survey, review
Introduction
The waters of India harbour an estimated 114 shark species (
Checklist of shark species occurring in the Andaman and Nicobar Islands.
|
Taxon |
Common name |
First report |
Validity |
||
| Family Alopiidae | |||||
|
1 |
Alopias pelagicus Nakamura, 1935 |
Pelagic Thresher |
|
Confirmed |
|
|
2 |
Alopias superciliosus Lowe, 1841 |
Bigeye Thresher |
|
Confirmed |
|
|
3 |
Alopias vulpinus (Bonnaterre, 1788) |
Common Thresher |
|
Needs confirmation |
|
| Family Carcharhinidae | |||||
|
4 |
Carcharhinus albimarginatus (Rüppell, 1837) |
Silvertip Shark |
|
Confirmed |
|
|
5 |
Carcharhinus altimus (Springer, 1950) |
Bignose Shark |
This study |
This study |
|
|
6 |
Carcharhinus amblyrhynchos (Bleeker, 1856) |
Grey Reef Shark |
|
Confirmed |
|
|
7 |
Carcharhinus amboinensis (Müller & Henle, 1839) |
Pigeye Shark |
This study |
This study |
|
|
8 |
Carcharhinus brevipinna (Müller & Henle, 1839) |
Spinner Shark |
|
Confirmed |
|
|
9 |
Carcharhinus dussumieri (Müller & Henle, 1839) |
Whitecheek Shark |
|
Confirmed |
|
|
10 |
Carcharhinus falciformis (Müller & Henle, 1839) |
Silky shark |
|
Confirmed |
|
|
11 |
Carcharhinus hemiodon (Müller & Henle, 1839) |
Pondicherry Shark |
|
Needs confirmation |
|
|
12 |
Carcharhinus leucas (Müller & Henle, 1839) |
Bull Shark |
This study |
This study |
|
|
13 |
Carcharhinus limbatus (Müller & Henle, 1839) |
Blacktip Shark |
|
Confirmed |
|
|
14 |
Carcharhinus longimanus (Poey, 1861) |
Oceanic Whitetip Shark |
|
Confirmed |
|
|
15 |
Carcharhinus macloti (Müller & Henle, 1839) |
Hardnose Shark |
|
Confirmed |
|
|
16 |
Carcharhinus melanopterus (Quoy & Gaimard, 1824) |
Blacktip Reef Shark |
|
Confirmed |
|
|
17 |
Carcharhinus plumbeus (Nardo, 1827) |
Sandbar Shark |
|
Confirmed |
|
|
18 |
Carcharhinus sealei (Pietschmann, 1913) |
Blackspot Shark |
|
Needs confirmation |
|
|
19 |
Carcharhinus sorrah (Müller & Henle, 1839) |
Spottail Shark |
|
Confirmed |
|
|
20 |
Galeocerdo cuvier (Péron & Lesueur, 1822) |
Tiger Shark |
|
Confirmed |
|
|
21 |
Glyphis gangeticus (Müller & Henle, 1839) |
Ganges Shark |
|
Needs confirmation |
|
|
22 |
Loxodon macrorhinus Müller & Henle, 1839 |
Sliteye Shark |
|
Confirmed |
|
|
23 |
Negaprion acutidens (Rüppell, 1837) |
Sharptooth Lemon Shark |
|
Confirmed |
|
|
24 |
Prionace glauca (Linnaeus, 1758) |
Blue Shark |
|
Needs confirmation |
|
|
25 |
Rhizoprionodon acutus (Rüppell, 1837) |
Milk Shark |
|
Confirmed |
|
|
26 |
Rhizoprionodon oligolinx Springer, 1964 |
Grey Sharpnose Shark |
|
Confirmed |
|
|
27 |
Scoliodon laticaudus Müller & Henle, 1838 |
Spadenose Shark |
|
Confirmed |
|
|
28 |
Triaenodon obesus (Rüppell, 1837) |
Whitetip Reef Shark |
|
Confirmed |
|
| Family Centrophoridae | |||||
|
29 |
Centrophorus granulosus (Bloch & Schneider, 1801) |
Needle Dogfish |
|
Confirmed |
|
|
30 |
Centrophorus atromarginatus Garman, 1913 |
Dwarf Gulper Shark |
This study |
This study |
|
|
31 |
Centrophorus moluccensis Bleeker, 1860 |
Smallfin Gulper Shark |
|
Confirmed |
|
| Family Ginglymostomatidae | |||||
|
32 |
Nebrius ferrugineus (Lesson, 1831) |
Tawny Nurse Shark |
This study |
This study |
|
| Family Hemigaleidae | |||||
|
33 |
Chaenogaleus macrostoma (Bleeker, 1852) |
Hooktooth Shark |
|
Confirmed |
|
|
34 |
Hemigaleus microstoma Bleeker, 1852 |
Sicklefin Weasel Shark |
|
Confirmed |
|
|
35 |
Hemipristis elongata (Klunzinger, 1871) |
Snaggletooth Shark |
This study |
This study |
|
|
36 |
Paragaleus randalli Compagno, Krupp & Carpenter, 1996 |
Slender Weasel Shark |
This study |
This study |
|
| Family Hemiscylliidae | |||||
|
37 |
Chiloscyllium griseum Müller & Henle, 1838 |
Grey Bambooshark |
|
Confirmed |
|
|
38 |
Chiloscyllium hasseltii Bleeker, 1852 |
Indonesian Bambooshark |
This study |
This study |
|
|
39 |
Chiloscyllium indicum (Gmelin, 1789) |
Slender Bambooshark |
|
Confirmed |
|
|
40 |
Chiloscyllium punctatum Müller & Henle, 1838 |
Brownbanded Bambooshark |
|
Confirmed |
|
| Family Lamnidae | |||||
|
41 |
Isurus oxyrinchus Rafinesque, 1810 |
Shortfin Mako |
|
Confirmed |
|
| Family Odontaspididae | |||||
|
42 |
Carcharias taurus Rafinesque, 1810 |
Sandtiger shark |
This study |
This study |
|
| Family Proscyliidae | |||||
|
43 |
Eridacnis radcliffei Smith, 1913 |
Pygmy Ribbontail Catshark |
|
Confirmed |
|
|
44 |
Proscyllium magnificum Last & Vongpanich, 2004 |
Magnificent Catshark |
|
Confirmed |
|
| Family Pseudocarchariidae | |||||
|
45 |
Pseudocarcharias kamoharai (Matsubara, 1936) |
Crocodile shark |
|
Confirmed |
|
| Family Rhincodontidae | |||||
|
46 |
Rhincodon typus Smith, 1828 |
Whale Shark |
|
Confirmed |
|
| Family Scyliorhinidae | |||||
|
47 |
Apristurus investigatoris (Misra, 1962) |
Broadnose Catshark |
|
Confirmed |
|
|
48 |
Bythaelurus hispidus (Alcock, 1891) |
Bristly Catshark |
|
Confirmed |
|
|
49 |
Cephaloscyllium silasi (Talwar, 1974) |
Indian Swellshark |
|
Confirmed |
|
| Family Sphyrnidae | |||||
|
50 |
Eusphyra blochii (Cuvier, 1816) |
Winghead Shark |
|
Confirmed |
|
|
51 |
Sphyrna lewini (Griffith & Smith, 1834) |
Scalloped Hammerhead |
|
Confirmed |
|
|
52 |
Sphyrna mokarran (Rüppell, 1837) |
Great Hammerhead |
|
Confirmed |
|
|
53 |
Sphyrna tudes (Valenciennes, 1822) |
Smalleye Hammerhead |
|
Needs confirmation |
|
|
54 |
Sphyrna zygaena (Linnaeus, 1758) |
Smooth Hammerhead |
|
Confirmed |
|
| Family Stegostomatidae | |||||
|
55 |
Stegostoma fasciatum (Hermann, 1783) |
Zebra Shark |
|
Confirmed |
|
| Family Squalidae | |||||
|
56 |
Squalus hemipinnis White, Last & Yearsley, 2007 |
Indonesian Shortnose Spurdog |
This study |
This study |
|
|
57 |
Squalus megalops (Macleay, 1881) |
Shortnose Spurdog |
|
Needs confirmation |
|
| Family Triakidae | |||||
|
58 |
Hemitriakis indroyonoi W.T. White, Compagno & Dharmadi, 2009 |
Indonesian Houndshark |
This study |
This study |
|
|
59 |
Mustelus mosis Hemprich & Ehrenberg, 1899 |
Arabian Smoothhound Shark |
This study |
This study |
|
Oceanic islands are highly productive, harbour high species diversities and may function as critical stops on the ontogenetic or annual migratory route of species, serving as important breeding or feeding grounds (
Due to the distance of the archipelago from peninsular India, it has received limited attention in terms of ecological monitoring of its fisheries resources. Prior to the 1940s, there was no organised fishing sector on the archipelago (
Reporting by the Andaman and Nicobar Islands Directorate of Fisheries has broadly focused on commercial fish stocks and does not include species-specific or even group-level categories for chondrichthyans and all shark, ray and chimaera landing volumes are lumped together (
The main objectives of this study are to (1) update the species list of sharks occurring around the archipelago, (2) provide details of recent taxonomic revisions while correcting past misidentifications and (3) provide recommendations for future research and management opportunities to ensure the sustainability of shark stocks around these islands.
Methods
Study Area
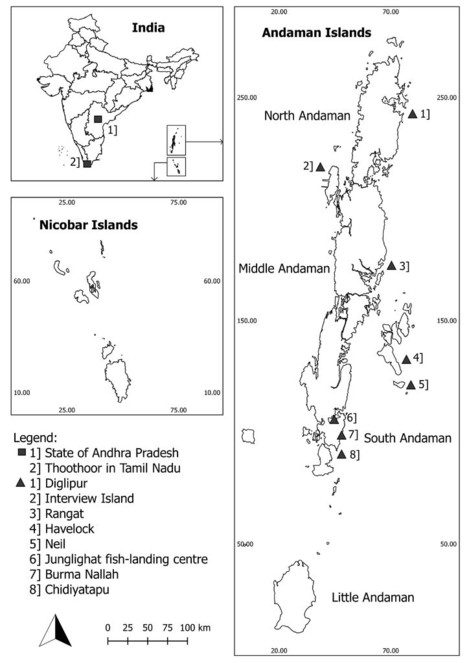
The archipelago is considered unique in its geographical location and biogeography and is situated in the Indian Exclusive Economic Zone (EEZ) in the Bay of Bengal (Fig.
Inset top left: Map of India showing the location of the state of Andhra Pradesh, Thoothoor in Tamil Nadu and the Andaman and Nicobar Islands. Inset bottom left: Map of the Nicobar Islands. Inset right: Map of the Andaman Islands showing Junglighat and Burma Nallah, the two main fish-landing centres of South Andaman Islands and the fishing grounds.
Fish landing surveys
Fish landing surveys were carried out at fish landing centres, namely, Junglighat situated in the capital city of Port Blair and Burma Nallah located south of Port Blair on the South Andaman Island (Fig.
Sharks were photo-documented to support identification using available literature (
Literature review
A comprehensive literature review was carried out by visiting the repositories of the Zoological Survey of India, Port Blair; Department of Fisheries, Andaman and Nicobar Islands; Fisheries Survey of India Port Blair; State Library of the Andaman; the Andaman Nicobar Environment Team (ANET); and through the Web of Science database. All available peer-reviewed articles and fisheries reports on shark diversity on the archipelago from 1871 to 2017 were collated and reviewed (Table
Literature published on the diversity of sharks in the Andaman and Nicobar Islands, India.
|
Authors |
Title |
Journal |
Remarks |
|
|
On the fishes of the Andaman Islands |
Zoological Society of London |
Survey |
|
|
Pisces: Natural history notes from H.M. Indian marine survey steamer 'Investigator' |
The Annals and Magazine of natural history |
Survey |
|
|
List of the fishes known from the Andaman Islands |
Memoirs of the Indian Museum |
Checklist |
|
|
New species of scyliorhinid from Andaman sea |
Zoological Survey of India |
New record |
|
|
A new scyliorhinid fish from the collections of the R.I.M.S. Investigator |
Proceedings of the All-India Congress of Zoology |
New record |
|
|
Living resources of the seas around India |
Central Marine Fisheries Institute |
Fisheries - opportunistic |
|
|
Fishes of the Andaman and Nicobar Islands |
Journal of Andaman Science Association |
Checklist |
|
|
Pelagic sharks in the Indian Ocean |
Bay of Bengal news |
Fisheries - opportunistic |
|
|
New records of rare fishes from Andaman Islands |
Journal of Andaman Science Association |
New record |
|
|
The trade in sharks and shark products in India - a preliminary survey |
TRAFFIC report |
Fisheries - opportunistic |
|
|
New records of fishes from the Andaman and Nicobar Islands |
Environmental Ecology |
New record |
|
|
Atlas of tunas, bill fishes and sharks in the Indian Exclusive Economic Zone around ANI |
Fisheries Survey of India |
Checklist |
|
|
An account of ichthyofauna of Andaman and Nicobar Islands |
Zoological Survey of India |
Checklist |
|
|
Elasmobranch fisheries of India - an appraisal |
Central Marine Fisheries Institute |
Fisheries - opportunistic |
|
|
Guide to reef fishes of Andaman and Nicobar Islands |
Zoological Survey of India |
Guide |
|
|
Poisonous and venomous fishes of Andaman Islands |
Zoological Survey of India |
Identification guide |
|
|
Handbook on sharks of Indian waters |
Zoological Survey of India |
Checklist |
|
|
A field guide to marine food fishes of Andaman and Nicobar Islands |
Zoological Survey of India |
Fisheries - opportunistic |
|
|
Distributional records and biological notes on two deep sea shark from Andaman waters |
Journal of Andaman Science Association |
Fisheries - opportunistic |
|
|
Decline in CPUE of Oceanic Sharks in the Indian EEZ |
Proceedings to the Indian Ocean Tuna Commission |
Fisheries - opportunistic |
|
|
Checklist of fishes of Andaman and Nicobar Islands |
Environmental Ecology |
Checklist |
|
|
Ichthyofaunal diversity in Great Nicobar Biosphere Reserve |
Journal of Threatened Taxa |
Checklist |
|
|
Spatio-temporal distribution, abundance and diversity of oceanic sharks occurring in the ANI |
Zoological Survey of India |
Fisheries - survey |
|
|
Bycatch in tuna longline fishery in the Indian EEZ around Andaman and Nicobar Islands |
Proceedings to the Indian Ocean Tuna Commission |
Fisheries - opportunistic |
|
|
Diversity and abundance of Chondrichthyes in the Andaman and Nicobar Islands |
Ecology of faunal communities on the Andaman and Nicobar Islands |
Checklist |
|
|
Diversity, distribution and abundance of oceanic resources around Andaman and Nicobar Islands |
Indian Journal of Fisheries |
Fisheries - opportunistic |
|
|
Emergence and transformation of marine fisheries in the Andaman Islands |
Dakshin Foundation and ANET |
Fisheries - literature review |
|
|
Fishes of Andaman and Nicobar Islands: A checklist |
Journal of Andaman Science Association |
Checklist |
|
|
Checklist of Condrichthyes in Indian waters |
Journal of Marine Biological Association of India |
Checklist |
|
|
Diversity, abundance and size structure of pelagic sharks caught in tuna longline survey in the Indian seas |
Indian Journal of Geo-Marine Science |
Survey |
|
|
First report of Magnificent catshark, Proscyllium magnificum Last and Vongpanich, 2004, from Bay of Bengal, Indian EEZ |
World Journal of Fish and Marine Sciences |
New record |
|
|
DNA barcoding reveals species composition of sharks and rays in the Indian commercial fisheries |
Mitochondrial DNA |
Checklist |
|
|
First incidence of three sharks off Andaman Islands, India |
Journal of Andaman Science Association |
New record |
|
|
New biogeographic data and DNA barcodes for the Indian swellshark, Cephaloscyllium silasi (Talwar, 1974) from Andaman waters |
Acta Ichthyologica Et Piscatoria |
New record |
|
|
Report of the crocodile shark Pseudocarcharias kamoharai (Matsubara, 1936) from deep waters of the Andaman Sea |
Marine Biodiversity |
New record |
|
|
A first record of the Smallfin Gulper Shark Centrophorus moluccensis Bleeker, 1860 (Chondrichthyes: Squaliformes: Centrophoridae) from the Andaman and Nicobar waters, Indian EEZ |
Journal of Threatened Taxa |
New record |
Results
Landing survey
A total of 3864 sharks were recorded over 123 sampling days representing 36 species. Twelve species, previously unreported from the study area including Indonesian houndshark Hemitriakis indroyonoi and Indonesian shortsnout dogfish Squalus hemipinnis, two new records from the Indian EEZ, were recorded. Details of each of these twelve species are provided below with diagnostic characteristics that allow identification to the species level using
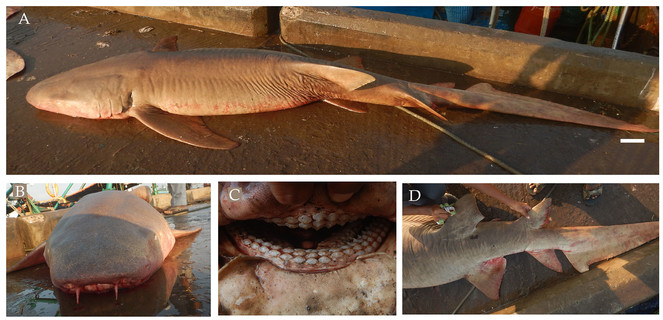
1. CARCHARHINIFORMES - CARCHARHINIDAE - Carcharhinus altimus (Springer, 1950)
From March 2017 to January 2018, three males and one female bignose shark Carcharhinus altimus (Fig.
Diagnostic features: Large, broad, moderately rounded and long snout, equal to or greater than mouth width (Fig.
2. CARCHARHINIFORMES - CARCHARHINIDAE - Carcharhinus amboinensis (Müller & Henle, 1839)
From February 2017 to February 2018, thirteen male and nineteen female specimens of the pigeye shark, Carcharhinus amboinensis (Fig.
Diagnostic features: Snout broad, short and bluntly rounded (Fig.
3. CARCHARHINIFORMES - CARCHARHINIDAE - Carcharhinus leucas (Müller & Henle, 1839)
From March 2017 to March 2018, ten male and fourteen female specimens of the bull shark, Carcharhinus leucas (Fig.
Diagnostic features: Snout broad, short and bluntly rounded (Fig.
4. CARCHARHINIFORMES - HEMIGALEIDAE - Hemipristis elongata (Klunzinger, 1871)
From February 2017 to April 2018, eighteen male and ten female specimens of the snaggletooth shark, Hemipristis elongata (Fig.
Diagnostic features: Broadly rounded snout with protruding teeth when mouth closed (Fig.
5. CARCHARHINIFORMES - HEMIGALEIDAE - Paragaleus randalli Compagno, Krupp & Carpenter, 1996
From January 2017 to April 2018, one hundred and fifty three individuals of the slender weasel shark Paragaleus randalli (Fig.
Diagnostic features: Snout with narrowly rounded tip and distinct dark lines (Fig.
6. CARCHARHINIFORMES - TRIAKIDAE - Mustelus mosis Hemprich & Ehrenberg, 1899
On 4th March 2017, three Arabian smoothhound sharks Mustelus mosis (Fig.
Diagnostic features: Snout short and bluntly angular (Fig.
7. CARCHARHINIFORMES - TRIAKIDAE - Hemitriakis indroyonoi W.T. White, Compagno & Dharmadi, 2009
In December 2017 and February 2018, two female Indonesian houndsharks were landed (Fig.
Diagnostic features: Snout long and narrow (Fig.
8. LAMNIFORMES – ODONTASPIDIDAE – Carcharias taurus Rafinesque, 1810
On 20th March 2018, one female sandtiger shark Carcharias taurus (Fig.
Diagnostic features: Conical short snout with large slender pointed teeth (Fig.
9. ORECTOLOBIFORMES - HEMISCYLLIDAE - Chiloscyllium hasseltii Bleeker, 1852
On 20th February, a female Indonesian bambooshark Chiloscyllium hasseltii (Fig.
Diagnostic features: Convex pectoral, pelvic and dorsal fins (Fig.
10. ORECTOLOBIFORMES - GINGLYMOSTOMATIDAE - Nebrius ferrugineus (Lesson, 1831)
From February 2017 to March 2018, three male and two female specimens of tawny nurse shark Nebrius ferrugineus (Fig.
Diagnostic features: Rounded snout with transverse, subterminal mouth well in front of eyes (Fig.
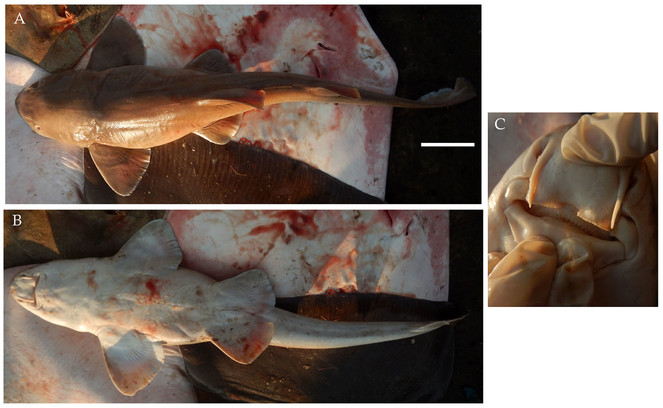
11. SQUALIFORMES - CENTROPHORIDAE – Centrophorus atromarginatus Garman, 1913
On 6th September, a male dwarf gulper shark Centrophorus atromarginatus (Fig.
Diagnostic features: Fairly long thick snout (Fig.
12. SQUALIFORMES - SQUALIDAE - Squalus hemipinnis White, Last & Yearsley, 2007
On 21st July, a female Indonesian shortsnout spurdog Squalus hemipinnis (Fig.
Diagnostic features: Narrow, short, bluntly pointed snout (Fig.
Literature review
We found 36 published accounts on sharks from the archipelago (Tables
In addition, there have been frequent misidentifications and doubtful records of several shark species (
Discussion
With systematic surveys carried out at fish landing sites, this study added twelve new species records to the known shark fauna of the Andaman and Nicobar Archipelago in a relatively short timeframe, highlighting the importance of monitoring landings at the species level. Ten of these species have been recorded and confirmed from mainland India (
The records of C. hasseltii and P. randalli increase their known total lengths from 61 cm to 88.5 cm TL and 83.6 cm to 95.8 cm TL, respectively (
Accurate species identification is fundamental to monitoring ecological trends in populations, informing about and assessing conservation actions, designing and implementating management plans and evaluating the status of ecosystems and species (
Reported landings of elasmobranchs (sharks and rays) from the archipelago have quadrupled from 467 mt in 2001 to 2,124 mt in 2011 (
Sharks are highly susceptible to fishing pressure and the lack of systematic monitoring of catch diversity and volumes, as well as the current lack of management, is a cause for concern with many species likely to have been overlooked and which could already have been overexploited (
Acknowledgements
We would like to thank the fishermen of Junglighat and Burma Nallah and the processing unit staff at Monsoon fisheries, South Andaman Islands, India for allowing us to collect information from specimens landed and for supporting our research project. We would also like to thank Tanmay Wagh, Evan Nazareth and Nairika Barucha for helping with data collection; Dr. Vardhan Patankar for guidance on field logistics; Andaman Nicobar Environment Team (ANET) for their support with the organisation of this project; and Dave Ebert for confirming the identification of Squalus hemipinnis. Finally, we would like to thank The Rufford Foundation (Rufford Small Grant: 21010-1) and Conservation Leadership Programme (Future Conservationist Award: 03332917) for providing funding to Z. Tyabji to carry out this study.
Ethics and security
All applicable international, national and/or institutional guidelines for the care and use of animals were followed by the authors. Further, there were no permits required to carry out sampling and field studies for this study.
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- Emergence and transformation of marine fisheries in the Andaman Islands.Dakshin Foundation and ANET1‑50.
- Deep-sea fishing for chondrichthyan resources and sustainability concerns—a case study from southwest coast of India.Indian Journal of Geo-Marine Sciences40(3):347‑355.
- Checklist of chondrichthyans in Indian waters.Journal of the Marine Biological Association of India56(1):109‑120. https://doi.org/10.6024/jmbai.2014.56.1.01750s-17
- Alcock AW (1891) Class Pisces. In: Natural history notes from H. M. Indian marine survey steamer 'Investigator', Commander R. F. Hoskyn, R. N., commanding. Series II, No. 1. On the results of deep-sea dredging during the season 1890-91. Annals and Magazine of Natural History.6.
- Field Guide to Look-alike Sharks and Rays Species of the Southeast Asian Region.SEAFDEC/MFRDMD/SP22:1‑107.
- Ecological impact assessment in the Andaman islands and observations in the Nicobar islands. The ground beneath the waves: Post-tsunami impact assessment of wildlife and their habitats in India.Wildlife Trust of India,New Delhi,78-103pp.
- Comparative feeding ecology of sea birds of a tropical oceanic Island.Connecticut: Peabody Museum Natural History Bulletin24:1‑131.
- Island biogeography-the forest birds of the Andaman Islands as a case study.Tigerpaper (FAO)33(1):30‑32.
- Species identification by experts and non-experts: comparing images from field guides.Scientific Reports6:33634. https://doi.org/10.1038/srep33634
- Effects of species misidentification on population assessment of overfished white marlin Tetrapturus albidus and roundscale spearfish T. georgii.Endangered Species Research9(2):81‑90. https://doi.org/10.3354/esr00234
- DNA barcoding reveals species composition of sharks and rays in the Indian commercial fishery.Mitochondrial DNA, Part A28(4):458‑472. https://doi.org/10.3109/19401736.2015.1137900
- Improving the conservation of Mediterranean chondrichthyans: The ELASMOMED DNA barcode reference library.PLoS ONE12(1):e0170244. https://doi.org/10.1371/journal.pone.0170244
- Internesting behavior of the green turtle, Chelonia mydas, at a Mid-Ocean Island breeding ground.Copeia703‑706. https://doi.org/10.2307/1442684
- Field guide to the sharks of the world.Harper Collins Publishers Ltd.,London,368pp.
- FAO species catalogue. Sharks of the World. An annotated and illustrated catalogue of shark species known to date.FAO Fisheries Synopsis4:1‑655.
- Compagno LJ (2001) Sharks of the world, an annotated and illustrated catalogue of shark species known to date. Bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes).2.FAO,FAO, Rome,269pp.
- Review of the diversity, ecology, and conservation of elasmobranchs in the Azores region, mid-north Atlantic.Frontiers in Marine Science4:354. https://doi.org/10.3389/fmars.2017.00354
- On the fishes of the Andaman Islands.Proceedings from Zoological Society of London3:667705.
- Devi K, Rao DV (2003) Poisonous and venomous fishes of Andaman Islands, Bay of Bengal. Zoological Survey of India.211.71pp.
- Fisheries management and conservation of sharks in Indonesia.African Journal of Marine Science37(2):249‑258. https://doi.org/10.2989/1814232X.2015.1045431
- Dingerkus G, DeFino TC (1983) A revision of the orectolobiform shark family Hemiscyllidae (Chondrichthyes, Selachii). Bulletin of the American Museum of Natural History.176.94pp.
- Provisional population total: rural-urban distribution, Andaman and Nicobar Islands.2.Directorate of Census Operations, Government of India,35pp.
- Long-Term Occupancy Trends in a Data-Poor Dugong Population in the Andaman and Nicobar Archipelago.PLoS ONE8(10):e76181. https://doi.org/10.1371/journal.pone.0076181
- Extinction risk and conservation of the world’s sharks and rays.Elife3:e00590. https://doi.org/10.7554/eLife.00590.001
- A Pocket Guide to Sharks of the World.Princeton University Press,Princeton, New Jersey,256pp.
- How you count counts: the importance of methods research in applied ecology.Journal of Applied Ecology45(5):1313‑1320. https://doi.org/10.1111/j.1365-2664.2008.01545.x
- Feeding grounds of the western South Atlantic humpback whale population.Marine Mammal Science25:964‑969. https://doi.org/10.1111/j.1748-7692.2009.00301.x
- Patterns and ecosystem consequences of shark declines in the ocean.Ecology Letters13:1055‑1071. https://doi.org/10.1111/j.1461-0248.2010.01489.x
- http://andssw1.and.nic.in/ecostat/basicstatPDF2011_12/06.%20Fisheries.pdf. Accessed on: 2017-12-21.
- Illegal and unreported fishing: global analysis of incentives and a case study estimating illegal and unreported catches from India, Doctoral dissertation.University of British Columbia
- Comparative anatomy, phylogeny and cladistics classification of the Order Orectolobiformes (Chondrichthyes, Elasmobranchii).Memoirs of the graduate school of fisheries sciences, Hokkaido University48(1):1‑100.
- The trade in shark and shark products in India – a preliminary survey.TRAFFIC India Publication1‑50.
- Noteworthy elasmobranch records from Oman.African Journal of Marine Science33(1):171‑175. https://doi.org/10.2989/1814232X.2011.572380
- A list of the fishes known from the Andaman Islands.Memoirs of the Indian Museum12:331‑403.
- Hornby C, Kumar MA, Bhathala BY, Pauly D, Zeller D (2015) Reconstruction of the Andaman and Nicobar Islands (India) marine fish catch from 1950-2010.PhD thesis, Fisheries sector, University of British Columbia,42pp.
- Shark and Ray Fisheries of Myanmar–status and socio-economic importance.12.32
- Troubled waters: Threats and extinction risk of the sharks, rays and chimaeras of the Arabian Sea and adjacent waters.Fish and Fisherieshttps://doi.org/10.1111/faf.12311
- Sharks of the Arabian Seas: an identification guide.The International Fund for Animal Welfare,Dubai,240pp.
- Shark diversity in the Arabian/Persian Gulf higher than previously thought: insights based on species composition of shark landings in the United Arab Emirates.Marine Biodiversity45(4):719‑731. https://doi.org/10.1007/s12526-014-0275-7
- Proceedings of the symposium on living resources of the seas around India, Mandapam camp.483-494pp.
- Atlas of Tunas, Billfishes and Sharks in the Indian EEZ around Andaman and Nicobar Islands.FSI/FC (FA)(3).
- Decline in CPUE of oceanic sharks in the Indian EEZ: urgent need for precautionary approach.Indian Ocean Turtle Commission‑17.
- Bycatch in tuna longline fishery in the Indian EEZ around Andaman and Nicobar Islands.Indian Ocean Tuna Commission Working Party on Ecosystems and Bycatch19.
- Proceedings of the symposium on living resources of the seas around India.Central Marine Fisheries Research Institute Special Publication,Kochi,387 - 389pp.
- First report of Magnificent Catshark, Proscyllium magnificum Last and Vongpanich, 2004 (Procyllidae: Carcharhiniformes) from Bay of Bengal, Indian EEZ.World Journal of Fish and Marine Sciences7(6):479‑481. https://doi.org/10.5829/idosi.wjfms.2015.7.6.101184
- New biogeographic data and DNA barcodes for the Indian Swellshark, Cephaloscyllium silasi (Talwar, 1974) (Elasmobranchii: Carcharhiniformes: Scyliorhinidae), from Andaman waters.Acta Ichthyologica et Piscatorial46(2):131‑135. https://doi.org/10.3750/AIP2016.46.2.10
- Sharks and rays of Borneo.CSIRO Publication, i–v,298pp.
- Global Diversity Hotspots and Conservation Priorities for Sharks.PLoS ONE6(5):e19356. https://doi.org/10.1371/journal.pone.0019356
- On a new species of Scyliorhinid fish from Andaman Sea, Bay of Bengal.Record from Zoological Survey of India8(2):87‑90.
- Proceedings of the First All-India Congress of Zoology,1959.636–638pp.
- Formicidae of the Andaman and Nicobar islands (Indian Ocean: Bay of Bengal).Journal of Insect Science10(1):172. https://doi.org/10.1673/031.010.14132.
- Population structure of South Pacific humpback whales and the origin of the eastern Polynesian breeding grounds.Marine Ecology Progress Series330:257‑268. https://doi.org/10.3354/meps330257
- Pelagic sharks in the Indian seas – their exploitation, trade, management and conservation.Central Marine Fisheries Research Institute Special Publication70:1‑95.
- A first record of the smallfin gulper shark Centrophorus moluccensis Bleeker, 1860 (Chondrichthyes: Squaliformes: Centrophoridae) from the Andaman & Nicobar waters, Indian EEZ.Journal of Threatened Taxa9(11):10899‑10903. https://doi.org/10.11609/jott.3315.9.11.10899-10903
- Report of the crocodile shark Pseudocarcharias kamoharai (Matsubara, 1936) from deep waters of the Andaman Sea.Marine Biodiversity47(2):535‑538. https://doi.org/10.1007/s12526-016-0499-9
- New records of rare fishes from the Andaman and Nicobar Islands.Journal of Andaman Science Association9:103‑106.
- Rajan PT (2003) A field guide to marine food fishes of Andaman and Nicobar Islands. Zoological Survey of India.Kolkata,260pp.
- Rajan PT, Sreeraj CR, Venkataraman K (2012) Diversity and abundance of Chondrichthyan fishes in Andaman and Nicobar Islands. Ecology of faunal communities on the Andaman and Nicobar Islands.117–126pp. https://doi.org/10.1007/978-3-642-28335-2
- Fishes of Andaman and Nicobar Islands: A checklist.Journal of the Andaman Science Association18(1):47‑48.
- First incidence of three sharks off Andaman Islands, India.Journal of the Andaman Science Association21(2):221‑228.
- Ichthyofaunal diversity in Great Nicobar Biosphere Reserve, Bay of Bengal.Journal of Threatened Taxa1(3):166‑169. https://doi.org/10.11609/JoTT.o1985.166-69
- Elasmobranch fisheries of India – an appraisal.Central Marine Fisheries Research Institute special publication71:76.
- New records of fishes from Andaman and Nicobar Islands.Environmental Ecology15:107‑112.
- Rao DV, Devi K, Rajan PT (2000) An account of Ichthyofauna of Andaman and Nicobar Islands, Bay of Bengal. Zoological Survey of India.178-434pp.
- Guide to reef fishes of Andaman and Nicobar Islands.Zoological Survey of India1‑555.
- Checklist of fishes of Andaman and Nicobar Islands, Bay of Bengal.Environmental Ecology27(1):334‑353.
- Ornithogeographic affinities of the Andaman and Nicobar Islands.Journal of Biogeography323‑332. https://doi.org/10.2307/2845224
- Marine biodiversity hotspots and conservation priorities for tropical reefs.Science295(5558):1280‑1284. https://doi.org/10.1126/science.1067728
- Diversity, distribution and abundance of oceanic resources around Andaman and Nicobar Islands.Indian Journal of Fisheries59(2):63‑67.
- The fishes of southwestern Thailand, the Andaman Sea-a review of research and a provisional checklist of species.Phuket Marine Biological Center Research Bulletin70:29‑77.
- The importance of research and public opinion to conservation management of sharks and rays: a synthesis.Marine and Freshwater Research62(6):518‑527. https://doi.org/10.1071/MF11086
- Sinha MK, Pandian PP, Pattanayak SK, Kar AB (2010) Spatio-temporal distribution, abundance, and diversity of oceanic sharks occurring in Andaman and Nicobar waters recent trends in biodiversity of Andaman and Nicobar Islands. Zoological Survey of India.373–385pp.
- Pelagic sharks in the Indian Ocean.Bay of Bengal News48:2‑7.
- Effects of including misidentified sharks in life history analyses: A case study on the grey reef shark Carcharhinus amblyrhynchos from Papua New Guinea.PLoS ONE11(4):e0153116. https://doi.org/10.1371/journal.pone.0153116
- The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems.ICES Journal of Marine Science57(3):476‑494. https://doi.org/10.1006/jmsc.2000.0724
- Distributional records and biological notes on two deep sea shark Centrophorus acus Garnan and Squalus megalops (Macleay) from Andaman waters.Journal of Andaman Science Association46(2):178‑184.
- First record of the sandbar shark, Carcharhinus plumbeus, (Chondrichthyes: Carcharhiniformes: Carcharhinidae) from Indian waters.Marine Biodiversity Records8:e126. https://doi.org/10.1017/s1755267215001025
- Fishes of the Andaman and Nicobar Islands: A synoptic analysis.Journal of Andaman Science Association6(2):72‑102.
- Diversity, abundance and size structure of pelagic sharks caught in tuna longline survey in the Indian seas.Indian Journal of Geo-Marine Science44(1):26‑36.
- Venkataraman K, John MM, Raghuram KP (2003) Handbook on sharks of Indian waters (Diversity, fishery status trade and conservation). Zoological Survey of India, Kolkata.113pp.
- An ill-thought ban.Samudra30:3‑9.
- DNA barcoding Australasian chondrichthyans: results and potential uses in conservation.Marine and Freshwater Research59(1):57‑71. https://doi.org/10.1071/MF07148
- Contribution to the taxonomy and distribution of six shark species (Chondrichthyes, Elasmobranchii) from the Gulf of Thailand.ISRN Zoology2012:1‑24. https://doi.org/10.5402/2012/860768
- Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity.Journal of Fish Biology88(3):837‑1037. https://doi.org/10.1111/jfb.12874
- Economically important sharks and rays of Indonesia.Australian Centre for International Agricultural Research, Monograph series24:1‑329.
- Squalus hemipinnis sp. nov, a new short-snout spurdog from eastern Indonesia. In: Last PR, White WT, Pogonoski JJ (Eds) Descriptions of New Dogfishes of the genus Squalus (Squaloidea: Squalidae).CSIRO Marine and Atmospheric Research14:101‑108.
- Chiloscyllium hasseltii.IUCN Red List of Threatened Specieshttps://doi.org/10.2305/iucn.uk.2009-2.rlts.t161557a5450824.en
- Hemitriakis indroyonoi sp. nov., a new species of houndshark from Indonesia (Carcharhiniformes: Triakidae).Zootaxa2110:41‑57.
- A review of the taxonomy of chondrichthyan fishes: a modern perspective.Journal of Fish Biology80(5):901‑917. https://doi.org/10.1111/j.1095-8649.2011.03192.x