|
Biodiversity Data Journal :
General research article
|
|
Corresponding author:
Academic editor: Stuart Longhorn
Received: 23 Sep 2015 | Accepted: 15 Dec 2015 | Published: 21 Dec 2015
© 2015 Adriano Kury, Abel Pérez-González
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Kury A, Pérez-González A (2015) A companion to Part 2 of the World Checklist of Opiliones species (Arachnida): Laniatores – Samooidea, Zalmoxoidea and Grassatores incertae sedis. Biodiversity Data Journal 3: e6663. https://doi.org/10.3897/BDJ.3.e6663
|
|
Abstract
Background
A series of databases is being prepared to list the valid species of Opiliones worldwide. This paper containing nomenclatural acts is meant to accompany Part 2, which includes the members of the infraorder Grassatores of the superfamilies Samooidea and Zalmoxoidea plus the Grassatores currently not allocated to any family (i.e. Grassatores incertae sedis).
New information
The following 32 taxonomic changes are proposed here:
(1-3) The Afrotropical genera Hovanoceros Lawrence, 1959, Malgaceros Lawrence, 1959 and Tetebius Roewer, 1949 (all currently in Samoidae) are all newly transferred to Biantidae.
(4-5) Microminua soerenseni Soares & Soares, 1954, from Brazil is newly transferred to Tibangara (Gonyleptoidea: Cryptogeobiidae), newly combined as Tibangara soerenseni new comb., new familial allocation for the species.
(6-7) The new genus Llaguenia Gen. nov is erected for the South American species Zamora peruviana Roewer, 1956, newly combined as Llaguenia peruviana new comb., and newly placed in Gonyleptoidea: Cranaidae (Prostygninae).
(8) Bebedoura Roewer, 1949, known from a single Brazilian species, is transferred from Tricommatinae to Grassatores incertae sedis.
(9) Microconomma Roewer, 1915, known from a single Cameroonian species, is transferred from Samoidae to Grassatores incertae sedis.
(10) Stygnomimus Roewer, 1927, with two Indomalayan species and hitherto included in the Stygnommatidae, is here formally considered Grassatores incertae sedis.
(11) Bichito González-Sponga, 1998, known from a single Venezuelan species, originally described in Phalangodidae: Phalangodinae, and currently in Grassatores incertae sedis is transferred to Samoidae.
(12) The Neotropical genus Microminua Sørensen, 1932, currently with two species, is newly transferred from Kimulidae to Samoidae.
(13-14) Cornigera González-Sponga, 1987 (currently in Samoidae), is newly considered a junior subjective synonym of Microminua, and its single species is combined under Microminua as Microminua flava (González-Sponga, 1987) new comb.
(15) Niquitaia González-Sponga, 1999 (originally in Phalangodidae: Phalangodinae, currently in Zalmoxidae), monotypic from Venezuela, is newly transferred to Samoidae.
(16) Heteroscotolemon Roewer, 1912 originally described in Phalangodidae: Phalangodinae, and currently in Grassatores incertae sedis is transferred to Zalmoxidae.
(17) While the Australasian genus Zalmoxista Roewer, 1949 is currently in Samoidae and some of its former species have been transferred to Zalmoxis Sørensen, 1886, Zalmoxista americana Roewer, 1952 from Peru, is here newly transferred to Zalmoxidae into Minuides Sørensen, 1932, forming the combination Minuides americanus (Roewer, 1952) new comb. (specific name inflected to match the masculine gender).
(18) Neobabrius Roewer, 1949 (currently in Phalangodidae), monotypic from Indonesia, is newly transferred to Zalmoxidae.
(19) While Crosbyella Roewer, 1927, belongs to Phalangodidae, Crosbyella roraima Goodnight & Goodnight, 1943 (originally Phalangodinae, but currently Zalmoxidae without generic assignment) is here transferred to Soledadiella González-Sponga, 1987, as Soledadiella roraima new comb. (Zalmoxoidea: Zalmoxidae).
(20) Zalmoxissus Roewer, 1949 is newly synonymized with Zalmoxis Sørensen, 1886 (Zalmoxidae).
(21) The original spelling Zalmoxis sorenseni Simon, 1892 is restored from the unjustified emendation soerenseni.
(22) The Neotropical genus Phalangodella Roewer, 1912 (originally in Phalangodidae: Tricommatinae, but currently in Grassatores incertae sedis) is newly transferred to Zalmoxoidea incertae sedis and (23-26) four other genera are newly synonymized with it: Phalangodella Roewer, 1912 = Exlineia Mello-Leitão, 1942 = Langodinus Mello-Leitão, 1949 = Cochirapha Roewer, 1949 = Phalpuna Roewer, 1949, generating the following new combinations (27-32): Phalangodella fulvescens (Mello-Leitão, 1943) new comb., Phalangodella milagroi (Mello-Leitão, 1942) new comb., Phalangodella rhinoceros (Mello-Leitão, 1945) new comb., Phalangodella flavipes (Mello-Leitão, 1949) new comb., Phalangodella rugipes (Roewer, 1949) new comb. and Phalangodella urarmata (Roewer, 1949) new comb.
Keywords
New genus, new familial assignments, new synonymies, genital morphology, Gonyleptoidea, Phalangodoidea, Brazil, Ecuador, Indonesia, Madagascar, Mozambique, Peru, Venezuela
Introduction
This work is a companion to the 2nd data paper in a series containing a checklist of the valid species of harvestmen in the World: "World Checklist of Opiliones species (Arachnida). Part 2: Laniatores – Samooidea, Zalmoxoidea and Grassatores incertae sedis" (
Harvestmen taxonomy, founded in the classic and typological Roewerian system (e.g.
We are currently in the middle of this systematic reorganization. Modern approaches are very different to the classical ones, and we now typically have a critical amount of new information about the arrangement of the major Opiliones lineages that is highly incongruent with the Roewerian system. But there is a huge impediment – a considerable fraction of harvestmen species are known only from their original and often insufficient descriptions, and their names remain tied to an obsolete taxonomic system. Huge efforts were made by modern opilionologists (e.g.
For that reason it is not rare for newly written compilations in Opiliones, such as catalogues, checklists, etc., to typically include several taxonomic and nomenclatural changes (e.g.
Material and methods
The large collections of our own musems, MNRJ (Museu Nacional/ UFRJ, Rio de Janeiro) and MACN (Museo Argentino de Ciencias Naturales "Bernardino Rivadavia", Buenos Aires), have been complemented along the years by several visits to major repositories in the world. Re-study of type specimens and search for homologies in genital morphology associated with a cladistic framework allowed us to dismantle Roewer's system. The advent of the internet and wide digitalization of literature allowed access to countless rare works, allowing spelling checks, and solidly anchoring checklists.
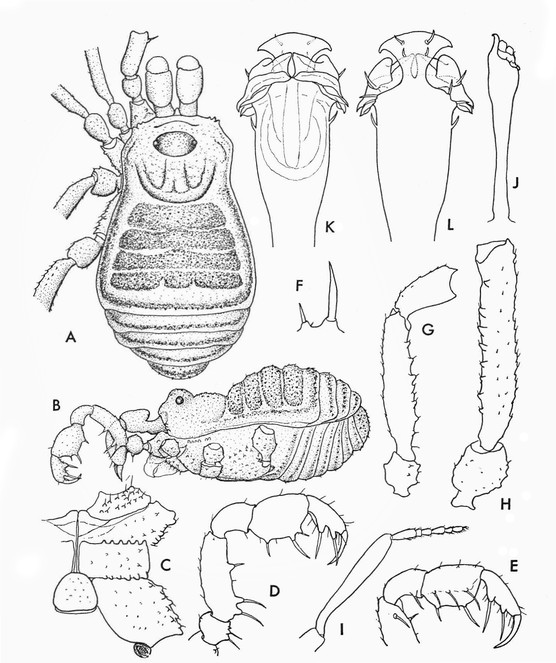
Optical photograph images were taken along the years with a variety of hardware. Most recent photos have been integrated with the stacker software CombineZP Suite (
Male genitalia preparation follows
Abbreviations: AK = Adriano Kury reference number; AMNH = American Museum of Natural History, New York, USA; FNMH = Field Natural History Museum, Chicago, USA; MCNC = Museo de Ciencias Naturales de Caracas, Caracas, Venezuela; MNHN = Muséum national d'Histoire naturelle, Paris, France; MSNG = Museo Civico di Storia Naturale "Giacomo Doria", Genoa, Italy; MZSP = Museu de Zoologia da Universidade de São Paulo, Brazil; SMF = Naturmuseum Senckenberg, Frankfurt am Main, Germany; ZMUC = Universitetets Zoologiske Museum Copenhagen, Denmark.
Results
Biantidae
There are two closely related genera from Madagascar and a third from Eastern Continental Africa which could all be closely related. They are here included in Biantidae.
Hovanoceros Lawrence, 1959, new familial allocation
Hovanoceros
Hovanoceros bison Lawrence, 1959
Hovanoceros bison
Type data. 2 ♂ syntypes (MNHN, not examined) MADAGASCAR, Ambodivoangi, Maroantsetra.
Historical systematic background.
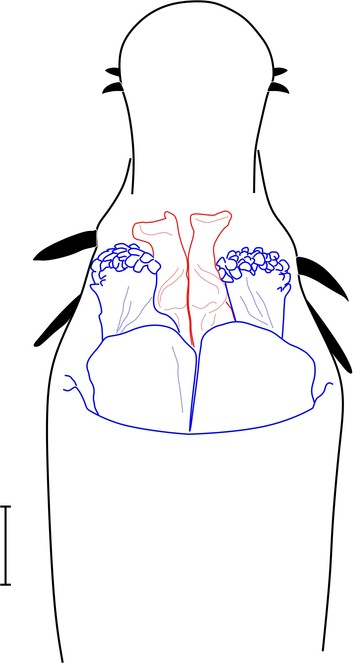
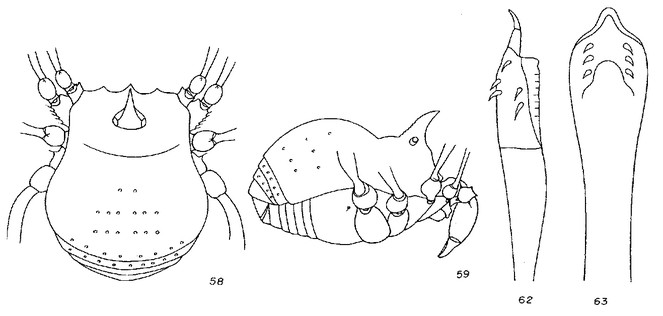
Rationale for the new familial allocation. External features include a huge protuberant ocularium and an unusual scutal armature, but they do not point conclusively to any specific family (See Fig.
Malgaceros Lawrence, 1959, new familial allocation
Malgaceros
Malgaceros boviceps Lawrence, 1959
Malgaceros boviceps
Type data. 3 ♂ 1 ♀ syntypes (MNHN, not examined), MADAGASCAR, Nosy Be.
Background and allocation. Malgaceros shares the same history in the literature as Hovanoceros.
Rationale for the new familial allocation. It is clearly closely related to Hovanoceros as mentioned in the original description, and is here transferred into the same family Biantidae.
Tetebius Roewer, 1949, new familial allocation
Tetebius
Historical systematic background. Tetebius is a monotypic genus with a species from Mozambique, which was originally placed in Phalangodidae: Phalangodinae. It was removed from the Phalangodidae but not placed anywhere else by
Rationale for the new familial allocation. Tetebius latibunus has not been examined by us. External features are inconclusive, but the sketch illustration of the penis as drawn by Roewer (1942a) in ventral view appears close to that of Hovanoceros (See Fig.
Cryptogeobiidae
As Cryptogeobiidae are members of the Gonyleptoidea, they are not treated in the Part 2 of this series, but rather in Part 5 (Lesser Gonyleptoidea).
Tibangara Mello-Leitão, 1940
Tibangara
Taxonomic background. Tibangara was recently transferred from Phalangodidae to Cryptogeobiidae (
Tibangara soerenseni (Soares & Soares, 1954) new comb., new familial allocation
Microminua soerenseni
Gen. sp. W:
Type data. ♀ holotype (MZSP 842, examined), ♀ paratypes (MZSP 833, examined), from BRAZIL, Rio de Janeiro, Rio de Janeiro, Corcovado.
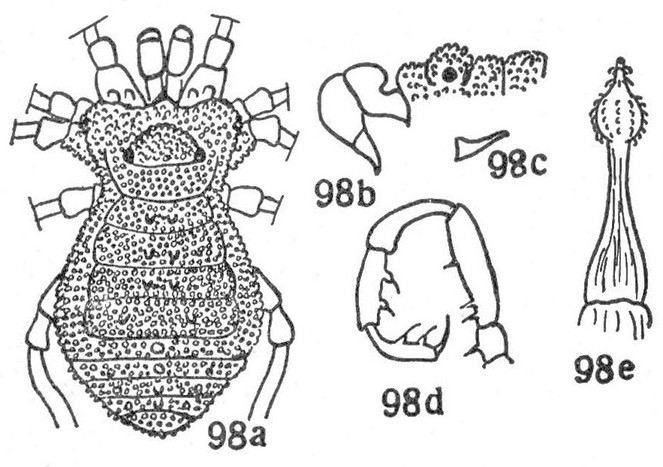
Rationale for the new familial and generic allocation of the species. Males and females of this species have been examined from the type locality (as well as the types) and based both on external features as in male genitalia they are close relatives of Tibangara nephelina. Their similarity with Kimulidae is only superficial (Fig.
Cranaidae
Cranaidae: Prostygninae
Llaguenia Gen. nov. /new genus
Type species. Zamora peruviana Roewer, 1956.
Etymology. Generic name derives from Hacienda Llaguén in Peru, the collection locality of the type species.
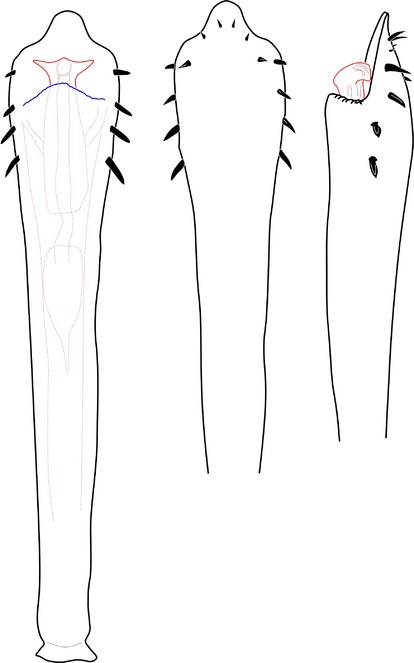
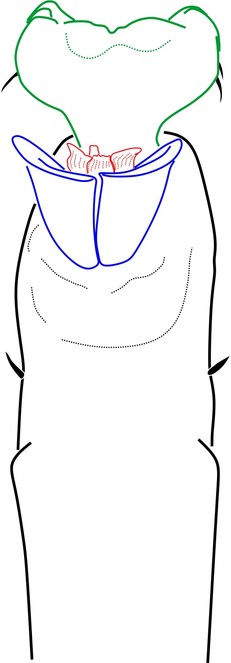
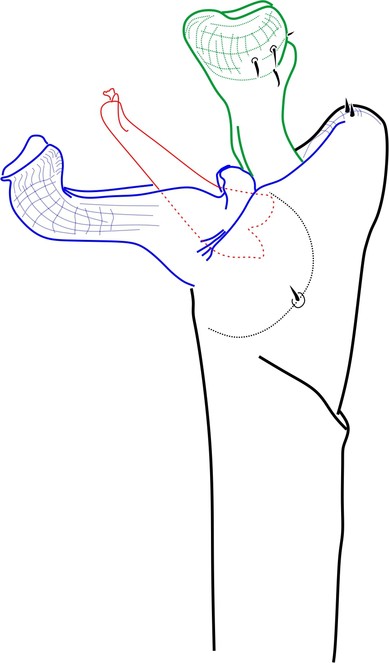
Diagnosis. In both sexes, dorsal scutum outline alpha with coda notably elongate and slightly divergent. Mesotergum divided into four areas. Area I divided into left and right halves. Scutum and free tergites smooth and unarmed. Ocularium extremely narrow. Scutal groove U-shaped, notably elongate. Cheliceral hand of male swollen. Pedipalpal femur cylindrical, unarmed ventrally. Coxa IV outline widely surpasses dorsal scutum in dorsal view, and parallel to main body axis. Femur IV of male incrassate and armed with row of eight proventral spines. Penis ventral plate subrectangular, with lateral and apical wide concavities, three pairs of small macrosetae (MS) C, one pair of small MS D, and two pairs of very long MS A. Stylus sinuous, with slightly squared head, and well developed thumb-like dorsal process. Cutervolus and Prostygnus have a sexually dimorphic ocularium sexually dimorphic, hugely developed in males, scutal area III armed with powerful erect acuminate spines, free tergite III with paired spines, cheliceral hand immensely swollen, pedipalpal femur with ventral row of spines (Prostygnus only), MS A short, distal border of ventral plate straight (Prostygnus) or strongly convex (Cutervolus).
Llaguenia peruviana (Roewer, 1956), new comb.
Zamora peruviana
Type data. ♂ holotype (SMF RII 9701, examined), 1 ♀ paratype (SMF RII 9702, examined), PERU, La Libertad, Hacienda Llaguén, forest of Rejo Cargaruay, 2650 m.
Rationale for the new familial allocation. The subfamily Prostygninae is currently in Cranaidae (
Assorted Grassatores
Phalangodidae was once a great repository of diverse species (e.g.
Grassatores incertae sedis
Bebedoura Roewer, 1949
Bebedoura
Bebedoura rugosa Roewer, 1949
Bebedoura rugosa
Type data. ♂ holotype (SMF RII 6897/17, examined), from BRAZIL, Pernambuco, Bebedouro (wrongly cited as in "São Paulo" state in).
Historical systematic background. Bebedoura was originally included in Phalangodidae: Tricommatinae. A recent cladistic analysis of this group (
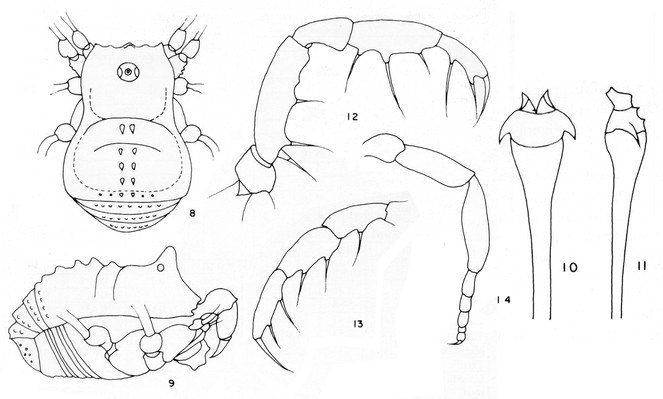
Rationale for the removal from Tricommatinae. The female holotype of Bebedoura rugosa has been studied by ABK (see Figs
Microconomma Roewer, 1915
Microconomma
Microconomma armatipes Roewer, 1915
Microconomma armatipes
Type data. ♂ holotype (SMF RI 1131, not examined), from CAMEROON, Kamerun Mountains, Bakossu, 400 m.
Historical systematic background. Monotypic genus, with one species from Cameroon. Microconomma was originally placed in Phalangodinae. It was transferred by
Rationale for the removal from Samoidae. At the moment we have no evidence to support either previously suggested assignment and so transfer this genus to Grassatores incertae sedis. The original description is too superficial to allow any judgement, but the species in question, as illustrated, doesn't fit the typical Samoidae habitus as defined by
Stygnomimus Roewer, 1927
Stygnomimus
Stygnomimus conopygus Roewer, 1927
Stignomimus conopygus Roewer 1927a: 305.
Type data. ♂ holotype (SMF RII/63/1, examined), [Indonesia, Riau Islands], Riouw Archipelago.
Stygnomimus malayensis Suzuki, 1969
Stignomimus malayensis Suzuki 1969: 32.
Type data. ♀ holotype (not examinined)Templer Park, Malaysia.
Historical systematic background The original assignment of this Indomalayan genus to Stygnommatidae by
Rationale for the removal from Stygnommatidae. There is no positive evidence yet to assign the genus to Stygnommatidae or even to Samooidea.
Samoidae
Bichito González-Sponga, 1998, new familial assignment
Bichito
Bichito pijiguaoensis González-Sponga, 1998
Bichito pijiguaoensis
Type data. ♂ holotype (MAGS 1222a, not examined); 1 ♀ paratype (MAGS 1222b, not examined); 5 ♂ 5 ♀ 3 juv. paratypes (MAGS, not examined), from VENEZUELA, Bolívar, Cedeño: Near bauxite mines of Los Pijiguaos, 06°35’20’’N, 66°45’12’’W, 80 m.
Historical systematic background. Originally described in Phalangodinae, removed to Grassatores incertae sedis by
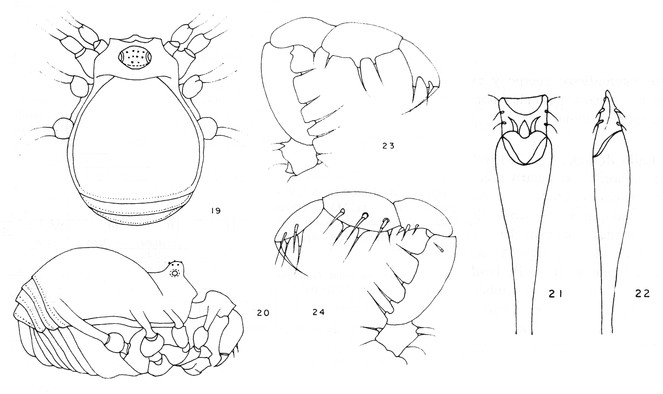
Rationale for the new placement. The original drawings of the male genitalia are poorly detailed, but they allow recognition of an everted penis with two conductors, capsula interna and glans without stragulum or modified follis (Fig.
Microminua Sørensen, 1932, new familial assignment
Microminua Sørensen in
Cornigera
Rationale for the new placement. The new family placement is fully supported by the male genital groundplan where the truncus is cylindrical, without a well-defined ventral plate as in Gonyleptoidea, with pars distalis, compressed dorsoventrally, not differentiated from pars basalis by any remarkable groove or constriction and laterally armed with strong spatulate (foliar) spines. The capsula interna is eversible and formed by a pair of conductors completely fused. The follis is not modified into a stragulum and not observed externally. This genital morphology matches the penial groundplan described for Samoidae by
Microminua flava (González-Sponga, 1987), new comb.
Cornigera flava
Type data. ♂ holotype (MCNC, not examined), VENEZUELA, Miranda, Zamora: Salmerón, 250 m.
Microminua parvula Sørensen, 1932
Microminua parvula Sørensen in
Type data. 30 ♂ ♀ syntypes (ZMUC; subsample at SMF RII/6237/165-4, examined), from VENEZUELA, Distrito Federal, Hacienda La Moka, right margin of Siquire River, 10 km NE Santa Lucia on road Caracas-Santa Lucia.
Historical systematic background. Microminua was originally established in Minuidae by Sørensen (
Rationale for the generic synonymy. The male genitalia of the type material of Microminua parvula (syntypes in SMF and ZMUC (See Fig.
Niquitaia González-Sponga, 1999, new familial assignment
Niquitaia
Niquitaia convexa González-Sponga, 1999
Niquitaia convexa
Type data. ♂ holotype (MAGS 1254a, not examined); 1 ♀ paratype (MAGS 1254b, not examined); 1 ♂ 3 ♀ paratypes (MAGS, not examined), VENEZUELA, Trujillo, Boconó: km 4 road Boconó-Niquitao, 1400 m.
Historical systematic background. Niquitaia was originally established in Phalangodidae: Phalangodinae along with the type species Niquitaia convexa from Venezuela. It was removed to Zalmoxidae by
Rationale for the new placement. The external morphology is very similar to Kalominua Sørensen, 1932 (Samoidae): body as an asymmetrical hourglass, anterior half of scutum much shorter, posterior half rounded, laterally convex appearance, male genitalia without stragulum, with pars distalis flattened dorso-ventrally with ventral region wide, undivided and armed with strong macrosetae (Fig.
Zalmoxidae
The Zalmoxidae are here augmented by the inclusion of two genera originally described in Phalangodidae and one species, originally included in a genus of Samoidae.
Heteroscotolemon Roewer, 1912 new familial assignment
Heteroscotolemon
Heteroscotolemon australis Roewer, 1912
Heteroscotolemon australis
Type data. ♀ [originally reported as ♂] holotype (SMF RI/207, examined), from “Guayana: Nieder-Oyopock” [FRENCH GUYANA, Lower Oyapock River, which marks the fro ntier with Brazil].
Historical systematic background. Heteroscotolemon was originally included in Phalangodinae, then transferred to Grassatores incertae sedis by Kury (2003).
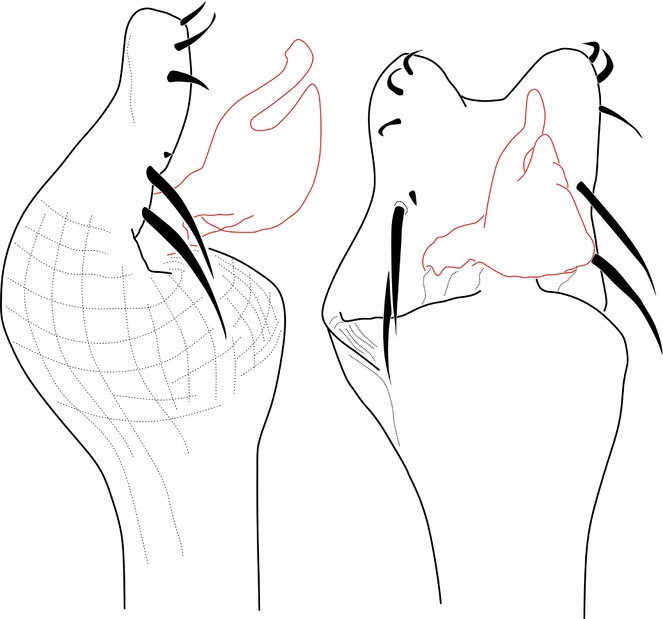
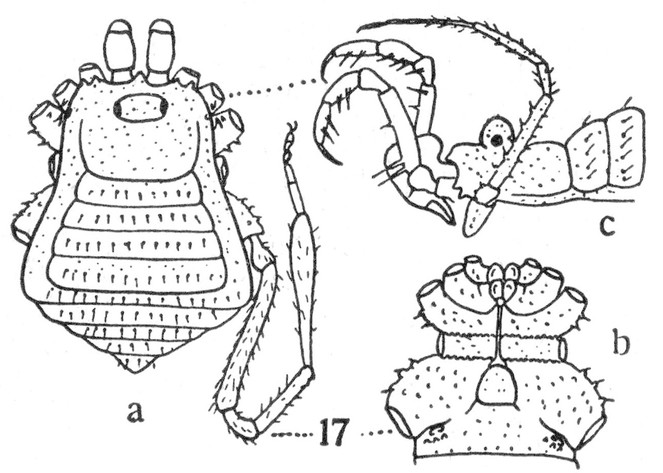
Rationale for the new placement. Evidence for the new placement as a large zalmoxid comes from the backward pointed scutal grooves, bimerous distitarsus I, strongly armed pedipalpal trochanter and femur. All other Laniatores recorded from French Guyana are either Gonyleptoidea, which typically have distitarsus I trimerous, or Stenostygninae, which do not have a common ocularium. Heteroscotolemon australis is very different from Parascotolemon ornatum Roewer, 1912, a typical local zalmoxid (See Figs
Minuides Sørensen, 1932
Minuides Sørensen in
Minuides americanus (Roewer 1952), new comb.
Zalmoxista americana
Type data. ♀ holotype (SMF RII 10226/240, not examined), PERU, Pasco, Laguna Punrun, 4400 m, near Cerro do Pasco in drainage of Junin Lake.
Historical systematic background. Roewer (1949a) originally created Zalmoxista in Phalangodinae to include two species from Australia and one from New Caledonia. Later he added a fourth species from Peru (Zalmoxista americana Roewer, 1952), in a very brief unillustrated description.
Rationale for the new placement. The identity of "Zalmoxista" americana is highly doubtful, but it may be recognized as a Zalmoxidae. Another zalmoxid genus which has the same tarsal counts and the same conformation of scutal areas is Minuides, which currently includes several Neotropical species of doubtful monophyly. Therefore this species is here included in Minuides (Zalmoxidae) pending further study.
Neobabrius Roewer, 1949, new familial assignment
Neobabrius
Neobabrius parvulus Roewer, 1949
Neobabrius parvulus
Type data. ♂ holotype, 1 ♂ 1 ♀ paratypes (SMF RII 3138/79, not examined), from INDONESIA, Jawa Timur, Bawean Island.
Historical systematic background. This monotypic genus was originally in Phalangodinae and represents a neglected taxon.
Rationale for the new placement. Roewer's description of Neobabrius parvulus (Fig.
Soledadiella González -Sponga, 1987
Soledadiella
Soledadiella roraima (Goodnight and Goodnight, 1943), new comb.
Crosbyella roraima
Pellobunus roraima:
“Crosbyella” roraima:
Type data. ♀ holotype (AMNH, examined only by photograph), from [VENEZUELA, Bolívar], “Rondon Camp, Mt. Roraima, 6900 feet.”
Historical systematic background. This species from the border of Brazil and Venezuela has originally been assigned to Crosbyella (Phalangodinae) and then Pellobunus (Samoidae).
Rationale for the placement. Examining two pictures of the female holotype of C. roraima (in AMNH, photos courtesy of R. Pinto-da-Rocha), suggests placement in the genus Soledadiella: Ocularium not greatly developed and situated far from margin of carapace; abdominal scutum much larger than carapace, widening posteriorly, with convex sides without constriction, its posterior border straight; scutal area I slightly longer than each of the others; scutal grooves gently curved, pointing backwards; scutal areas and free tergites densely covered by coarse rounded granules, while carapace is smooth. (See Figs
Zalmoxis Sørensen, 1886
Zalmoxis
Zalmoxissus
Historical systematic background. Roewer (
Fundamentation of the synonymy. All of those but the monotypic Zalmoxissus were synonymized under Zalmoxis by
Zalmoxis sorenseni Simon, 1892 original spelling restored
Zalmoxis sorenseni
Zalmoxis soerenseni [unjustified emended spelling]:
Comment. Zalmoxis sorenseni Simon, 1892 appears in the literature wrongly as Zalmoxis soerenseni Simon, 1892. The change of spelling proposed by
Zalmoxissus tristis (Thorell, 1891)
Zalmoxis tristis
Zalmoxida tristis:
Type data. ♂(?) holotype (MCSN) from PAPUA NEW GUINEA, National Capital District, Yule Island (“Yule-Roro”).
Zalmoxoidea (no familial inclusion)
Phalangodella Roewer, 1912, new superfamilial assignment
Phalangodella
Erxlineia
Exlineia
Langodinus
Cochirapha
Phalpuna
Historical systematic background. The monotypic genus Phalangodella was originally placed in Phalangodidae Tricommatinae. It was transferred to Grassatores incertae sedis by
Rationale for the placement. The male genitalia of Phalangodella sp. has a jackknife structure exclusive to Zalmoxoidea with a foldable capsula externa (stragulum). The presence of a rudimentary pergula and rutrum justifies its inclusion in Zalmoxoidea, closest to Zalmoxidae and Icaleptidae, although it is not a perfect match with either (Figs
Included species.
Phalangodella aequatorialis Roewer, 1912 (type species)
Phalangodella flavipes (Mello-Leitão, 1949) new comb. for Langodinus flavipes
Phalangodella fulvescens (Mello-Leitão, 1943) new comb. for Exlineia fulvescens
Phalangodella milagroi (Mello-Leitão, 1942) new comb. for Erxlineia milagroi
Phalangodella rhinoceros (Mello-Leitão, 1945) new comb. for Exlineia rhinoceros
Phalangodella rugipes (Roewer, 1949) new comb. for Cochirapha rugipes
Phalangodella urarmata (Roewer, 1949) new comb. for Phalpuna urarmata
Generic diagnosis. Dorsal scutum campaniform elongate, densely covered by long-haired setiferous tubercles. Carapace elongate, with ocularium very narrow, marginal as a blunt protuberance. Mesotergum divided into 4 areas by substraight grooves, last groove curved backwards. Both male and female possess femur IV thickened, curved and with distal prolateral spine and tibia IV moderately incrassate. Penis with short scattered macrosetae; rudimentary proto-pergula (projected distal ring) and proto-rutrum (thick mushroom-shaped apical process). Capsula externa jackknife-like unfolding by means of a long stragulum, divided into left and right halves. Capsula interna without lateral sclerites, and with apical expansion as a parastyllar collar. Truncus cylindrical with subdistal fold encircling lateral and ventral parts.
Acknowledgements
We thank Petra Sierwald (FMNH) for the loan of Malagasy Biantidae. APG wants to thank Peter Jäger and Nikolaj Scharff for hospitiality in his visit to their museums. All illustrations from the literature are used here under written permission of the respective copyright holders. This study has been supported by grant # 562149/2010-4 (PROTAX – OPESC project) and scholarship # 302116/2010-9 (PQ - AMMA project) from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to ABK. The SEM micrographs were taken in the SEM Lab of Marine Diversity of the MNRJ (financed by PETROBRAS), with the kind assistance of Elivaldo de Lima / Amanda Veiga.
Author contributions
ABK surveyed the taxonomy and nomenclatural acts on the literature, visited museums, revised material, type or otherwise, and drafted the manuscript.
APG studied the complex male genitalia of non-gonyleptoid Laniatores, tried to justify systematic arrangements, proposed homologies for morphological strucutures and studied alternative placements for extraneous genera.
Both authors have worked decades on the systematics of Laniatores, doing all parts of this research, including fieldwork, and imaging to slide/stub preparations, and organization of morphological matrices.
References
- Acosta LE, Pérez-González A, Tourinho AL (2007) Methods and Techniques of Study: Methods for taxonomic study. In: Acosta LE, Pérez-González A, Tourinho AL (Eds) Harvestmen, the biology of Opiliones.Harvard University Press,Cambridge,597pp.
- Aracnidos de Venezuela. Opiliones Laniatores I. Familias Phalangodidae y Agoristenidae.Academia de Ciencias Fisicas, Matematicas y Naturales,Caracas,562pp.
- Aracnidos de Venezuela. Cinco nuevos generos y cinco nuevas especies de microopiliones en la hojarasca del bosque tropical (Opiliones: Laniatores: Phalangodidae).Acta Biologica Venezuelica18(4):27‑41.
- Aracnidos de Venezuela. Cinco nuevos géneros y cinco nuevas especies de microopiliones hemiedaficos (Opiliones Laniatores Phalangodidae).Acta Biologica Venezuelica19(2):55‑69.
- Phalangida from South America.American Museum Novitates1234:1‑19.
- Phalangida from Tropical America.32(1):1‑58.
- Opiliones.Insects of Micronesia, Bernice P. Bishop Museum, Honolulu3(2):71‑83.
- CombineZP: GNU public license software.32-bit. URL: http://www.hadleyweb.pwp.blueyonder.co.uk
- Descriptiones Laniatorum (Arachnidorum Opilionum Subordinis) fecit William Sørensen. Opus posthumum recognovit et edidit Kai L. Henriksen.Det Kongelige Danske Videnskabernes Selskabs skrifter, Naturvidenskabelig og Mathematisk Afdeling (ser. 9)3(4):197‑422.
- The prickly blade mapped: establishing homologies and a chaetotaxy for macrosetae of penis ventral plate in Gonyleptoidea (Arachnida, Opiliones, Laniatores).Zool J Linn Soc174(1):1‑46. https://doi.org/10.1111/zoj.12225
- Annotated catalogue of the Laniatores of the New World (Arachnida, Opiliones).Revista Ibérica de Aracnología, vol especial monográfico1:1‑337.
- First report of the male of Zamora granulata Roewer 1928, with implications on the higher taxonomy of the Zamorinae (Opiliones, Laniatores, Cranaidae).Zootaxa3546:29‑42.
- Why does the Tricommatinae position bounce so much within Laniatores? A cladistic analysis, with description of a new family of Gonyleptoidea (Opiliones, Laniatores).Zoological Journal of the Linnean Society172:1‑48. https://doi.org/10.1111/zoj.12165
- World Checklist of Opiliones species (Arachnida). Part 2: Laniatores – Samooidea, Zalmoxoidea and Grassatores incertae sedis.Biodiversity Data Journal3:e6482. https://doi.org/10.3897/BDJ.3.e6482
- Arachnides-Opilions. Faune de Madagascar.Publications de L'Institut de Recherche Scientifique Tananarive Tsimbazaza9:1‑121.
- Considerações sobre os Phalangodoidea Soer. com descrição de novas formas.Annaes da Academia Brasileira de Sciencias10(2):135‑145.
- Mais alguns novos Opiliões Sul-Americanos.Annaes da Academia Brasileira de Sciencias12(2):93‑107.
- Oito novos Laniatores do Equador.Anais da Academia Brasileira de Ciências14(4):315‑325.
- Arácnidos recogidos en el Ecuador y el Perú por la Señora H. E. Frizell Don.Comunicaciones zoologicas del Museo de Historia natural de Montevideo1(5):1‑8.
- Considerações sôbre o genero Eusarcus Perty e descrição de quatro novos Laniatores.Anais da Academia Brasileira de Ciências17(2):149‑162.
- Famílias, subfamília, espécies generos novos de opiliões e notas de sinonimia.Boletim do Museu Nacional, (Nova Série Zoologia)94:1‑33.
- Pérez-González A (2006) Revisão sistemática e análise filogenética de Stygnommatidae (Arachnida, Opiliones).Unpublished Ph.D. Thesis. Programa de Pós-Graduação em Zoologia, UFRJ,Rio de Janeiro,308pp.
- Pérez-González A, Kury AB (2007) Kimulidae Pérez González, Kury and Alonso-Zarazaga, new name, pp 207–209. In: Pinto-da-Rocha R, Machado G, Giribet G (Eds) Harvestmen: the biology of the Opiliones.Harvard University Press,Cambridge and London,597pp.
- Pérez-González A, Kury AB (2007) Samoidae Sørensen, 1886. In: Pinto-da-Rocha R, Machado G, Giribet G (Eds) Harvestmen: the biology of the Opiliones.Harvard University Press,Cambridge and London,597pp.
- Die Familien der Assamiiden und Phalangodiden der Opiliones-Laniatores. (= Assamiden, Dampetriden, Phalangodiden, Epedaniden, Biantiden, Zalmoxiden, Samoiden, Palpipediden anderer Autoren).Archiv für Naturgeschichte, AbtA, Original-Arbeiten78(3):1‑242.
- 106 neue Opilioniden.Archiv für Naturgeschichte, Abt A, Original-Arbeiten81(3):1‑152.
- Weitere Weberknechte I. (1. Ergänzung der: "Weberknechte der Erde," 1923).Abhandlungen der Naturwissenschaftlichen Verein zu Bremen26(2):261‑402.
- Über Phalangodidae II. Weitere Weberknechte XIV.Senckenbergiana30(4):247‑289.
- Einige neue Gattungen der Phalangodidae (Opiliones).Veröffentlichungen aus dem Museum für Natur-, Völker- u. Handelskunde in Bremen, Bremen, Reihe A Naturwissenschaften1:143‑144.
- Über Phalangodiden I. (Subfam. Phalangodinae, Tricommatinae, Samoinae.) Weitere Weberknechte XIII.Senckenbergiana30(1):11‑61.
- Neotropische Arachnida Arthrogastra, zumeist aus Peru [I].Senckenbergiana33(1):37‑58.
- Arachnida Arthrogastra aus Peru, II.Senckenbergiana Biologica37(5):429‑445.
- New Australasian Zalmoxidae (Opiliones: Laniatores) and a new case of male polymorphism in Opiliones.Zootaxa3236:1‑35.
- The evolutionary and biogeographic history of the armoured harvestmen – Laniatores phylogeny based on ten molecular markers, with the description of two new families of Opiliones (Arachnida).Invertebrate Systematics25:106‑142. https://doi.org/10.1071/is11002
- The Zalmoxidae (Arachnida: Opiliones: Laniatores) of the Paleotropics: a catalogue of Southeast Asian and Indo-Pacific species.Zootaxa2972:37‑58.
- A new family of Laniatores (Arachnida: Opiliones) from the Afrotropics.Invertebrate Systematics25:143‑154. https://doi.org/10.1071/IS11003
- Arachnides des îles Philippines.Annales de la Société Entomologique de France, Paris (séries 6)61:35.
- Algumas notas sobre opiliões com descrição de novas formas.Papéis avulsos do Departamento de Zoologia11(25):401‑507.
- Sørensen WE (1886) Opiliones, pp. 53–86. In: Koch L, Keyserling Ev (Eds) Die Arachniden Australiens nach der Natur beschrieben und abgebildet.2.Bauer & Raspe,Nürnberg.
- Harvestmen (Opiliones) from the Mascarene Islands and resurrection of the family Zalmoxidae.Annals of the Natal Museum30:1‑8.
- An annotated check-list of harvestmen, excluding Phalangiidae, of the Afrotropical Region (Opiliones).Annals of the Natal Museum33(2):271‑336.
- On a collection of opilionids from Southeast Asia.Journal of Science of the Hiroshima University, Series B, Division 1 (Zoology),22(2):11‑77.
- Opilioni nuovi o poco conosciuti dell´Arcipelago Malese.Annali del Museo Civico di Storia Naturale di Genova10:669‑770.