|
Biodiversity Data Journal :
Taxonomic paper
|
|
Corresponding author:
Academic editor: Jukka Salmela
Received: 02 Feb 2015 | Accepted: 03 Mar 2015 | Published: 05 Mar 2015
© 2015 Torsten Dikow
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Dikow T (2015) Review of Anasillomos Londt, 1983 with the description of a new species (Insecta: Diptera: Asilidae). Biodiversity Data Journal 3: e4652. https://doi.org/10.3897/BDJ.3.e4652
|
|
Abstract
The southern African assassin-fly genus Anasillomos Londt, 1983 is reviewed. A new species, Anasillomos juergeni sp. n., is described from the Namib desert and represents the second species in the genus. Descriptions/re-descriptions, photographs, and identification keys are provided to aid in the identification. Distribution, occurrence in biodiversity hotspots sensu Conservation International, and seasonal incidence are discussed.
Keywords
Afrotropical, assassin flies, identification keys, cybertaxonomy, data sharing
Introduction
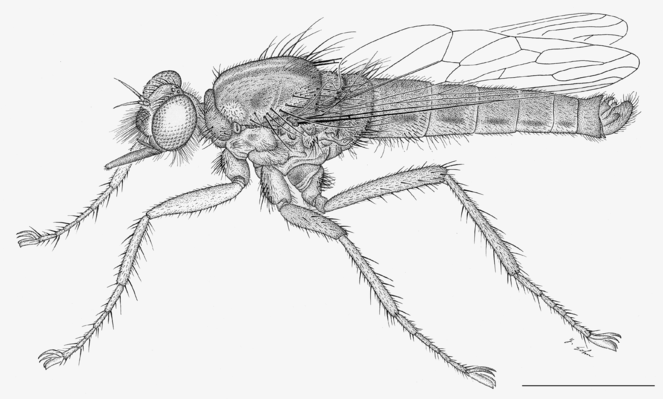
The genus Anasillomos Londt, 1983 (Fig.
Habitat photographs of Namib sand dunes at Gobabeb and Karoo near Grünau. Anasillomos juergeni sp. n. was collected at Gobabeb and Anasillomos chrysopos and Anasillomos juergeni sp. n. were collected near Grünau.
b: large, mostly bare sand dunes and partly vegetated interdune valleys at Gobabeb (23°34'17"S 015°02'52"E)
c: small, vegetated sand dunes at Gobabeb (23°33'50"S 015°02'01"E)
d: habitat near Grünau (27°30'45"S 017°50'01"E)
This review was instigated by the collection of 10 specimens of Anasillomos at the Gobabeb Research and Training Centre (www.gobabebtrc.org) by the author in February 2012. Even though only a single male specimen was collected during this field trip, comparison with the original description and the illustrations of the male terminalia provided by
Materials and methods
Morphological features were examined using a Zeiss SteREO Discovery.V12 stereo microscope. Wing length is measured from the tegula to the distal tip of the wing. The female genitalia and male terminalia were first excised and macerated in 10% potassium hydroxide (KOH) at 55°C followed by neutralization in acetic acid (CH3COOH) and rinsing in distilled water (H2O). They were temporarily stored in 75% ethanol (C2H5OH) for examination and illustration and eventually sealed in polyethylene vials containing 100% glycerine (C3H8O3) and attached to the specimen's pin.
Terminology
Terminology follows
Species descriptions and re-descriptions
Species descriptions are based on composites of all specimens and not exclusively on the holotype and are compiled from a character matrix of 205 features and 242 character states assembled with Lucid Builder (version 3.5) and eventually exported as natural-language descriptions. These species descriptions have been deposited in the Zenodo data depository and can be accessed in XML-format following the SDD (Structure of Descriptive Data) standard (see also Suppl. material
Specimen occurrence data
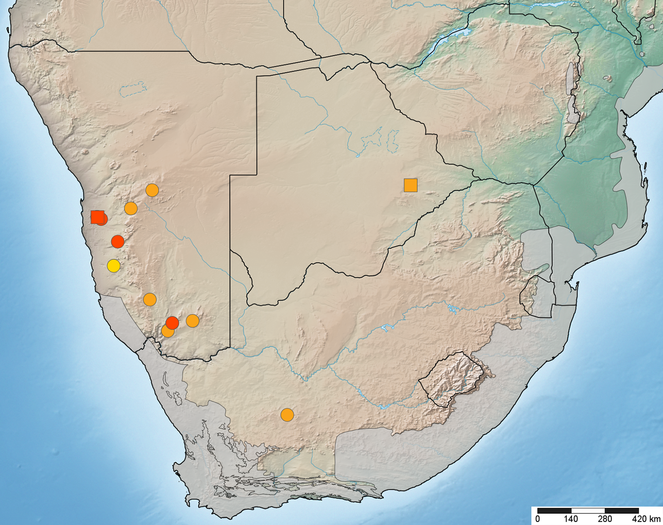
The following data on species occurrences are given (where available): country, state/province, county, locality, geographic co-ordinates (formatted in both decimal and degrees minutes seconds latitude/longitude), elevation (in meters), date of collection (format: yyyy-mm-dd), habitat information, sampling protocol (if other than hand netting), collector, catalog number (a unique specimen number and any other identifying number), depository (institution and collection code), number of specimens, sex, life stage, name of person who identified the specimen, and any other previous identifications. Each specimen is listed with a unique specimen number (either an institutional catalog number or an AAM-XXXXXX number used by the author) that will allow the re-investigation as well as provide a unique Life Science Identifier (LSID). The occurrence of all species is illustrated in distribution maps plotted with SimpleMappr with all of those localities for which co-ordinates are available. Type localities are plotted with a square symbol while all other specimens are plotted with a circular symbol. The distribution map includes Biodiversity Hotspots sensu Conservation International. The specimen occurrence data are deposited as a Darwin Core Archive (DwC-A) in the Global Biodiversity Information Facility (GBIF) using the Integrated Publishing Toolkit (IPT) at the NMNH.
Photographs
Whole habitus photographs of pinned specimens were taken using a Visionary Digital Passport II system (base and StackShot only), an Olympus E-30 digital SLR, a 50 mm macro lens (equivalent to 100 mm focal length in 35 mm photography), and a 25 mm extension tube. The specimens were illuminated by a Falcon FLDM-i200 LED dome-light for even and soft light. Photographs of the female and male terminalia were taken on a Zeiss SteREO Discovery.V12 stereo microscope at 50x magnification and an attached Olympus PEN E-PL5 digital camera. The dissected terminalia were placed in 75% ethanol in a glass dish and illuminated by a Schott VisiLED light source utilizing mixed bright-field (dorsal), dark-field (lateral), and transillumination (ventral). Adobe DNG-format images were stacked using HeliconFocus software. All photographs have been deposited in Morphbank:: Biological Imaging. These images will be automatically harvested by the Encyclopedia of Life (EOL) and are available under the respective species page.
Keys
The dichotomous, interactive key has been build with Lucid Phoenix and the multi-access, matrix-based key with Lucid Builder and both have been deposited in the IdentifyLife archive, registered in Lucidcentral, and made available on the author's research web-site.
Institutions providing specimens
Institutions providing specimens are listed below, together with the abbreviations used in the text when citing depositories (institutionCode), a link to the record in the Global Registry of Biodiversity Repositories (GRBio), and the people who kindly assisted: NMNW – National Museum of Namibia, Windhoek, Khomas, Namibia (A. Kirk-Spriggs); NMSA – KwaZulu-Natal Museum, Pietermaritzburg, KwaZulu-Natal, South Africa (B. Muller, J. Londt); SAMC – Iziko South African Museum, Cape Town, Western Cape, South Africa (D. Lawson, S. van Noort); USNM – National Museum of Natural History, Smithsonian Institution, Washington, DC, USA.
Data resources
Morphbank: image collection ID 851000.
Zenodo: natural-language species descriptions from Lucid Builder in SDD format (also available as Suppl. material
GBIF: specimen occurrence data-set – d61dc8a1-83f7-4905-8863-d19a5a89a0a4 – DOI 10.15468/bd6wxn.
SimpleMappr: distribution map – 2986 (as in Fig.
Map of southern Africa with elevational relief and biodiversity hotspots (in grey) showing distribution of Anasillomos chrysopos (orange) and A. juergeni sp. n. (red). Possible A. juergeni sp. n. from Awasib in yellow (see Discussion). Type localities with square symbol. Map data available in Google Earth KML file and also through GBIF (data-set d61dc8a1-83f7-4905-8863-d19a5a89a0a4).
Lucid Builder: illustrated, multi-entry, matrix-based identification key – asiloidflies.si.edu and IdentifyLife (also available as Suppl. material
Lucid Phoenix: illustrated, dichotomous identification key – asiloidflies.si.edu.
Taxon treatments
Anasillomos
Type species
Diagnosis
Anasillomos has been included in the most recent key to Afrotropical Stenopogoninae sensu
Taxon discussion
The phylogenetic studies on Asilidae based on both morphological (
Anasillomos chrysopos
Nomenclature
Anasillomos chrysopos Londt, 1983: 284.
-
scientificName: Anasillomos chrysopos Londt, 1983; scientificNameID:urn:lsid:zoobank.org:act:38457B23-1147-4C3D-8263-6E93473A0156; family:Asilidae; genus:Anasillomos; specificEpithet:chrysopos; scientificNameAuthorship:Londt, 1983; country:Botswana; stateProvince:Central; locality:Serowe; verbatimCoordinates:22°23'00"S 026°43'00"E; decimalLatitude:-22.38333; decimalLongitude:26.71667; eventDate:1982-08-29; individualCount:1; sex:male; lifeStage:adult; catalogNumber:NMSA-DIP-06957; recordedBy:Forchhammer, P.; identifiedBy:Londt, J.; dateIdentified:1983; institutionCode:NMSA; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos chrysopos Londt, 1983; scientificNameID:urn:lsid:zoobank.org:act:38457B23-1147-4C3D-8263-6E93473A0156; family:Asilidae; genus:Anasillomos; specificEpithet:chrysopos; scientificNameAuthorship:Londt, 1983; country:Botswana; stateProvince:Central; locality:Serowe; verbatimCoordinates:22°23'00"S 026°43'00"E; decimalLatitude:-22.38333; decimalLongitude:26.71667; eventDate:1982-08-29; individualCount:1; sex:male; lifeStage:adult; catalogNumber:NMSA-DIP-66448; recordedBy:Forchhammer, P.; identifiedBy:Londt, J.; dateIdentified:1983; institutionCode:NMSA; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos chrysopos Londt, 1983; scientificNameID:urn:lsid:zoobank.org:act:38457B23-1147-4C3D-8263-6E93473A0156; family:Asilidae; genus:Anasillomos; specificEpithet:chrysopos; scientificNameAuthorship:Londt, 1983; country:Botswana; stateProvince:Central; locality:Serowe; verbatimCoordinates:22°23'00"S 026°43'00"E; decimalLatitude:-22.38333; decimalLongitude:26.71667; eventDate:1982-08-29; individualCount:1; sex:male; lifeStage:adult; catalogNumber:NMSA-DIP-66449; recordedBy:Forchhammer, P.; identifiedBy:Londt, J.; dateIdentified:1983; institutionCode:NMSA; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos chrysopos Londt, 1983; scientificNameID:urn:lsid:zoobank.org:act:38457B23-1147-4C3D-8263-6E93473A0156; family:Asilidae; genus:Anasillomos; specificEpithet:chrysopos; scientificNameAuthorship:Londt, 1983; country:Namibia; stateProvince:Karas; county:Bethanie; locality:Riverside No. 135; verbatimCoordinates:26°38'29"S 016°59'50"E; decimalLatitude:-26.64139; decimalLongitude:16.99722; eventDate:1971-10-23–1971-10-26; habitat:hover near ground among small shrubs; individualCount:1; sex:male; lifeStage:adult; catalogNumber:NMSA-DIP-66450; identifiedBy:Londt, J.; dateIdentified:1983; institutionCode:NMSA; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos chrysopos Londt, 1983; scientificNameID:urn:lsid:zoobank.org:act:38457B23-1147-4C3D-8263-6E93473A0156; family:Asilidae; genus:Anasillomos; specificEpithet:chrysopos; scientificNameAuthorship:Londt, 1983; country:Namibia; stateProvince:Karas; county:Bethanie; locality:Riverside No. 135; verbatimCoordinates:26°38'29"S 016°59'50"E; decimalLatitude:-26.64139; decimalLongitude:16.99722; eventDate:1971-10-23–1971-10-16; habitat:hover near ground among small shrubs; individualCount:1; sex:male; lifeStage:adult; catalogNumber:NMSA-DIP-06963; identifiedBy:Londt, J.; dateIdentified:1983; institutionCode:NMSA; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos chrysopos Londt, 1983; scientificNameID:urn:lsid:zoobank.org:act:38457B23-1147-4C3D-8263-6E93473A0156; family:Asilidae; genus:Anasillomos; specificEpithet:chrysopos; scientificNameAuthorship:Londt, 1983; country:Namibia; stateProvince:Karas; locality:Ai-Ais Nature Reserve, NW Grünau (road D601); verbatimCoordinates:27°30'45"S 017°50'01"E; decimalLatitude:-27.5125; decimalLongitude:17.83361; eventDate:1999-12-09; individualCount:1; sex:female; lifeStage:adult; catalogNumber:NMSA-DIP-66452; recordedBy:Dikow, T.; previousIdentifications:Anasillomos sp. by Londt, J.; Dikow, T. in 2000; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:NMSA; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos chrysopos Londt, 1983; scientificNameID:urn:lsid:zoobank.org:act:38457B23-1147-4C3D-8263-6E93473A0156; family:Asilidae; genus:Anasillomos; specificEpithet:chrysopos; scientificNameAuthorship:Londt, 1983; country:Namibia; stateProvince:Karas; locality:Ai-Ais Nature Reserve, NW Grünau (road D601); verbatimCoordinates:27°30'45"S 017°50'01"E; decimalLatitude:-27.5125; decimalLongitude:17.83361; eventDate:1999-12-09; individualCount:1; sex:female; lifeStage:adult; catalogNumber:NMSA-DIP-66451; recordedBy:Dikow, T.; previousIdentifications:Anasillomos sp. by Londt, J.; Dikow, T. in 2000; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:NMSA; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos chrysopos Londt, 1983; scientificNameID:urn:lsid:zoobank.org:act:38457B23-1147-4C3D-8263-6E93473A0156; family:Asilidae; genus:Anasillomos; specificEpithet:chrysopos; scientificNameAuthorship:Londt, 1983; country:South Africa; stateProvince:Northern Cape; locality:Carnarvon, 2 km NE; verbatimElevation:1350 m; verbatimCoordinates:30°56'07"S 022°07'03"E; decimalLatitude:-30.93528; decimalLongitude:22.1175; eventDate:1986-11-14; habitat:flat scrubland; individualCount:1; sex:female; lifeStage:adult; catalogNumber:NMSA-DIP-06965; recordedBy:Londt, J.; identifiedBy:Dikow, T.; dateIdentified:2000; institutionCode:NMSA; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos chrysopos Londt, 1983; scientificNameID:urn:lsid:zoobank.org:act:38457B23-1147-4C3D-8263-6E93473A0156; family:Asilidae; genus:Anasillomos; specificEpithet:chrysopos; scientificNameAuthorship:Londt, 1983; country:Namibia; stateProvince:Karas; county:Karasburg; locality:Ai Ais, 30 km NE, Altdorn No. 3; verbatimElevation:640 m; verbatimCoordinates:27°48'16"S 017°40'02"E; decimalLatitude:-27.80444; decimalLongitude:17.66722; samplingProtocol:Malaise trap; eventDate:1996-11-19; habitat:dry wash; individualCount:1; sex:female; lifeStage:adult; catalogNumber:INHS-33959; recordedBy:Irwin, M.; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:INHS; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos chrysopos Londt, 1983; scientificNameID:urn:lsid:zoobank.org:act:38457B23-1147-4C3D-8263-6E93473A0156; family:Asilidae; genus:Anasillomos; specificEpithet:chrysopos; scientificNameAuthorship:Londt, 1983; country:Namibia; stateProvince:Karas; locality:Awasib; verbatimCoordinates:25°23'00"S 015°39'00"E; decimalLatitude:-25.38333; decimalLongitude:15.65; eventDate:1971-11-09–1971-11-10; individualCount:1; sex:male; lifeStage:adult; identifiedBy:Londt, J.; dateIdentified:1983; institutionCode:NMNW; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos chrysopos Londt, 1983; scientificNameID:urn:lsid:zoobank.org:act:38457B23-1147-4C3D-8263-6E93473A0156; family:Asilidae; genus:Anasillomos; specificEpithet:chrysopos; scientificNameAuthorship:Londt, 1983; country:Namibia; stateProvince:Khomas; locality:Windhoek; verbatimCoordinates:22°34'00"S 017°05'00"E; decimalLatitude:-22.56667; decimalLongitude:17.08333; eventDate:1984-09-26; individualCount:1; sex:female; lifeStage:adult; catalogNumber:NMNW-H60955; recordedBy:Irish, J.; identifiedBy:Londt, J.; dateIdentified:2005; institutionCode:NMNW; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos chrysopos Londt, 1983; scientificNameID:urn:lsid:zoobank.org:act:38457B23-1147-4C3D-8263-6E93473A0156; family:Asilidae; genus:Anasillomos; specificEpithet:chrysopos; scientificNameAuthorship:Londt, 1983; country:Namibia; stateProvince:Khomas; locality:Windhoek; verbatimCoordinates:22°34'00"S 017°05'00"E; decimalLatitude:-22.56667; decimalLongitude:17.08333; eventDate:1973-10-07–1973-10-12; individualCount:1; sex:female; lifeStage:adult; identifiedBy:Londt, J.; Dikow, T.; dateIdentified:2000; institutionCode:NMNW; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos chrysopos Londt, 1983; scientificNameID:urn:lsid:zoobank.org:act:38457B23-1147-4C3D-8263-6E93473A0156; family:Asilidae; genus:Anasillomos; specificEpithet:chrysopos; scientificNameAuthorship:Londt, 1983; country:Namibia; stateProvince:Khomas; locality:Windhoek; verbatimCoordinates:22°34'00"S 017°05'00"E; decimalLatitude:-22.56667; decimalLongitude:17.08333; eventDate:1973-10-07–1973-10-12; individualCount:1; sex:undetermined; lifeStage:adult; identifiedBy:Londt, J.; Dikow, T.; dateIdentified:2000; institutionCode:NMNW; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos chrysopos Londt, 1983; scientificNameID:urn:lsid:zoobank.org:act:38457B23-1147-4C3D-8263-6E93473A0156; family:Asilidae; genus:Anasillomos; specificEpithet:chrysopos; scientificNameAuthorship:Londt, 1983; country:Namibia; stateProvince:Khomas; locality:Windhoek; verbatimCoordinates:22°34'00"S 017°05'00"E; decimalLatitude:-22.56667; decimalLongitude:17.08333; eventDate:1973-10-04–1973-10-08; individualCount:1; sex:female; lifeStage:adult; identifiedBy:Londt, J.; Dikow, T.; dateIdentified:2000; institutionCode:NMNW; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos chrysopos Londt, 1983; scientificNameID:urn:lsid:zoobank.org:act:38457B23-1147-4C3D-8263-6E93473A0156; family:Asilidae; genus:Anasillomos; specificEpithet:chrysopos; scientificNameAuthorship:Londt, 1983; country:Namibia; stateProvince:Karas; locality:Great Karas Mountains; verbatimCoordinates:27°26'00"S 018°35'22"E; decimalLatitude:-27.43333; decimalLongitude:18.58944; eventDate:1936-11-00; individualCount:1; sex:male; lifeStage:adult; catalogNumber:SAM-DIP-A008264; recordedBy:SAM Museum Staff; identifiedBy:Londt, J.; institutionCode:SAMC; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos chrysopos Londt, 1983; scientificNameID:urn:lsid:zoobank.org:act:38457B23-1147-4C3D-8263-6E93473A0156; family:Asilidae; genus:Anasillomos; specificEpithet:chrysopos; scientificNameAuthorship:Londt, 1983; country:Namibia; stateProvince:Khomas; locality:Hakos Mountains, 191 km E Walvis Bay; verbatimCoordinates:23°14'43"S 016°17'22"E; decimalLatitude:-23.24528; decimalLongitude:16.28944; eventDate:1983-11-13; individualCount:1; sex:female; lifeStage:adult; catalogNumber:USNMENT00995574; recordedBy:Moore, A.; previousIdentifications:Anasillomos sp. by Londt, J. in 1994; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:USNM; basisOfRecord:PreservedSpecimen
Description
Head: wider than high, black; vertex slightly depressed (less than 60° angle on median margin of compound eye); facial swelling extending over lower ¾ of face, white pubescent; mystax white macrosetose on ventral margin and light brown macrosetose elsewhere, extending over lower ¾ of face; ommatidia of different size, at least some median ommatidia distinctly larger; postgena posterior margin simple, smooth; frons (at level of antennal insertion) more or less parallel-sided, apubescent antero-medially, grey to golden pubescent otherwise, white setose and light brown macrosetose laterally; ocellar tubercle golden pubescent, light brown setose and macrosetose; vertex golden pubescent, asetose; median occipital sclerite (m ocp scl) with several light brown macrosetae; postocular (pocl) setae straight, light brown macrosetae; occiput dorso-medially golden pubescent and laterally grey pubescent, dorso-medially with V-shaped apubescent stripes, white setose.
Proboscis and maxillary palpus: proboscis straight, black; postmentum plate-like, straight, ventral margin entirely smooth, white setose ventrally; prementum circular in cross section proximally, with dorso-median flange, white setose proximo-ventrally; labella reduced, fused to prementum entirely, small, only forming distal tip of proboscis, apex rounded; maxillary palpus two-segmented, black, distal palpomere cylindrical, yellowish setose and macrosetose; stipites fused entirely medially, apubescent, long white setose.
Antenna: light brown, lightly grey pubescent; scape 2x as long as pedicel, short white and light brown setose dorsally and long light brown macrosetose ventrally; pedicel white and light brown setose distally; postpedicel cylindrical (same diameter throughout), 1.5x as long as scape and pedicel combined, sparsely white setose dorso-distally; stylus comprised of 1 element, asetose; apical seta-like sensory element situated apically on stylus.
Thorax: brown to dark brown, postpronotal lobes and lateral scutum light brown to orange; prosternum apubescent, separated from proepisternum, square to rectangular in shape (straight dorsally); proepisternum grey pubescent, white setose and light brown macrosetose; cervical sclerite long white setose; antepronotum golden pubescent, white setose and light brown macrosetose; postpronotum golden pubescent, white setose and light brown macrosetose, postpronotal lobe white setose and long light brown macrosetose; pleuron grey and golden pubescent; proepimeron white setose; anepisternum anterior half asetose, dorso-medially white to light brown macrosetose (6–7 macrosetae), posterior half sparsely long white setose, supero-posteriorly white setose (not macrosetose); anterior basalare asetose, posterior basalare asetose; anepimeron predominantly asetose, antero-dorsally white setose, katepisternum predominantly asetose, postero-dorsally white setose, katepimeron asetose, katatergite white setose and long yellowish macrosetose; meron + metanepisternum asetose, metakatepisternum asetose, metepimeron sparsely white setose; anatergite long white setose; scutum predominantly light brown pubescent, median stripe (extending beyond transverse suture) grey pubescent, paramedian stripes (reaching transverse suture) light brown pubescent, narrow grey pubescent stripes (merging with median stripe beyond transverse suture) grey pubescent, broad stripes (anteriorly wide and posteriorly narrow) brown pubescent (especially visible in anterior and lateral view), sub–lateral spots (1 anterior and 2 posterior to transverse suture) brown pubescent; scutum setation: short white setose anterior and long white setose posterior to transverse suture, setae with small sockets, 3 npl setae, 4 spa setae, 4 pal setae, 2 light brown presutural dc macrosetae and 3–4 light brown postsutural dc macrosetae, acr setae white, presuturally short and longer postsuturally, median posterior scutum (between dc setae) long white setose, setae directed posteriorly; scutellum grey pubescent, ds sctl setae present, long white setae on posterior margin, ap sctl setae present, 6–8 long light brown macrosetae; postmetacoxal area entirely membranous.
Leg: light brown to brown, apubescent, all setae circular in cross section; pro coxa brown, grey pubescent, white setose and light brown macrosetose; pro femur brown, dorsally dark brown, short white setose, light brown macrosetose: 1–2 antero-median, 8–12 postero-dorsal, and 4–5 ventral macrosetae; pro tibia light brown to brown, short white setose, yellowish macrosetose: 7 in 1 antero-dorsal row, 5 in 1 postero-dorsal row, 7 in 1 postero-ventral row, 3 long in 1 postero-ventral row, distally with 6–7 long white macrosetae; mes coxa brown, grey pubescent, white setose and yellowish macrosetose; mes femur brown, dorsally dark brown, short white setose, light brown macrosetose: 2 antero-median, 6–8 postero-dorsal (longest distally), and 5–6 ventral macrosetae; mes tibia brown, short white setose, yellowish macrosetose: 4 in 1 antero-dorsal row, 5 in 1 dorsal row, 5 in 1 posterior row, 3 in 1 postero-ventral row; met coxa brown, grey pubescent, white setose and light brown macrosetose, anteriorly without any protuberance; met trochanter white setose and yellowish macrosetose, cylindrical, medially without protuberance; met femur brown, dorsally dark brown, short white setose, light brown macrosetose: 6–8 anterior, 4–5 ventral, and 3 posterior macrosetae; met tibia brown, straight, short white setose, yellowish macrosetose: 3–4 in 1 antero-dorsal row, 3–4 in 1 dorsal row, 3 in 1 ventral row; proximal pro, mes, and met tarsomeres as long as following 2 tarsomeres combined, pro, mes, and met tarsomeres white setose dorsally, pro tarsomeres white macrosetose disto-laterally and disto-ventrally, mes and met tarsomeres white macrosetose laterally and ventrally; pulvilli well-developed (as long as claw); claw abruptly angled distally, pointed; empodium setiform, well-developed (as long as pulvilli).
Wing: length 8.1–9.5 mm, wing membrane hyaline, without microtrichia; C circumambient (developed around entire wing), anterior wing margin straight; R₂₊₃ distally distinctly arching anteriorly, r₁ open; R₄ terminating anterior to wing apex, distinctly arching anteriorly, stump vein (R₃) absent; r₄ open, R₄ and R₅ diverging from each other; R₅ not reaching C (or wing margin); r₅ closed and petiolate; M₁ not reaching C (or wing margin); cell d closed by base of M₂ and m-m, M₂ and m-m not aligned, r-m situated in distal half of cell d; m₃ closed at C (non-petiolate) or open, M₃ and M₄ approximating at C; cua closed at C (non-petiolate); alula well-developed; microtrichia on posterior wing margin arranged in a single plane.
Abdomen: brown to black, laterally light brown to orange, tergites smooth, setae with small sockets only; T1 white setose, laterally long light brown macrosetose, grey pubescent, entirely sclerotized medially, dorsal surface smooth, without protuberances; T2–8 entirely sclerotized, predominantly black, sometimes brown to orange laterally, predominantly grey pubescent, T2 with apubescent stripe from antero-lateral corner to postero-median margin, T3–5 with narrower apubescent stripes as on T2, white setose, yellowish macrosetae postero-laterally on T2–4 (most pronounced on T2); marginal macrosetae present on T2–4, medial macrosetae present on T2; S1–8 black, lightly grey pubescent, white, erect setose.
Female: T7 and S7 without modifications, ovipositor comprised of 8th and following segments, T6–8 apubescent, setation directed anteriorly on T6–7 and erect on T8; T8 anteriorly with internal rectangular apodeme (entirely fused to T), S8 plate-like, hypogynial valves extending; T9 and T10 entirely fused, sclerites not distinguishable, T10 divided into two heavily sclerotized acanthophorite plates, with 6–7, yellowish acanthophorite spurs per plate; 3 spermathecae, all equally large, reaching anterior end of segment 6; common spermathecal duct short, not extending beyond tip of furca, individual spermathecal ducts long; ejection apparatus absent; spermathecal reservoirs formed by more or less expanded and coiled ducts, weakly sclerotized; furca (S9) formed by single, inverted V-shaped sclerite, median sclerite (at posterior tip) absent, anterior furcal apodeme absent.
Male (Fig.
Diagnosis
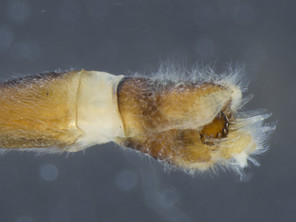
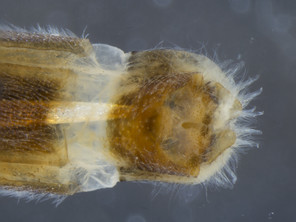
The species is distinguished from its congener by the dorso-medially white macrosetose anepisternum (in addition to white setae, Figs
Distribution
Known from Botswana, Namibia, and South Africa and therefore widely distributed in southern Africa, but rarely collected (Fig.
Taxon discussion
The type locality of A. chrysopos lies in eastern Botswana, far removed from the majority of specimens collected in western Namibia. There is little morphological variation within the species except for the setation color, which can vary from light brown to yellow.
Type locality
Botswana: Central: Serowe (22°23'00"S 026°43'00"E).
Biodiversity hotspot
Not known to occur in any of the southern African biodiversity hotspots (Cape Floristic Region, Maputaland-Pondoland-Albany, or Succulent Karoo).
Anasillomos juergeni , sp. n.
-
scientificName: Anasillomos juergeni sp. n.; scientificNameID: ; family:Asilidae; genus:Anasillomos; specificEpithet:juergeni; country:Namibia; stateProvince:Erongo; locality:Namib-Skeleton Coast National Park, Gobabeb; verbatimElevation:441 m; verbatimCoordinates:23°34'17"S 015°02'52"E; decimalLatitude:-23.57139; decimalLongitude:15.04778; eventDate:2012-02-07; habitat:on dune with grass boulders, perching on sand; individualCount:1; sex:male; lifeStage:adult; catalogNumber:USNMENT00832201; recordedBy:Dikow, T.; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:NMNW; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos juergeni sp. n.; scientificNameID: ; family:Asilidae; genus:Anasillomos; specificEpithet:juergeni; country:Namibia; stateProvince:Erongo; locality:Namib-Skeleton Coast National Park, Gobabeb; verbatimElevation:441 m; verbatimCoordinates:23°34'17"S 015°02'52"E; decimalLatitude:-23.57139; decimalLongitude:15.04778; eventDate:2012-02-07; habitat:on dune with grass boulders, perching on sand; individualCount:1; sex:female; lifeStage:adult; catalogNumber:USNMENT00832190; recordedBy:Dikow, T.; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:USNM; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos juergeni sp. n.; scientificNameID: ; family:Asilidae; genus:Anasillomos; specificEpithet:juergeni; country:Namibia; stateProvince:Erongo; locality:Namib-Skeleton Coast National Park, Homeb; verbatimElevation:445 m; verbatimCoordinates:23°38'34"S 015°10'55"E; decimalLatitude:-23.64278; decimalLongitude:15.18194; eventDate:2012-02-06; habitat:on dune, perching on sand; individualCount:1; sex:female; lifeStage:adult; catalogNumber:USNMENT00832192; recordedBy:Dikow, T.; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:USNM; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos juergeni sp. n.; scientificNameID: ; family:Asilidae; genus:Anasillomos; specificEpithet:juergeni; country:Namibia; stateProvince:Erongo; locality:Namib-Skeleton Coast National Park, Gobabeb; verbatimElevation:393 m; verbatimCoordinates:23°33'50"S 015°02'01"E; decimalLatitude:-23.56389; decimalLongitude:15.03361; eventDate:2012-02-05; habitat:small vegetated dunes and adjacent grassy flats, perching on low dead vegetation; individualCount:1; sex:female; lifeStage:adult; catalogNumber:USNMENT00832195; recordedBy:Dikow, T.; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:USNM; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos juergeni sp. n.; scientificNameID: ; family:Asilidae; genus:Anasillomos; specificEpithet:juergeni; country:Namibia; stateProvince:Erongo; locality:Namib-Skeleton Coast National Park, Gobabeb; verbatimElevation:441 m; verbatimCoordinates:23°34'17"S 015°02'52"E; decimalLatitude:-23.57139; decimalLongitude:15.04778; eventDate:2012-02-07; habitat:on dune with grass boulders, perching on sand; individualCount:1; sex:female; lifeStage:adult; catalogNumber:USNMENT00832196; recordedBy:Dikow, T.; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:USNM; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos juergeni sp. n.; scientificNameID: ; family:Asilidae; genus:Anasillomos; specificEpithet:juergeni; country:Namibia; stateProvince:Erongo; locality:Namib-Skeleton Coast National Park, Gobabeb; verbatimElevation:393 m; verbatimCoordinates:23°33'50"S 015°02'01"E; decimalLatitude:-23.56389; decimalLongitude:15.03361; eventDate:2012-02-05; habitat:small vegetated dunes and adjacent grassy flats, perching on low dead vegetation; individualCount:1; sex:female; lifeStage:adult; catalogNumber:USNMENT00832197; recordedBy:Dikow, T.; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:NMNW; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos juergeni sp. n.; scientificNameID: ; family:Asilidae; genus:Anasillomos; specificEpithet:juergeni; country:Namibia; stateProvince:Erongo; locality:Namib-Skeleton Coast National Park, Gobabeb; verbatimElevation:441 m; verbatimCoordinates:23°34'17"S 015°02'52"E; decimalLatitude:-23.57139; decimalLongitude:15.04778; eventDate:2012-02-07; habitat:on dune with grass boulders, perching on sand; individualCount:1; sex:female; lifeStage:adult; catalogNumber:USNMENT00832198; recordedBy:Dikow, T.; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:USNM; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos juergeni sp. n.; scientificNameID: ; family:Asilidae; genus:Anasillomos; specificEpithet:juergeni; country:Namibia; stateProvince:Erongo; locality:Namib-Skeleton Coast National Park, Gobabeb; verbatimElevation:441 m; verbatimCoordinates:23°34'17"S 015°02'52"E; decimalLatitude:-23.57139; decimalLongitude:15.04778; eventDate:2012-02-07; habitat:on dune with grass boulders, perching on sand; individualCount:1; sex:female; lifeStage:adult; catalogNumber:USNMENT00832200; recordedBy:Dikow, T.; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:NMNW; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos juergeni sp. n.; scientificNameID: ; family:Asilidae; genus:Anasillomos; specificEpithet:juergeni; country:Namibia; stateProvince:Erongo; locality:Namib-Skeleton Coast National Park, Gobabeb; verbatimElevation:441 m; verbatimCoordinates:23°34'17"S 015°02'52"E; decimalLatitude:-23.57139; decimalLongitude:15.04778; eventDate:2012-02-07; habitat:on dune with grass boulders, perching on sand; individualCount:1; sex:female; lifeStage:adult; catalogNumber:USNMENT00832202; recordedBy:Dikow, T.; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:USNM; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos juergeni sp. n.; scientificNameID: ; family:Asilidae; genus:Anasillomos; specificEpithet:juergeni; country:Namibia; stateProvince:Karas; locality:Ai-Ais Nature Reserve, NW Grünau (road D601); verbatimCoordinates:27°30'45"S 017°50'01"E; decimalLatitude:-27.5125; decimalLongitude:17.83361; eventDate:1999-12-09; individualCount:1; sex:female; lifeStage:adult; catalogNumber:NMSA-DIP-06951; recordedBy:Dikow, T.; previousIdentifications:Anasillomos sp. by Londt, J.; Dikow, T. in 2000; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:NMSA; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos juergeni sp. n.; scientificNameID: ; family:Asilidae; genus:Anasillomos; specificEpithet:juergeni; country:Namibia; stateProvince:Karas; locality:Ai-Ais Nature Reserve, NW Grünau (road D601); verbatimCoordinates:27°30'45"S 017°50'01"E; decimalLatitude:-27.5125; decimalLongitude:17.83361; eventDate:1999-12-09; individualCount:1; sex:female; lifeStage:adult; catalogNumber:NMSA-DIP-66453; recordedBy:Dikow, T.; previousIdentifications:Anasillomos sp. by Londt, J.; Dikow, T. in 2000; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:NMSA; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos juergeni sp. n.; taxonRemarks:Paratype of Anasillomos chrysopos Londt, 1983; scientificNameID: ; family:Asilidae; genus:Anasillomos; specificEpithet:juergeni; country:Namibia; stateProvince:Hardap; county:Maltahöhe; locality:Sesriem No. 137; verbatimCoordinates:24°29'00"S 015°48'00"E; decimalLatitude:-24.48333; decimalLongitude:15.8; eventDate:1973-02-15–1973-02-17; individualCount:1; sex:female; lifeStage:adult; catalogNumber:NMSA-DIP-06970; previousIdentifications:Anasillomos chrysopos by Londt, J. in 1983; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:NMSA; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos juergeni sp. n.; taxonRemarks:Paratype of Anasillomos chrysopos Londt, 1983; scientificNameID: ; family:Asilidae; genus:Anasillomos; specificEpithet:juergeni; country:Namibia; stateProvince:Erongo; locality:Gobabeb, Kuiseb River; verbatimCoordinates:23°33'37"S 015°02'00"E; decimalLatitude:-23.56028; decimalLongitude:15.03333; eventDate:1976-10-07; habitat:on dry river bed; individualCount:1; sex:female; lifeStage:adult; catalogNumber:NMSA-DIP-06958; recordedBy:Cunningham, A.; previousIdentifications:Anasillomos chrysopos by Londt, J. in 1983; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:NMSA; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos juergeni sp. n.; scientificNameID: ; family:Asilidae; genus:Anasillomos; specificEpithet:juergeni; country:Namibia; stateProvince:Erongo; locality:Namib-Skeleton Coast National Park, Gobabeb; verbatimElevation:441 m; verbatimCoordinates:23°34'17"S 015°02'52"E; decimalLatitude:-23.57139; decimalLongitude:15.04778; eventDate:2012-02-07; habitat:on dune with grass boulders, perching on sand; individualCount:1; sex:undetermined; lifeStage:adult; catalogNumber:USNMENT00914550; recordedBy:Dikow, T.; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:USNM; basisOfRecord:PreservedSpecimen
-
scientificName: Anasillomos juergeni sp. n.; scientificNameID: ; family:Asilidae; genus:Anasillomos; specificEpithet:juergeni; country:Namibia; stateProvince:Erongo; locality:Gobabeb, Namib Desert Research Station; verbatimCoordinates:23°33'37"S 015°02'26"E; decimalLatitude:-23.56028; decimalLongitude:15.04056; eventDate:1983-11-17; individualCount:1; sex:male; lifeStage:adult; catalogNumber:USNMENT00995573; recordedBy:Moore, A.; previousIdentifications:Anasillomos sp. by Londt, J. in 1994; identifiedBy:Dikow, T.; dateIdentified:2014; institutionCode:USNM; basisOfRecord:PreservedSpecimen
Description
Head: wider than high, black; vertex slightly depressed (less than 60° angle on median margin of compound eye); facial swelling extending over lower ¾ of face, white pubescent; mystax white macrosetose, extending over lower ¾ of face; ommatidia of different size, at least some median ommatidia distinctly larger; postgena posterior margin simple, smooth; frons (at level of antennal insertion) more or less parallel-sided, apubescent antero-medially, grey to golden pubescent otherwise, white setose and macrosetose laterally; ocellar tubercle golden pubescent, white setose and macrosetose; vertex golden pubescent, asetose; median occipital sclerite (m ocp scl) with several white macrosetae; postocular (pocl) setae straight, white macrosetose; occiput predominantly grey pubescent, dorso-medially with V-shaped apubescent stripes and medially with longitudinal light brown pubescent stripe, white setose.
Proboscis and maxillary palpus: proboscis straight, black; postmentum plate-like, straight, ventral margin entirely smooth, white setose ventrally; prementum circular in cross section proximally, with dorso-median flange, white setose proximo-ventrally; labella reduced, fused to prementum entirely, small, only forming distal tip of proboscis, apex rounded; maxillary palpus two-segmented, black, distal palpomere cylindrical, yellowish setose, distally white macrosetose; stipites fused entirely medially, apubescent, long white setose.
Antenna: light brown, lightly grey pubescent; scape 2x as long as pedicel, short white setose dorsally and long white macrosetose ventrally; pedicel white setose distally; postpedicel cylindrical (same diameter throughout), almost 2x as long as scape and pedicel combined, sparsely white setose dorso-distally; stylus comprised of 1 element, asetose; apical seta-like sensory element situated apically on stylus.
Thorax: dark brown to black, postpronotal lobes light brown to orange; prosternum apubescent, separated from proepisternum, square to rectangular in shape (straight dorsally); proepisternum grey pubescent, white setose and macrosetose; cervical sclerite long white setose; antepronotum golden pubescent, white setose and macrosetae; postpronotum golden pubescent, medially white setose, laterally long yellowish macrosetose, postpronotal lobe white setose and long yellowish macrosetose; pleuron predominantly grey and golden pubescent, katepimeron and antero-dorsal meron + metanepisternum apubescent; proepimeron white setose; anepisternum anterior half asetose, posterior half long white setose, supero-posteriorly white setose (not macrosetose); anterior basalare asetose, posterior basalare asetose; anepimeron predominantly asetose, antero-dorsally white setose, katepisternum predominantly asetose, postero-dorsally white setose, katepimeron asetose, katatergite white setose and long yellowish macrosetose; meron + metanepisternum asetose, metakatepisternum asetose, metepimeron sparsely white setose; anatergite long white setose; scutum predominantly grey pubescent, narrow paramedian stripes (just reaching past transverse suture) and sub–lateral spots (1 anterior and 2 posterior to transverse suture) brown pubescent; scutum setation: short white setose anterior and long white setose posterior to transverse suture, setae with small sockets, 3 npl setae, 3–4 spa setae, 4 pal setae, 1 white presutural dc macroseta and 3 white postsutural dc macrosetae, acr setae white, presuturally short and longer postsuturally, median posterior scutum (between dc setae) long white setose, setae directed posteriorly; scutellum grey pubescent, ds sctl setae present, long white setae in posterior half, ap sctl setae present, 5–6 long white macrosetae; postmetacoxal area entirely membranous.
Leg: light brown to brown, apubescent, all setae circular in cross section; pro coxa dark brown, grey pubescent, white setose and macrosetose; pro femur brown, dorsally dark brown, short white setose, white macrosetose: 1 antero-median, 5–6 postero-dorsal, and 6–7 ventral macrosetae; pro tibia brown, short white setose, white macrosetose: 5 in 1 antero-dorsal row, 5 in 1 postero-dorsal row, 4 long in 1 postero-ventral row, distally with 6–7 long white macrosetae; mes coxa dark brown, grey pubescent, white setose and macrosetose; mes femur brown, dorsally dark brown, short white setose, white macrosetose: 2 antero-median, 6–7 postero-dorsal, and 5–6 ventral macrosetae; mes tibia brown, short white setose, white macrosetose: 3 in 1 antero-dorsal row, 2 in 1 antero-ventral row, 4 in 1 dorsal row, 3 in 1 postero-ventral row; met coxa brown, grey pubescent, white setose and macrosetose, anteriorly without any protuberance; met trochanter white setose and macrosetose, cylindrical, medially without protuberance; met femur brown, dorsally dark brown, short white setose, white macrosetose: 6 anterior, 6–7 ventral, and 4–5 postero-ventral macrosetae; met tibia brown, straight, short white setose, white macrosetose: 4 in 1 antero-dorsal row, 3 in 1 dorsal row, 4–5 in 1 ventral row; proximal pro, mes, and met tarsomeres as long as following 2 tarsomeres combined, pro, mes, and met tarsomeres white setose dorsally, pro tarsomeres white macrosetose disto-laterally and disto-ventrally, mes and met tarsomeres white macrosetose laterally and ventrally; pulvilli well-developed (as long as claw); claw abruptly angled distally, pointed; empodium setiform, well-developed (as long as pulvilli).
Wing (Fig.
Abdomen: brown to black, laterally light brown to orange, tergites smooth, setae with small sockets only; T1 white setose, laterally long white macrosetose, grey pubescent, entirely sclerotized medially, dorsal surface smooth, without protuberances; T2–8 entirely sclerotized, predominantly black, sometimes brown to orange laterally, lightly grey pubescent, medially with distinct longitudinal dark grey pubescent stripe, white setose, setae longest on T2, medial setae directed laterally; marginal macrosetae absent from T2–8, medial macrosetae absent from T2–8; S1–8 black, lightly grey pubescent, white, erect setose.
Female (Fig.
Male (Fig.
Diagnosis
The species is distinguished from its congener by the dorso-posterior white setose anepisternum (not macrosetose, Figs
Etymology
The species is named after and dedicated to the memory of my late father, Jürgen Dikow, who has always been supportive of my entomological work and was excited to hear about every new species discovery I made.
Distribution
The species is so far only known from Namibia and in particular from the eastern edge of the Namib desert sand dunes (Gobabeb, Homeb, and Sesriem) as well as the Karoo in southern Namibia (near Grünau) (Fig.
Biology
All recently collected specimens during field work at or near the Gobabeb Research and Training Center conducted in February 2012 were perching on sand. The majority of specimens were collected on the large sand dunes south of the station and Kuiseb river bed (Fig.
Four female specimens have been captured with prey at or near Gobabeb of which three were feeding on Bombyliidae (Diptera) and one on Ichneumonidae (Hymenoptera).
Type locality
Namibia: Erongo: Namib-Skeleton Coast National Park, Gobabeb (dunes south of station at 23°34'17"S 015°02'52"E).
Biodiversity hotspot
Not known to occur in any of the southern African biodiversity hotspots (Cape Floristic Region, Maputaland-Pondoland-Albany, or Succulent Karoo).
Identification keys
|
Key to Anasillomos species |
||
| 1 | Anepisternum dorso-medially white to light brown macrosetose (6–7 short macrosetae) (Fig. |
Anasillomos chrysopos |
| – | Anepisternum dorso-medially only white setose (without short macrosetae) (Figs |
Anasillomos juergeni sp. n. |
Discussion
Seasonal incidence
Anasillomos has been primarily collected between August (Southern Hemisphere late winter) and February (late summer) with the notable exception of January (see Table
Seasonal incidence of Anasillomos species. Numbers refer to specimens / collecting events in particular month. Note that the December records represent a single collecting event near Grünau where both species were collected sympatrically.
|
species |
Jun |
Jul |
Aug |
Sep |
Oct |
Nov |
Dec |
Jan |
Feb |
Mar |
Apr |
May |
|
A. chrysopos |
3/1 |
1 |
5/3 |
5/5 |
2/1 |
|||||||
|
A. juergeni sp. n. |
1 |
1 |
2/1 |
11/4 |
Habitat, sympatry, and a central Namibian locality
On first examination, the two Anasillomos species appear to be occurring in different habitats in that A. chrysopos lives in savanna and Karoo biomes whereas A. juergeni sp. n. occurs in the Namib desert (Fig.
Unfortunately, specimens previously studied by
Acknowledgements
I would like to thank the museum curators who made specimens available through loans and for their hospitality when visiting the collections. Dawn Larson and Simon van Noort (SAMC) provided a photograph of the sole Anasillomos specimen in their care that facilitated re-identification. Furthermore, I would like to thank the staff at the Gobabeb Research and Training Centre for their hospitality during my visit and in particular Theo Wassenaar for facilitating my research, assistance with the export permit, and sending the collected specimens to me after I left Namibia. The Namibian Ministry of Environment and Tourism is thanked for providing collecting and export permits in support of my field work. This project is funded by a Field Dreams award from the Field Museum of Natural History, Chicago, IL under project entitled, "Exploring enigmatic flies in the Namib desert", which supported field work that led to the discovery of the new species. Partial funding is provided by a U.S. National Science Foundation REVSYS Grant (DEB 0919333; PI T. Dikow, Co-PI David Yeates). Any opinions, findings, and conclusions or recommendations expressed in this manuscript are those of the author and do not necessarily reflect the views of the National Science Foundation. I thank Y. Sohn (NMNH, Smithsonian Institution) for skillfully illustrating the male holotype of A. juergeni sp. n. Thanks also to the peer reviewers for suggestions that enhanced the manuscript.
References
- Cumming JM, Wood DM (2009) Adult morphology and terminology. In: Brown BV, Borkent A, Cumming JM, Wood DM, Woodley NE, Zumbado MA (Eds) Manual of Central American Diptera.1.NRC Press,Ottawa,9-50pp. [InEnglish].
- Phylogeny of Asilidae inferred from morphological characters of imagines (Insecta: Diptera: Brachycera: Asiloidea).Bulletin of the American Museum of Natural History319:1‑175. [InEnglish]. URL: http://hdl.handle.net/2246/5949
- A phylogenetic hypothesis for Asilidae based on a total evidence analysis of morphological and DNA sequence data (Insecta: Diptera: Brachycera: Asiloidea).Organisms Diversity & Evolution9(3):165‑188. [InEnglish]. https://doi.org/10.1016/j.ode.2009.02.004
- A review of the genera Anasillomos Londt, 1983, Oratostylum Ricardo, 1925, and Remotomyia Londt, 1983, with description of a new genus and two new species (Diptera: Asilidae: Stenopogoninae).Annals of the Natal Museum41:107‑121. [InEnglish]. https://doi.org/10.5281/zenodo.11581
- World catalogue of the genera of the family Asilidae (Diptera).Studia dipterologica10(2):473‑526. [InEnglish]. https://doi.org/10.5281/zenodo.14704
- The genus Daspletis Loew, 1858 and the description of two new genera, Anasillomos and Remotomyia (Diptera: Asilidae: Stenopogoninae).Journal of the Entomological Society of Southern Africa46(2):283‑308. [InEnglish]. https://doi.org/10.5281/zenodo.11781
- Fishermyia stuckenbergi, a new genus and species of Afrotropical robber-fly from Madagascar (Diptera: Asilidae: Stenopogoninae).African Invertebrates53(1):221‑230. [InEnglish]. URL: http://africaninvertebrates.org/ojs/index.php/AI/article/view/35
- The Torre-Bueno Glossary of Entomology.The New York Entomological Society,New York City,840pp. [InEnglish].
- Studies of Asilidae (Diptera) systematics and evolution. I. A preliminary classification in subfamilies.Arquivos de Zoologia23(3):217‑274. [InEnglish]. URL: http://archive.is/I5BJ
- Zoobank: Developing a nomenclatural tool for unifying 250 years of biological information.Zootaxa1950:39‑50. [InEnglish]. URL: http://www.mapress.com/zootaxa/2008/f/zt01950p050.pdf
- Antennal evolution in the Brachycera (Diptera), with a reassessment of terminology relating to the flagellum.Studia dipterologica6(1):33‑48. [InEnglish]. https://doi.org/10.5281/zenodo.12390
- The implications of function on the origin and homologies of the dipterous wing.Systematic Entomology14(4):507‑520. [InEnglish]. https://doi.org/10.1111/j.1365-3113.1989.tb00300.x
Supplementary materials
Natural-language species descriptions of Anasillomos as exported from Lucid Builder 3.5 in XML SDD format.
Multi-entry, matrix-based identification key to Anasillomos as exported from Lucid Builder 3.5 in XML SDD format