|
Biodiversity Data Journal :
Research Article
|
|
Corresponding author: Mikko Pasi Tapani Paajanen (mikko.paajanen@botany.ubc.ca)
Academic editor: Torsten Dikow
Received: 13 Mar 2020 | Accepted: 29 Apr 2020 | Published: 06 May 2020
© 2020 Mikko Paajanen, Quentin Cronk
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Paajanen MPT, Cronk Q (2020) Moth versus fly: a preliminary study of the pollination mode of two species of endemic Asteraceae from St Helena (Commidendrum robustum and C. rugosum) and its conservation implications. Biodiversity Data Journal 8: e52057. https://doi.org/10.3897/BDJ.8.e52057
|
|
Abstract
Commidendrum robustum (Roxb.) DC. (St Helena gumwood) and C. rugosum (Dryand.) DC. (St Helena scrubwood) are ecologically important, endemic woody Asteraceae from the isolated South Atlantic island of St Helena. Once very abundant, they now exist in sparse fragmented populations due to 500 years of environmental destruction. They are sister taxa that evolved on the island and are reported to hybridise. Commidendrum rugosum has a saucer-like erect capitulum, whereas C. robustum has a somewhat globular hanging capitulum. Using daytime timelapse photography to follow capitula through their life cycle, we found that C. rugosum appears to be myophilous, visited largely by flies (including the endemic syrphid, Sphaerophoria beattiei Doesburg & Doesburg) and occasionally by Lepidoptera. Commidendrum robustum, on the other hand, although visited by flies, strongly attracts moths (especially noted at the Millennium Forest site). Our data suggest that moth visits may reduce visits from flies due to the sensitivity of flies to interference by other insects. We conclude that C. robustum may have a mixed syndrome of myophily/phalaenophily and that there is apparently some divergence of the pollination niche between the two species. Its potential in attracting moths, coupled with its former abundance, suggests that it may have been a major food source for adults of the numerous endemic moths. Pollinator activity was measured by insect visitation rates (mean visits per capitulum per day, V) and insect residence time (mean pollinator kiloseconds per capitulum per day, R). Both are higher for C. robustum (C. rugosum, V = 16.4, R = 3.101; C. robustum, V = 34.0, R = 8.274), reflecting the abundance of moths on the capitula at the Millennium Forest site. The conservation implications of the pollination mode are that: (1) there is considerable pollinator activity on the capitula and pollination is not currently a limiting factor for plant reproduction; (2) gene exchange between geographically-isolated populations of C. rugosum is likely to be minimal due to the apparent reliance of the species for pollination on small flies (especially Sphaerophoria beattiei), which are believed to be not effective as pollinators over long distances (> 1 km). A possible exception is the strong-flying drone-fly, Eristalis tenax Linn. which, although not as abundant as Sphaerophoria, does visit the flowers; (3) there is considerable overlap between the two species in flower visitors and interspecific pollen transfer is possible where the two species grow intermixed (which has potential positive and negative implications for species survival).
Keywords
endemic plants, conservation, interspecific hybridisation, gene flow, fragmented populations, myophily, phalaenophily
Introduction
St Helena, an isolated Miocene volcanic oceanic island of 121.7 km2, has a remarkable endemic flora (
Commidendrum robustum (Roxb.) DC. (St Helena gumwood) and C. rugosum (Dryand.) DC. (St Helena scrubwood) are closely related (
Ecological speciation is believed to have played an important role in the divergence of Commidendrum lineages from each other (
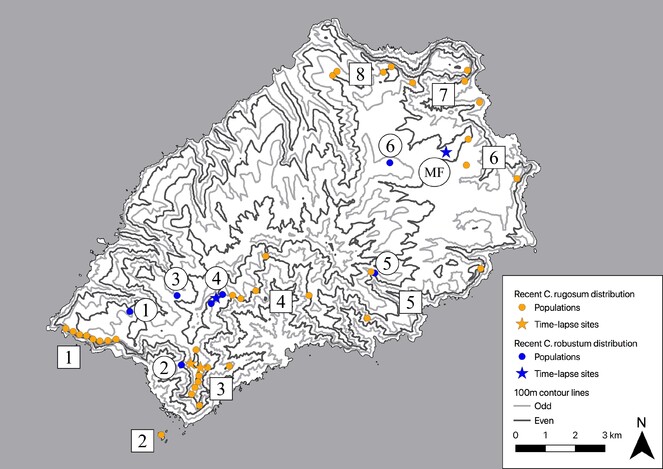
Due to the small geographical size of St Helena, these two species have always been growing relatively close to each other (Fig.
Map of recent (extant and < 50 yrs ago) Commidendrum robustum and C. rugosum populations on St Helena. Key to C. rugosum populations (square): 1. Man & Horse cliffs, 2. Speery Island, 3. Blue Point area, 4. Sandy Bay inland cliffs, 5. Eastern valleys, 6. Prosperous Bay Plain area, 7. Turk’s Cap and the Barn, 8. Flagstaff and Pipe Ridge area. Key to C. robustum populations (round): 1. Man & Horse (extinct), 2. Devil’s Cap Ridge (extinct), 3. Thompson’s Wood, 4. Peak Dale, 5. Deep Valley, 6. Piccolo Hill, Longwood. Note that two of these populations are marked as recently extinct. The former distribution (> 100 yrs ago) was considerably more extensive. The Millennium Forest gumwood site (planted) is also indicated (MF). Other planted sites are not shown.
Pollination mode (defined here as the type, mechanism and effectiveness of pollination) is important basic data for any species conservation programme. As pollinator-mediated seed set and gene flow in rare island endemic plants is relevant to many aspects of species biology (e.g. successful seed reproduction, genetic diversity, hybridisation and evolution) and hence to conservation and extinction, our aim in this study was to characterise, in a quantitative manner, insect visitation to the capitula of the two species. For this, we used time lapse photography, a relatively new and underused method for recording invertebrate visitation on flowers (
Material and methods
Time lapse footage was taken on St Helena using a Brinno TLC200 pro time lapse camera (
Time lapse footage was reviewed, flower visitors identified to species (most main visitors) or morphotaxa and the duration of each visit recorded and graphed. Duration of visit was recorded from first photographic record to last photographic record and it is thus a conservative measure (minimum duration). Insects in a single frame were recorded for presence, but received no duration. For graphing purposes, insects were pooled into taxonomic groups (order or suborder). Figures for pollinator activities include all most likely potential pollinators, i.e. Lepidoptera, Diptera and Hymenoptera, but exclude beetles, spiders and other arthropods, which were minor visitors and considered less likely to be effective pollinators for the two Commidendrum species. The original data are available on Figshare (https://doi.org/10.6084/m9.figshare.11971884.v1).
Site details
Time lapse imagery was recorded in three places on St Helena, Commidendrum robustum capitula in Millennium Forest and Peak Dale and C. rugosum capitula in Blue Point (Fig.
Results
General patterns of visit
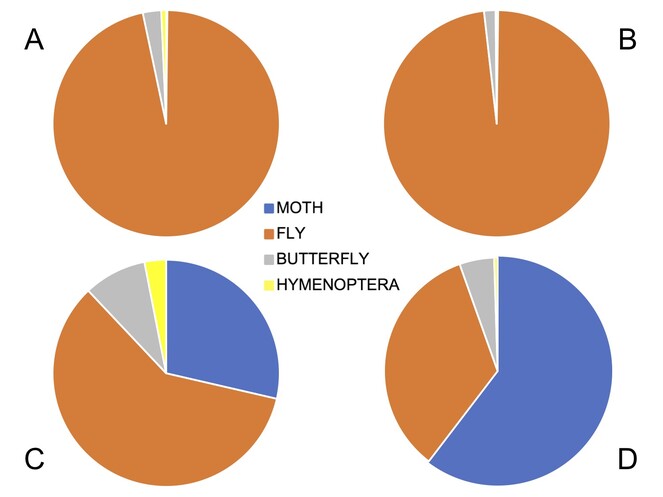
According to the time-lapse data, the main diurnal visitors are Lepidoptera and Diptera (Table
Regular visitors to the gumwood and scrubwood capitula. Species recorded only once are omitted from the table. Key: ** = endemic; * = native; ++ = common, + = frequent; (+) = occasional; - = absent.
|
Order |
Family |
Scientific name |
Common name |
Commidendrum robustum |
Commidendrum rugosum |
|---|---|---|---|---|---|
|
Lepidoptera |
Pyralidae |
Spoladea recurvalis (Fabricius, 1775) |
Beet weed moth |
++ |
- |
|
Lepidoptera |
Pyralidae |
Herpetogramma licarsisalis (Walker, 1858) |
Grass webworm |
++ |
(+) |
|
Lepidoptera |
Lycaenidae |
Lampides boeticus (Linnaeus, 1767) |
Long-tailed blue |
+ |
+ |
|
Diptera |
Syrphidae |
Sphaerophoria beattiei (Doesburg & Doesburg, 1977)** |
Loveridge's hoverfly |
++ |
++ |
|
Diptera |
Syrphidae |
Syritta stigmatica Loew, 1858* |
+ |
+ |
|
|
Diptera |
Syrphidae |
Eristalis tenax (Linnaeus, 1758)* |
Drone-fly |
+ |
+ |
|
Hymenoptera |
Apidae |
Apis mellifera Linnaeus, 1758 |
Honey Bee |
+ |
+ |
|
Diptera |
Syrphidae |
Eristalinus aeneus (Scopoli, 1763) |
+ |
+ |
|
|
Coleoptera |
Mordellidae |
Glipostenoda mellissiana (Wollaston, 1870)** |
Melliss's tumbling flower beetle |
(+) |
- |
|
Diptera |
Anthomyiidae/ Muscidae |
Unidentified |
"Small Black Flies" |
(+) |
++ |
|
Diptera |
Calliphoridae/ Muscidae |
Lucilia/Dasyphora | "Blue fly" |
+ |
(+) |
General morphology and anthesis of the gumwood and scrubwood capitula (typical numbers).
|
Characteristics |
Gumwood |
Scrubwood |
|
No. of ray florets |
40 |
50 |
|
No. of disc florets |
180 |
70 |
|
No. of disc florets opening per day |
42 |
26 |
|
No. of days florets are open |
4 |
4 |
|
No. of days capitulum receptive for pollinators |
6 |
10 |
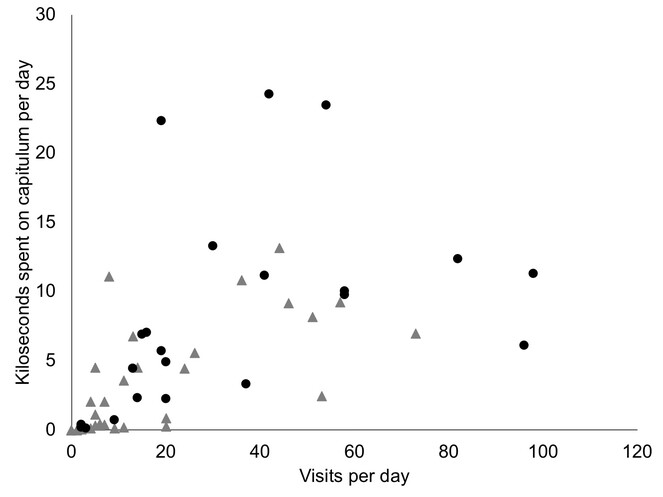
Overall, there is considerable pollinator activity in both the species (Fig.
Daily totals of pollinator activity per capitulum, with total number of visits per day plotted against total pollinator time spent on a capitulum. Each dot represents a capitulum on a particular day. Pollinator activity varies between good days and poor days depending mainly on weather (wind and rain). Grey triangles = Commidendrum rugosum, mainly flies. Black dots = C. robustum), moths and flies.
Finally, we noted that moths and syrphid flies interact differently with capitulum morphology. Commidendrum robustum has longer projecting anther tubes, ca. 1.8 mm versus 0.9 mm in C. rugosum. Moths alight on the tops of the projecting styles and anther tubes of C. robustum and may be seen to probe the corolla tubes from above with their probosces.
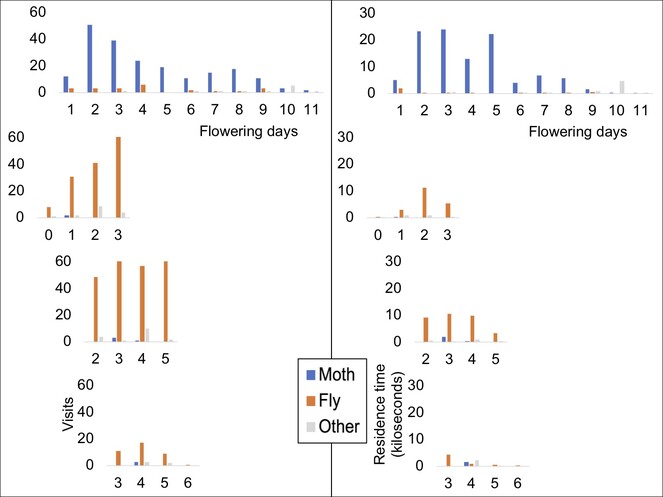
Flower visitation on Commidendrum robustum
Patterns of visits to individual C. robustum capitula are shown in Fig.
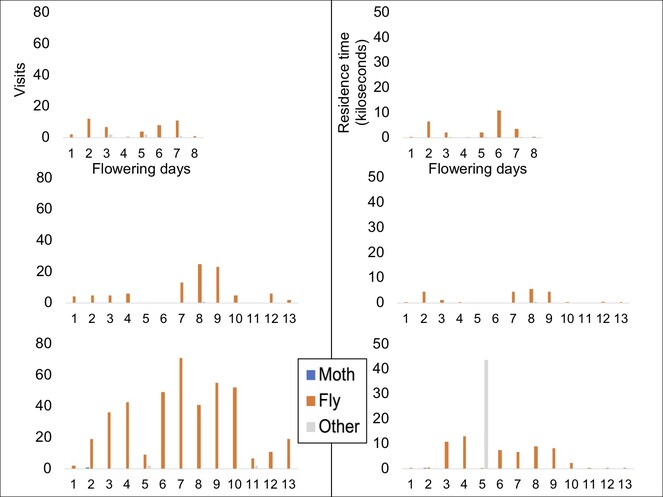
Flower visitation on Commidendrum rugosum
Patterns of visits to individual C. rugosum capitula are shown in Fig.
Insect-insect interaction and pollinator interference
The absence of insects on a capitulum occupied by a spider (noted above) is an example of an arthropod interaction affecting pollination. However, we were also interested in whether there were interactions between pollinator insects (particularly between moths and flies) that could affect pollination mode. To gain evidence about this, we first looked at patterns of “double occupancy”, cases in which two insects shared the same capitulum. We found this to be relatively rare, including 77 instances of moth-moth double occupancy (all on C. robustum), resulting from a total number of moth visits recorded in our study of 215 (35.8%). This compared with only 22 cases of fly-fly double occupancy (20 in C. rugosum, two in C. robustum), resulting from a total of 997 fly visits (2.2%). Most of these cases (14) involved the “small black fly” morphotaxon alone, rather than syrphids. In only two instances did we find two syrphids sharing a capitulum. There was only one case of moth-fly double occupancy (involving the “small black fly”).
To further investigate the possible sensitivity of flies to other insects, of larger or smaller size, we examined instances of “replacement”, where one insect is immediately replaced by another in a timelapse series, indicating the possibility of an interaction between the two. We found 19 replacements involving flies. There were 12 instances of fly replacing fly, six cases of which were of a larger fly replacing a smaller, two cases of a smaller fly replacing a larger and four same size replacements. There were seven instances of flies being replaced by non-fly flower visitors, in all cases the other visitors were larger in size. Conversely, we found no instances of flies replacing non-flies. Although the numbers are small, the patterns are consistent with flies (especially syrphids) not being attracted to capitula occupied by other organisms and, if on a capitulum, being readily disturbed by other organisms (particularly if larger than themselves).
Discussion
Evidence for differentiation in pollination niche between gumwood and scrubwood
Commidendrum robustum (St Helena gumwood) and C. rugosum (St Helena scrubwood) are clearly distinct species with distinct morphology and ecologies (
As noted in the Results, moths tend to alight on top of the long-projecting anther tubes on the C. robustum capitulum. In this position they are likely to accumulate pollen on their undersides and upper legs and thus effect pollination. By contrast, flies visiting C. robustum capitula, because of their short proboscis (ca. 1–2 mm), have to probe with their heads at the mouth of the corolla tube (i.e. below the anther tubes) in order to access nectar. In this position, they may potentially avoid the pollen and styles presented above. The C. rugosum capitula with their shorter anther tubes may be better suited to fly pollination, as the head of the fly (covered with short hairs and thus suitable for pollen transfer) remains at a similar level as the presented pollen and styles at the top of the short anther tubes. We therefore hypothesise that the difference in anther-tube length may be a key feature in differentiating the pollination niche.
It is possible that inter-insect behaviour reinforces the difference in fly vs. moth visitation rates, as hoverflies seem to be sensitive to interference and avoid visiting capitula occupied by moths and other larger insects. A study of wild roses visited by bumblebees and syrphids showed that the presence of a bumblebee on flowers deterred syrphids (
It should be noted that our limited study cannot rule out the possibility of moths being more common visitors to C. rugosum capitula at other times. Nevertheless, the almost complete failure of C. rugosum to attract abundant and nearly ubiquitous species of day flying moths in our study does indicate that scrubwood capitula may be less attractive to moths and, in general field observations, we failed to observe nocturnal moth activity on C. rugosum while finding it frequently on C. robustum capitula. Further studies, including nocturnal observations and observations at all seasons, would be valuable for further confirmation.
The role of Syrphid flies as pollinators in St Helena
Many fly groups are anthophilous and contribute to pollination, but of these, the hoverflies or flower flies (Diptera: Syrphidae) are generally thought to be especially important (
Moths as pollinators in St Helena
There are currently almost 140 moth species known in St Helena, out of which some 60 are endemic to the island (
According to
In the Galapagos, members of the woody Asteraceae genus Scalesia not only have capitula reminiscent of C. robustum (i.e. rather globular capitula on long peduncles), they are also visited by a mixture of flies and moths (
Conclusions
Conservation implications of pollination in Commidendrum
In cases where habitats are altered or fragmented, pollinator-plant mutualisms can be altered or destroyed with impacts on plant fitness and survival (
The second concern, inter-population gene flow, is more serious. The reliance of C. rugosum on small insect pollinators, such as the endemic syrphid Sphaerophoria beattiei, that are generally not considered effective pollinators at distances of over 1 km (
Finally, there is the issue of interspecific gene flow. A low level of interspecific gene flow has probably always occurred between the two species considered here and some apparent hybrids may be found. The overlap of pollinators, shown in this study, indicates how this may occur and highlights the importance of spatial distance, rather than pollinator specificity, in the isolation of these species. The wholesale destruction of habitat and ecological shifts, occurring over the last 500 years may have made interspecific gene flow less, or more, likely depending on specific locality. A limited amount of interspecific gene flow may have conservation benefits (
Acknowledgements
General advice on insect identification was generously provided by Dr Roger Key (Bedale, UK; formerly Natural England). Dr Timm Karisch (Museum für Naturkunde und Vorgeschichte Dessau) kindly provided information on the moth fauna of St Helena and advised on the moth identification. Dr Adrian Pont (Oxford University Museum of Natural History) provided invaluable help with the likely identification of certain fly groups, despite the lack of specimens or high-resolution photography. We thank Roger Key for helpful comments on the manuscript. We thank the two expert referees and the editor for their valuable comments on the manuscript. We also thank the numerous people on St Helena who have shared their advice and knowledge over the course of this investigation, especially Rebecca Cairns-Wicks, Liza Fowler, Vanessa Thomas-Williams, David Pryce and Derek Henry. We acknowledge funding under the NSERC (Discovery Grant) program to QC (RGPIN-2019-04041).
Author contributions
Mikko Paajanen planned the work, conducted the field work and analysed data. Quentin Cronk assisted with data analysis and interpretation. Both authors contributed to the writing of the paper.
References
- St Helena and Ascension Island: a natural history.Anthony Nelson
- Pollination patterns and plant breeding systems in the Galápagos: a review.Annals of Botany110(7):1489‑1501. https://doi.org/10.1093/aob/mcs132
- The endemic flora of St Helena.Anthony Nelson
- The past and present vegetation of St Helena.Journal of Biogeography16(1):47‑64. https://doi.org/10.2307/2845310
- Distance-dependent pollen limitation of seed set in some insect-pollinated dioecious plants.Acta Oecologica28(3):331‑335. https://doi.org/10.1016/j.actao.2005.07.001
- Evolution of St Helena arborescent Astereae (Asteraceae): relationships of the genera Commidendrum and Melanodendron.Botanical Journal of the Linnean Society144(1):69‑83. https://doi.org/10.1111/j.0024-4074.2004.00238.x
- Long-term time-lapse video provides near complete records of floral visitation.Journal of Pollination Ecology16:91‑100. https://doi.org/10.26786/1920-7603(2015)16
- The consequences of habitat fragmentation for plant–pollinator mutualisms.International Journal of Tropical Insect Science24(1):29‑43. https://doi.org/10.1079/ijt20049
- Adaptive introgression as a resource for management and genetic conservation in a changing climate.Conservation Biology30(1):33‑41. https://doi.org/10.1111/cobi.12574
- Darwin-Plus Project DPLUS040: Securing the future for St Helena’s endemic invertebrates: Report Lepidoptera. http://www.trust.org.sh/wp-content/uploads/2018/10/Timm-Karisch-Lepidoptera-report-2018.pdf
- Endangered mutualisms: The conservation of plant-pollinator interactions.Annual Review of Ecology and Systematics29(1):83‑112. https://doi.org/10.1146/annurev.ecolsys.29.1.83
- Insects from the middle of the Adriatic Sea.Entomologica Croatica14:75‑84.
- Flowering plants & ferns of St Helena (Ed. A Darlow).Pisces Publications for St Helena Nature Conservation Group.
- Lane MA (1996) Pollination biology of Compositae. In: Royal Botanic Gardens K Compositae: Biology & Utilization 2.
- Flies and flowers: taxonomic diversity of anthophiles and pollinators.The Canadian Entomologist133(4):439‑465. https://doi.org/10.4039/ent133439-4
- Pollination of Scalesia baurii ssp. hopkinsii (Asteraceae) on Pinta Island.Noticias de Galápagos(53)25‑28.
- Interactions among syrphid flies and bumblebees on flowers.Ecology62(1):81‑88. https://doi.org/10.2307/1936671
- The forgotten flies: the importance of non-syrphid Diptera as pollinators.Proceedings of the Royal Society B: Biological Sciences282:20142934. https://doi.org/10.1098/rspb.2014.2934
- Conservation in relation to mating system in Nesohedyotis arborea (Rubiaceae), a rare endemic tree from St Helena.Biological Conservation80(2):135‑145. https://doi.org/10.1016/s0006-3207(96)00130-9
- Pollination by a guild of fluctuating moth populations: Option for unspecialization in Silene vulgaris.The Journal of Ecology79(3). https://doi.org/10.2307/2260655
- Adaptive introgression: a plant perspective.Biology Letters14:20170688. https://doi.org/10.1098/rsbl.2017.0688
- Hybridization and extinction.Evolutionary Applications(9)892‑908. https://doi.org/10.1111/eva.12367
- Pollinator-driven ecological speciation in plants: new evidence and future perspectives.Annals of Botany113(2):199‑212. https://doi.org/10.1093/aob/mct290
- Pollination failure in plants: why it happens and when it matters.Trends in Plant Science7(6):270‑277. https://doi.org/10.1016/s1360-1385(02)02258-6
- Notes on Lepidoptera of St. Helena, with descriptions of new species.Annals and Magazine of Natural History5:415‑441. https://doi.org/10.1080/00222937908562413
Supplementary materials
Diagram of Commidendrum rugosum (left) and C. robustum (right) florets in a capitulum showing the Fibonacci patterning (divergence angle 137.4 degrees) and the daily opening of cohorts of disc flowers centripetally (alternating orange and yellow cohorts indicate successive days). Larger outer circles indicate ray flowers.
Night-time visitors to Commidendrum robustum (gumwood) at Peak Dale (Helenoscoparia scintillulalis) and Millennium Forest (all others). Species represented, left panel: Helenoscoparia scintillulalis, other photographs: top row left to right: Hypocala rostrata, Hypocala rostrata dark form; middle row left to right: Spodoptera littoralis, Trichoplusia ni; bottom row left to right: Herpetogramma licarsisalis, Spoladea recurvalis. All photographs taken 21 February 2014 (Peak Dale) and 24 February 2014 (Millenium Forest). Note: observations on a solitary C. rugosum (scrubwood) at Horse Point (nearby to Millennium Forest) on the same night (24 February 2014) did not reveal any nocturnal pollinators.
Effective dispersal distances of syrphid flies (Sphaerophoria beattiei and Syritta stigmatica) pollinating females of the St Helena dogwood (Nesohedyotis arborea) inferred from fruit set at different distances to the nearest male. The same species visit flowers of Commidendrum robustum and C. rugosum. Pollination effectiveness, measured by percentage fruit set (% fecundity), is highly efficient at short distances, but declines rapidly with increasing distance (exponential fitted curve). (Data redrawn from: Percy & Cronk, 1997).