|
Biodiversity Data Journal :
Taxonomic paper
|
|
Corresponding author:
Academic editor: Pierfilippo Cerretti
Received: 26 Jan 2015 | Accepted: 07 Aug 2015 | Published: 11 Aug 2015
© 2015 AJ Fleming, D. Monty Wood, Daniel Janzen, Winnie Hallwachs, M. Alex Smith
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Fleming A, Wood D, Janzen D, Hallwachs W, Smith M (2015) Three new species of Trigonospila Pokorny (Diptera: Tachinidae), from Area de Conservación Guanacaste, northwestern Costa Rica, with a key for their identification. Biodiversity Data Journal 3: e4595. https://doi.org/10.3897/BDJ.3.e4595
|
|
Abstract
We describe three new species of Trigonospila Pokorny (Tachinidae: Blondeliini) from Area de Conservación Guanacaste (ACG), northwestern Costa Rica. All were reared from various species of ACG caterpillars during an ongoing inventory of caterpillars, their food plants and their parasitoids in dry forest, rain forest and cloud forest. By coupling morphology, photographic documentation, life history and molecular data, we provide a clear and concise description of each species. All species published as new, are known to be previously undescribed as a result of careful study of the genus by DMW. This study builds on the current knowledge of the genus by adding three new species to the current 7 described in the New World. Trigonospila edwinbermudezi sp. n., Trigonospila uniformis sp. n., and Trigonospila josemariamoragai sp. n. are all authored and described as new by Fleming and Wood, with a key to their identification. The authors also offer a new record and description of the previously unknown male of Trigonospila panamensis (Townsend), reared from ACG caterpillars.
Keywords
Trigonospila, Diptera, Tachinidae, tropical rain forest, tropical dry forest, parasitoid fly, host-specificity, caterpillars
Introduction
The tachinid genus Trigonospila
The genus Trigonospila is widely distributed, occurring throughout the Old World, in Eurasia, Australia, Oriental and in the Afrotropical regions (
Herein we describe three new species of the genus Trigonospila, and describe the male of T. panamensis (Townsend), all reared from wild-caught caterpillars collected from Area de Conservación Guanacaste, in northwestern Costa Rica. The decision that these three are previously undescribed is based on examination of New World Trigonospila, and observation of differences in external morphology, and CO1 gene sequences. By coupling CO1 data with morphological descriptions we are able to show that the coloration patters of the abdomens are not only differences between males and females but they are consistent within species making them useful in visual species identification. This paper adds to the existing knowledge of Trigonospila by providing new records relating to distribution and host preference.
Materials and methods
As this paper forms part of a larger series dedicated to naming the tachinid fauna of ACG, the methods described herein are in referenced and adapted from earlier works by the authors (
Acronyms for depositories.
BMNH - The Natural History Museum, London, United Kingdom
CNC - Canadian National Collection of Insects, Arachnids and Nematodes, Ottawa, Canada
USNM - U.S. National Museum of Natural History, Washington, D.C., USA
INBIO - Instituto Nacional de Biodiversidad, Santo Domingo de Heredia, Costa Rica
Geographic area of the study and rearing intensity
All flies and rearing information described here were found by the 35+ year–old ongoing inventory of the caterpillars, their food plants and their parasitoids of the dry forest, rain forest, cloud forest, and intergrades, in the 125,000+ ha terrestrial portion of Area de Conservación Guanacaste (ACG) in northwestern Costa Rica (
This inventory has reared about 600,000 wild-caught caterpillars since 1978. All frequencies of parasitism reported here need to be considered against this background inventory. Equally, it is patently obvious that the inventory searches some kinds of vegetation and height off the ground much more thoroughly than others, and it also searches throughout the year. Comparison of reared species of parasitoids with those collected by net or Malaise traps demonstrates that to date, the caterpillar inventory has so far encountered well less than half the species of caterpillar parasitoids present in ACG. The largest unsampled void is the upper foliage of the canopy above about 3–4 m above the ground.
The treatment reported here is focused on placing names on the species reared, thereby preparing them for later detailed ecological and behavioral accounts and studies that will normally extend across ACG ecological groups, whole ecosystems, and taxonomic assemblages much larger than a genus.
Imaging
Our descriptions of new species are deliberately brief and only include some differentiating descriptions of body parts and colors that are commonly used in tachinid identification. These brief descriptions are complemented with an extensive series of color photos of every species to illustrate the readily-observed differences among them.
Habitus photographs were taken using a Canon T3i digital SLR, equipped with a 65mm Macro Photo Lens 1:2.8 (MP–E 65mm), mounted on a microscope track stand (AmScope, Model: TS200) modified to accept a Manfrotto QR 200PL–14 quick release plate. Images were shot in aperture priority, allowing the camera to control shutter speed at f/4.5 and take 40 images at equal distance increments. Illumination was provided with a homemade reflective dome (instruction for dome creation can be found at: http://www.cdfa.ca.gov/plant/ppd/entomology/Dome/kd-200.html) placed over a 144 LED ringlight (AmScope, Model: LED–144–YK).
The photographic series was processed from RAW format using Photoshop CS6, and digitally stacked. Each final composite image was created using Zerene Stacker Software v1.04 maximizing image quality and depth of field.
All specimens listed as examined are considered paratypes, except for the holotype which is noted separately.
Wherever a specimen label has been examined, the information is presented using the following symbols: /, indicates the end of a line; //, indicates the end of a label. Labels are presented from top (closest to the specimen) to bottom, with any comments about the label being given in square brackets.
Voucher specimen management
All caterpillars reared from the ACG efforts receive a unique voucher code in the format of yy–SRNP–xxxxx. Any parasitoid emerging from this caterpillar receives the same voucher code, and then if/when later the parasitoid is dealt with individually, it receives a second voucher code unique to it, in the format of DHJPARxxxxxxx. The voucher codes and collateral data assigned to both host and emergent parasitoids are available at http://janzen.bio.upenn.edu/caterpillars/database.lasso. To date, all DHJPARxxxxxxx coded tachinids have had one leg removed for attempted DNA barcoding at the Biodiversity Institute of Ontario (BIO) in the University of Guelph, with all collateral data and all successful barcodes permanently and publically deposited in the Barcode of Life Data System (BOLD, http://www.boldsystems.org) (
Inventoried Tachinidae were collected under Costa Rican government research permits issued to DHJ since 1978, and likewise exported under permit by DHJ from Costa Rica to Philadelphia, and then to the final depository in the Canadian National Insect collection in Ottawa, Canada. Tachinid identifications for the inventory were done by DHJ in coordination with a) visual inspection by AJF and DMW, b) DNA barcoding by BIO, MAS, and BOLD, and c) correlation with host caterpillar identifications by DHJ and WH through the inventory itself. Dates of capture of each reared fly in the inventory are the dates of eclosion of the fly, and not the date of capture of the caterpillar. This is because the fly eclosion date is much more representative of the time when that fly species is on the wing than is the time of capture of the caterpillar or (rarely) finding a parasitized pupa. However, the collector listed is the parataxonomist who found the caterpillar, rather than the person who retrieved the newly eclosed fly from its rearing bag or bottle, and processed it by freezing, pinning, labeling and oven–drying. Fly biology and degrees of parasitization by these flies will be the detailed subject of later papers.
DNA barcoding
DNA barcodes (the standard 5’ region of the mitochondrial cytochrome c oxidase I (COI) gene) for all ACG inventory specimens were obtained using DNA extractions made from single legs using a glass fiber protocol (
Generic synonyms of Trigonospila Pokorny
Trigonospila
Zosteromyia
Succingulum
Panacemyia
Gymnamedoria
Zosteromyiopsis
Nimiocauda
New World species previously included in Trigonospila Pokorny
In the process of species determination, specimens provided from ACG were examined in comparison to the entire known seven-member fauna of the New World Trigonospila by both AJF and DMW. These comparisons were made based on geographical proximity of the species, as well as any similarities in life history and morphology. It was found only one species reared in ACG matches any of the known species, Trigonospila panamensis (Townsend). Differentiating comparisons are discussed in the descriptions, when necessary. Wherever possible, holotypes were compared to ACG specimens. However, it should be noted if holotype material was unavailable, direct comparisons were made with specimens present at the CNC.
erilis
melaleuca
pallipes
panamensis
solitaria
trinitatis
verticalis
Diagnosis of the genus Trigonospila Pokorny
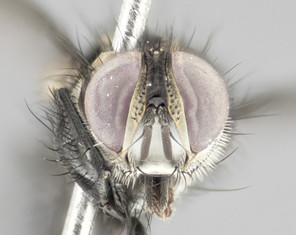
Head: male lacking proclinate fronto-orbital bristles; reclinate orbital bristles of male indistinguishable from frontals; ocellar seta hair-like, nearly parallel to each other in male, parallel or divergent in female; eye bare, or with minute inconspicuous hairs; parafacial bare, extremely narrow; lower margin of face at level of vibrissa not visible in profile; facial ridge with a few small recumbent setae on lower third or less; subvibrissal ridge short, usually with 3 or fewer bristles; anterior margin of postgena concave anteriorly, sloping anteroventrally toward vibrissal angle, without genal dilation; first flagellomere of male about as long as that of female; arista minutely to short pubescent, thickened on basal fourth to fifth.
Thorax: prosternum bare (this character state, a rarity in Blondeliini, is contrary to
Abdomen (Figs
According to
Taxon treatments
Trigonospila josemariamoragai , sp. n.
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Sendero Navarro; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:21-Mar-2011; individualID:DHJPAR0042272; individualCount:1; sex:M; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042272; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-70422; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Del Oro; verbatimLocality:Quebrada Salazar; verbatimElevation:560; verbatimLatitude:11.002; verbatimLongitude:-85.463; verbatimCoordinateSystem:Decimal; decimalLatitude:11.002; decimalLongitude:-85.463; samplingProtocol:reared from caterpillar of Stenoma exarata (Elachistidae); verbatimEventDate:03-Jan-2011; individualID:DHJPAR0040982; individualCount:1; sex:M; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0040982; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:10-SRNP-22521; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Sendero Navarro; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:25-Mar-2011; individualID:DHJPAR0042259; individualCount:1; sex:F; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042259; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-70435; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Sendero Navarro; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:22-Mar-2011; individualID:DHJPAR0042260; individualCount:1; sex:F; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042260; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-70449; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Sendero Navarro; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:22-Mar-2011; individualID:DHJPAR0042261; individualCount:1; sex:M; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042261; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-70451; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Sendero Navarro; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:22-Mar-2011; individualID:DHJPAR0042262; individualCount:1; sex:F; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042262; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-70440; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Sendero Navarro; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:21-Mar-2011; individualID:DHJPAR0042263; individualCount:1; sex:M; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042263; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-70438; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Sendero Navarro; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:20-Mar-2011; individualID:DHJPAR0042264; individualCount:1; sex:F; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042264; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-70454; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Sendero Navarro; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:20-Mar-2011; individualID:DHJPAR0042265; individualCount:1; sex:F; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042265; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-70426; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Sendero Navarro; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:20-Mar-2011; individualID:DHJPAR0042266; individualCount:1; sex:M; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042266; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-70439; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Sendero Navarro; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:19-Mar-2011; individualID:DHJPAR0042267; individualCount:1; sex:M; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042267; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-70433; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Sendero Navarro; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:18-Mar-2011; individualID:DHJPAR0042268; individualCount:1; sex:M; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042268; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-70434; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Sendero Navarro; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:21-Mar-2011; individualID:DHJPAR0042270; individualCount:1; sex:F; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042270; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-70445; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Sendero Navarro; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:21-Mar-2011; individualID:DHJPAR0042271; individualCount:1; sex:M; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042271; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-70429; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Sendero Navarro; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:20-Mar-2011; individualID:DHJPAR0042273; individualCount:1; sex:F; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042273; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-70453; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Sendero Navarro; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:20-Mar-2011; individualID:DHJPAR0042274; individualCount:1; sex:M; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042274; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-70444; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Sendero Navarro; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:21-Mar-2011; individualID:DHJPAR0042275; individualCount:1; sex:M; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042275; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-70443; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Sendero Navarro; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:18-Mar-2011; individualID:DHJPAR0042278; individualCount:1; sex:F; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042278; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-70427; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila josemariamoragai; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:josemariamoragai; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Rincon Rain Forest; verbatimLocality:Quebrada Bambu; verbatimElevation:109; verbatimLatitude:10.9301; verbatimLongitude:-85.25205; verbatimCoordinateSystem:Decimal; decimalLatitude:10.9301; decimalLongitude:-85.25205; samplingProtocol:reared from caterpillar of Egchiretes Poole01 (Nolidae); verbatimEventDate:08-Aug-2013; individualID:DHJPAR0052422; individualCount:1; sex:F; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0052422; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:13-SRNP-76881; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
Description
Male (Fig.
Female (Fig.
Diagnosis
Small black and yellow fly, with 4 prominent black stripes on the thorax, these smudging together so that it appears as 2 large thoracic vittae and a golden scutellum. Males with a straight conical, and apically pointed abdomen, with 3 gold bands interspersed with black wrapping the abdomen, terminating in a black tip. Female abdomen with a strong down-pointing curve abdominal bands mid-dorsally pointed joining the next segment’s gold band so that 4 small black triangles become apparent on abdomen.
Etymology
Trigonospila josemariamoragai is named in honor of Jose Mario Moraga, in recognition of his frequent rescues of ACG parataxonomists' computers.
Distribution
Costa Rica, ACG, Prov. Guanacaste, rain forest.
Ecology
Reared from, Nolidae, Steniscadia polyodonta; Elachistidae, Stenoma spp. (19 records). One fly larva per caterpillar.
Trigonospila uniformis , sp. n.
-
scientificName: Trigonospila uniformis; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:uniformis; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Alajuela; county:Area de Conservacion Guanacaste; locality:Sector Rincon Rain Forest; verbatimLocality:Estacion Llanura; verbatimElevation:135; verbatimLatitude:10.933; verbatimLongitude:-85.253; verbatimCoordinateSystem:Decimal; decimalLatitude:10.933; decimalLongitude:-85.253; samplingProtocol:reared from caterpillar of Stenoma Janzen44 (Elachistidae); verbatimEventDate:Jul-15-2009; individualID:DHJPAR0035709; individualCount:1; sex:M; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0035709; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:09-SRNP-44688; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila uniformis; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:uniformis; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; stateProvince:Alajuela; county:Area de Conservacion Guanacaste; locality:Sector Rincon Rain Forest; verbatimLocality:Quebrada Bambu; verbatimElevation:109; verbatimLatitude:10.93; verbatimLongitude:-85.252; verbatimCoordinateSystem:Decimal; decimalLatitude:10.93; decimalLongitude:-85.252; samplingProtocol:reared from caterpillar of Antaeotricha spurca (Elachistidae); verbatimEventDate:26-Apr-2010; individualID:DHJPAR0040672; individualCount:1; sex:F; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0040672; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:10-SRNP-75629; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
Description
Male (Fig.
Female (Fig.
Diagnosis
Small black and yellow fly, with 4 prominent black stripes on the thorax, these smudging together so that it appears as 2 large thoracic vittae. Males have a black scutellum, straight conical, and apically pointed abdomen, with 3 narrow gold bands interspersed with black wrapping the abdomen, terminating in a black tip. Female abdomen with a strong down-pointing curve abdominal, 3 grayish abdominal bands lacking mid-dorsal point.
Etymology
From the Latin “uniformis”, for not changing in form or character, in reference to the uniform nature of the pruinose bands on the abdomen.
Distribution
Costa Rica, ACG, Prov. Alajuela, rain forest, 109–135 m elevation.
Ecology
Reared from, Elachistidae, Stenoma Janzen44 and Antaeotricha spurca (2 records). One fly larva per caterpillar.
Trigonospila edwinbermudezi , sp. n.
-
scientificName: Trigonospila edwinbermudezi; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:edwinbermudezi; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector Pitilla; verbatimLocality:Medrano; verbatimElevation:380; verbatimLatitude:11.01602; verbatimLongitude:-85.38053; verbatimCoordinateSystem:Decimal; decimalLatitude:11.01602; decimalLongitude:-85.38053; samplingProtocol:reared from caterpillar of Paridnea holophaealis (Pyralidae); verbatimEventDate:Aug-16-2014; individualID:DHJPAR0056126; individualCount:1; sex:M; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0056126; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:14-SRNP-71290; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila edwinbermudezi; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:edwinbermudezi; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector El Hacha; verbatimLocality:Finca Araya; verbatimElevation:295; verbatimLatitude:11.015; verbatimLongitude:-85.511; verbatimCoordinateSystem:Decimal; decimalLatitude:11.015; decimalLongitude:-85.511; samplingProtocol:reared from caterpillar of Omphalocera cariosa (Pyralidae); verbatimEventDate:Sep-11-2002; individualID:DHJPAR0018447; individualCount:1; sex:F; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0018447; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:02-SRNP-28022; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila edwinbermudezi; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:edwinbermudezi; scientificNameAuthorship:Fleming & Wood, 2015; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Area de Conservacion Guanacaste; locality:Sector San Cristobal; verbatimLocality:Quebrada Garcia; verbatimElevation:495; verbatimLatitude:10.861; verbatimLongitude:-85.426; verbatimCoordinateSystem:Decimal; decimalLatitude:10.861; decimalLongitude:-85.426; samplingProtocol:reared from caterpillar of Paridnea holophaealis (Pyralidae); verbatimEventDate:03-Aug-2011; individualID:DHJPAR0044883; individualCount:1; sex:F; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0044883; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-2384; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
Description
Male (Fig.
Female (Fig.
Diagnosis
Small black and gold fly, with 4 prominent black stripes on the thorax, appearing as 2 large thoracic vittae with a golden divider, and a golden scutellum. Female abdomen with a strong down-pointing curve abdominal bands mid-dorsally pointed joining the next segment’s gold band so that 4 small black triangles become apparent on abdomen. The tip of the abomen golden.
Etymology
Trigonospila edwinbermudezi is named in honor of Edwin Bermudez, the first Encargado de Sector for Sector El Hacha of ACG.
Distribution
Costa Rica, ACG, Prov. Guanacaste, rain forest and dry forest-rain forest interface, 295–495 m elevation.
Ecology
Reared from, Pyralidae, Omphalocera cariosa and Paridnea holophaealis (3 records). One fly larva per caterpillar.
Trigonospila panamensis
-
scientificName: Trigonospila panamensis; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:panamensis; scientificNameAuthorship:Townsend, 1919; continent:Central America; country:Costa Rica; countryCode:CR; stateProvince:Guanacaste; county:Sector Horizontes; locality:Area de Conservacion Guanacaste; verbatimLocality:Sitio La Dama; verbatimElevation:105; verbatimLatitude:10.786; verbatimLongitude:-85.558; verbatimCoordinateSystem:Decimal; decimalLatitude:10.786; decimalLongitude:-85.558; samplingProtocol:Host Collection; verbatimEventDate:04-Oct-1996; individualID:DHJPAR0018446; individualCount:1; sex:F; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0018446; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:96-SRNP-9740; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila panamensis; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:panamensis; scientificNameAuthorship:Townsend, 1919; continent:Central America; country:Costa Rica; stateProvince:Guanacaste; county:Sector Santa Rosa; locality:Area de Conservacion Guanacaste; verbatimLocality:Cafetal; verbatimElevation:280; verbatimLatitude:10.858; verbatimLongitude:-85.611; verbatimCoordinateSystem:Decimal; decimalLatitude:10.858; decimalLongitude:-85.611; samplingProtocol:Host Collection; verbatimEventDate:26-Jun-2009; individualID:DHJPAR0035626; individualCount:1; sex:F; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0035626; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:09-SRNP-13144; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila panamensis; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:panamensis; scientificNameAuthorship:Townsend, 1919; continent:Central America; country:Costa Rica; stateProvince:Guanacaste; county:Sector Santa Rosa; locality:Area de Conservacion Guanacaste; verbatimLocality:Cafetal; verbatimElevation:280; verbatimLatitude:10.858; verbatimLongitude:-85.611; verbatimCoordinateSystem:Decimal; decimalLatitude:10.858; decimalLongitude:-85.611; samplingProtocol:Host Collection; verbatimEventDate:16-Jun-2009; individualID:DHJPAR0036435; individualCount:1; sex:M; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0036435; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:09-SRNP-13124; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila panamensis; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:panamensis; scientificNameAuthorship:Townsend, 1919; continent:Central America; country:Costa Rica; stateProvince:Guanacaste; county:Sector Santa Rosa; locality:Area de Conservacion Guanacaste; verbatimLocality:Cafetal; verbatimElevation:280; verbatimLatitude:10.858; verbatimLongitude:-85.611; verbatimCoordinateSystem:Decimal; decimalLatitude:10.858; decimalLongitude:-85.611; samplingProtocol:Host Collection; verbatimEventDate:15-Jun-2009; individualID:DHJPAR0036444; individualCount:1; sex:M; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0036444; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:09-SRNP-13112; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
-
scientificName: Trigonospila panamensis; phylum:Arthropoda; class:Insecta; order:Diptera; family:Tachinidae; genus:Trigonospila; specificEpithet:panamensis; scientificNameAuthorship:Townsend, 1919; continent:Central America; country:Costa Rica; stateProvince:Alajuela; county:Sector Rincon Rain Forest; locality:Area de Conservacion Guanacaste; verbatimLocality:Baches; samplingProtocol:Host Collection; verbatimEventDate:15-Feb-2011; individualID:DHJPAR0042319; individualCount:1; sex:M; lifeStage:adult; preparations:pinned; catalogNumber:DHJPAR0042319; occurrenceDetails: ; recordedBy:D.H. Janzen & W. Hallwachs; otherCatalogNumbers:11-SRNP-67043; identifiedBy:AJ Fleming; dateIdentified:2015; language:en; institutionCode:CNC; collectionCode:Insects; basisOfRecord:Pinned Specimen
Description
Male, previously unknown from the original description of T. panamensis
Female (Fig.
The authors wish to caution that this species-level identification is based solely on morphology, since no DNA barcoded specimens of T. panamensis are available for molecular comparison.
Diagnosis
Small black and gray fly, with 4 prominent black stripes on the thorax, these do not smudge together and remain distinctively separate in females, scutellum gold. Males with a straight conical, and apically pointed abdomen, with 3 gold bands interspersed with black wrapping the abdomen, terminating in a black tip. Female abdomen with a strong down-pointing curved abdomen, abdominal bands mid-dorsally pointed joining the next segment’s gold band so that 4 small black dashes become apparent on abdomen.
Distribution
Panama, Taboga Island; Costa Rica, ACG, Prov. Alajuela and Guanacaste, rain forest and dry forest, 105–280 m elevation.
Ecology
Reared from, Crambidae, Elachistidae, Tortricidae, and Pyralidae (7 records). One fly larva per caterpillar.
Identification keys
|
Key to the species of Trigonospila reared from caterpillars in Area de Conservación Guanacaste, Northwestern Costa Rica This key was prepared based on the specimens collected as a result of the 40+ year inventory still being conducted in ACG. Our key is intended to identify the fauna present within the confines of the ACG. |
||
| 1 | Proclinate orbital bristles present (♀) (Figs |
2 |
| – | Proclinate orbital bristles absent (♂) (Figs |
5 |
| 2 | Abdominal tergites dark velvety black, with dull, grayish bands covering up to 1/2 of tergal surface; bands are transverse with no distinctive mid-dorsal extension posteriorly (Fig. |
T. uniformis sp. n. |
| – | Abdominal tergites dark velvety black, with dull, grayish or bright yellow bands covering at least 1/2 of tergal surface; transverse bands possessing mid-dorsal extensions posteriorly creating an apparent dorsocentral stripe extending the length of the abdomen (Fig. |
3 |
| 3 | Abdominal banding extending to cover more than ½ of T3, banding on T4 covering all but 1/5th of tergal surface (Fig. |
T. panamensis (Townsend) |
| – | Abdominal banding extending to cover up to ½ of T3, and T4, when coupled with dorsocentral stripe 4 black triangles become evident (Fig. |
4 |
| 4 | Abdominal banding extending out onto posterior margin of ST1+2 extending beyond the insertion point of median marginal bristles on ST1+2 (Fig. |
T. edwinbermudezi sp. n. |
| – | Abdominal banding extending out onto posterior margin of ST1+2 extending up to but not beyond the insertion point of median marginal bristles on ST1+2 (Fig. |
T. josemariamoragai sp. n. |
| 5 | Scutellum with white pruinosity only at tip (occupying 1/3 or less of total area); abdominal tergites dark velvety black, with bright, narrow yellow bands covering up to 1/5th of tergal surface, bands not wrapping around to underside of tergites; bright yellow bands straddling the margin between ST1+2 and T3, and the anterior margin of T4 flat, with no distinctive mid-dorsal peaks (Fig. |
T. uniformis sp. n. |
| – | Scutellum bearing white or yellowish pruinosity over 2/3 or more of total area; abdominal tergites dark velvety black, with bright, narrow yellow bands covering 1/3rd or more of tergal surface, bands wrapping around to underside of tergites; bright yellow bands straddling the margin between ST1+2 and T3, and the anterior margin of T4, either with rough edging or the presence of a distinct mid-dorsal peak (Fig. |
6 |
| 6 | Tergal bands not possessing a sharp mid-dorsal peak, instead the margins of the bands appearing as jagged on both T3 and T4. | T. panamensis (Townsend) |
| – | Tergal bands possessing a sharp mid-dorsal peak figuring prominently on both T3 and T4. | 7 |
| 7 | Mid-dorsal peak extending almost to hind margin of T3; parafacial with no traces of silver; thoracic vittae fused throughout their entire length. | T. josemariamoragai sp. n. |
| – | Mid-dorsal peak not extending to hind margin T3 of adjacent tergite; parafacial with silver on lower half; thoracic vittae not fused pre-suturally. | T. edwinbermudezi sp. n. |
Acknowledgements
We gratefully acknowledge the unflagging support of the team of ACG parataxonomists (Janzen et al. 2009, Janzen & Hallwachs 2011) who found and reared the specimens used in this study, and the team of biodiversity managers who protect and manage the ACG forests that host these tachinids and their caterpillar hosts. The study has been supported by U.S. National Science Foundation grants BSR 9024770 and DEB 9306296, 9400829, 9705072, 0072730, 0515699, and grants from the Wege Foundation, International Conservation Fund of Canada, Jessie B. Cox Charitable Trust, Blue Moon Fund, Guanacaste Dry Forest Conservation Fund, Area de Conservación Guanacaste, Permian Global, and University of Pennsylvania (DHJ&WH). This study has been supported by the Government of Canada through its ongoing support of the Canadian National Collection, Genome Canada, the Biodiversity Institute of Ontario, and the Ontario Genomics Institute (2008–0GI–ICI–03) (MAS), and by a Discovery Grant from Natural Sciences and Engineering Research Council of Canada (MAS).
References
- Die Zweiflügler des Kaiserlichen Museums zu Wien. V. Vorarbeiten zu einer Monographie der Muscaria schizometopa (exclusive Anthomyidae). Pars II.Denkschriften der Kaiserlichen Akademie der Wissenschaften. Wien. Mathematisch-Naturwissenschaftliche Klasse58:305‑446.
- A conspectus of the Tachinidae (Diptera) of Australia, including keys to the supraspecific taxa and taxonomic and host catalogues.21:1‑221.
- A taxonomic conspectus of the Tachinidae (Diptera) of the Oriental Region.Bulletin of the British Museum (Natural History) Entomology26:1‑358.
- Crosskey RW (1980) [Chapter] 93. Family Tachinidae pp. 822–882. In: Crosskey RW (Ed.) Catalogue of the Diptera of the Afrotropical Region.British Museum (Natural History),London.
- Curran C (1926) Appendix. New Diptera from Jamaica pp. 102–114. In: Gowdey CC (Ed.) Entomological Bulleting No. 4 Parts 1 and 2: Catalogus insectorum jamaicensis.Jamaica Government Printing Office,Kingston,114pp.
- Review of Apanteles sensu stricto (Hymenoptera, Braconidae, Microgastrinae) from Area de Conservación Guanacaste, northwestern Costa Rica, with keys to all described species from Mesoamerica.ZooKeys383:1‑565. https://doi.org/10.3897/zookeys.383.6418
- Seven new species of Spathidexia Townsend (Diptera: Tachinidae) reared from caterpillars in Area de Conservación Guanacaste, Costa Rica.Biodiversity Data Journal3:e4597. https://doi.org/10.3897/bdj.3.e4597
- Revision of the New World species of Houghia Coquillett (Diptera, Tachinidae) reared from caterpillars in Area de Conservación Guanacaste, Costa Rica.Zootaxa3858:1‑1. https://doi.org/10.11646/zootaxa.3858.1.1
- A new species of Cordyligaster Macquart, reared from caterpillars in Area de Conservacion Guanacaste, northwestern Costa Rica.Biodiversity Data Journal2:4174‑4174. https://doi.org/10.3897/BDJ.2.e4174
- An inexpensive, automation-friendly protocol for recovering high-quality DNA.Molecular Ecology Notes6(4):998‑1002. https://doi.org/10.1111/j.1471-8286.2006.01428.x
- Joining inventory by parataxonomists with DNA barcoding of a large complex tropical conserved wildland in northwestern Costa Rica.PLoS ONE6:e18123. https://doi.org/10.1371/journal.pone.0018123
- Integration of DNA barcoding into an ongoing inventory of complex tropical biodiversity.Molecular Ecology Resources9:1‑26. https://doi.org/10.1111/j.1755-0998.2009.02628.x
- Reading the complex skipper butterfly fauna of one tropical place.PloS one6:e19874. https://doi.org/10.1371/journal.pone.0019874
- Diptères exotiques nouveaux ou peu connus.Memoires de la Société (Royale) des sciences, de l'agriculture et des arts à Lille4:134‑294.
- Essai sur les Tachinaires (Larvaevoridae).7:1‑67.
- World genera of the Tachinidae (Diptera) and their regional occurrence. Version 8.0.Published on the internet,Ottawa, Canada,87pp. URL: http://www.nadsdiptera.org/Tach/WorldTachs/Genera/Worldgenera.htm
- Catalogue of the Tachinidae (Diptera) of America north of Mexico.Memoirs on Entomology, International18:1‑410.
- Études sur les Muscides de France 2e partie.Revue D'Entomologie Publiée par la Société Française D'Entomologie13:1‑113.
- Études sur les muscides de France 2e partie.Revue d'Entomologie Publiée par la Société Francaise d'Entomologie15:1‑230.
- Vier neue österreichische Dipteren.Wiener Entomologische Zeitung5:191‑196.
- BOLD: The Barcode of Life Data System (http://www.barcodinglife.org).Molecular Ecology Notes7(3):355‑364. https://doi.org/10.1111/j.1471-8286.2007.01678.x
- New Tachinidae from northeastern United States (Diptera).Bulletin of the Brooklyn Entomological Society38:78‑90.
- Notes on muscoid synonymy with descriptions of three new species (Diptera).Proceedings of the Entomological Society of Washington.55:243‑247.
- Extrapolations from field studies and known faunas converge on dramatically increased estimates of global microgastrine parasitoid wasp species richness (Hymenoptera: Braconidae).Insect Conservation and Diversity6(4):530‑536. https://doi.org/10.1111/icad.12003
- The neighbor-joining method: a new method for reconstructing phylogenetic trees.Molecular Biology and Evolution4:406‑425.
- A Host-Parasite Catalog of Tachinidae (Diptera) of Japan.Makunagi/ Acta Dipterologica. Supplement2:1‑171.
- Hyperparasitoid wasps (Hymenoptera, Trigonalidae) reared from dry forest and rain forest caterpillars of Area de Conservación Guanacaste, Costa Rica.Journal of Hymenoptera Research29:119‑144. https://doi.org/10.3897/jhr.29.3233
- DNA barcode accumulation curves for understudied taxa and areas.Molecular Ecology Resources9:208‑216. https://doi.org/10.1111/j.1755-0998.2009.02646.x
- DNA barcodes affirm that 16 species of apparently generalist tropical parasitoid flies (Diptera, Tachinidae) are not all generalists.Proceedings of the National Academy of Sciences104(12):4967‑4972. https://doi.org/10.1073/pnas.0700050104
- DNA barcodes reveal cryptic host-specificity within the presumed polyphagous members of a genus of parasitoid flies (Diptera: Tachinidae).Proceedings of the National Academy of Sciences103(10):3657‑3662. https://doi.org/10.1073/pnas.0511318103
- Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology, and collections.Proceedings of the National Academy of Sciences105(34):12359‑12364. https://doi.org/10.1073/pnas.0805319105
- Estudio preliminar de Trigonospila sp. (Diptera: Tachinidae), parasitoide de Compsus viridilineatus (Coleoptera: Curculionidae).Boletín Científico Centro de Museos, Museo de Historia Natural15:150‑159.
- The tachinids of Trinidad. 11. Echinomyiines, Dexiines, and allies.Canadian Journal of Zoology41:335‑576. https://doi.org/10.1139/z63-029
- New muscoid genera species and synonymy. (Diptera).Insecutor Inscitiae Menestruus6:157‑182.
- New Muscoid flies in the collection of the Deutsches Entomologisches Institut in Berlin.Entomologica Mitteilungen16:277‑287.
- New genera and species of Old World oestromuscoid flies.40:439‑479.
- A taxonomic conspectus of the Blondeliini of North and Central America and the West Indies (Diptera: Tachinidae).Memoirs of the Entomological Society of Canada132:1‑132. https://doi.org/10.4039/entm117132fv
- Wood DM, Zumbado MA (2010) Tachinidae (tachinid flies, parasitic flies) pp. 1343–1417. In: Brown BV, Borkent A, Cumming JM, Wood DM, Woodley NE, Zumbado MA (Eds) Manual of Central American Diptera.2.NRC Research Press,Ottawa,xvi + 715–1442pp.
- Wulp F (1890) Family Muscidae. In: Godman FD, Salvin O (Eds) Biologia Centrali-Americana Insecta, Diptera.2.
- Diptera Scandinaviæ disposita et descripta.Ex Officina Lundbergiaiva, Sumtibus Regiis.,Lund,435pp.
Supplementary material
Neighbor-joining tree of DNA barcodes from ACG inventory Trigonospila as of January, 2015. The inventory is ongoing and as new specimens are added, they can be accessed on BOLD.