|
Biodiversity Data Journal :
Taxonomic Paper
|
|
Corresponding author:
Academic editor: Pavel Stoev
Received: 24 Nov 2015 | Accepted: 17 May 2016 | Published: 19 May 2016
© 2016 Daniil Korobushkin, Irina Semenyuk, Ivan Tuf
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Korobushkin D, Semenyuk I, Tuf I (2016) An annotated checklist of the Chilopoda and Diplopoda (Myriapoda) of the Abrau Peninsula, northwestern Caucasus, Russia. Biodiversity Data Journal 4: e7308. https://doi.org/10.3897/BDJ.4.e7308
|
|
Abstract
Background
The Abrau Peninsula is located in northwestern Caucasus between the cities of Novorossiysk and Anapa, Krasnodar Province, Russia. This paper contains an annotated checklist of the Chilopoda and Diplopoda inhabiting the Abrau Peninsula.
New information
The fauna of the Abrau Peninsula comprises 17 centipede (4 orders) and 16 millipede (6 orders) species. Henia taurica, hitherto known only from the Crimea, has now been reported from several localities in the studied region. The study also reveals two possibly new millipede species. Statistical analyses showed that habitat preferences of myriapod species within the Abrau Peninsula are caused by species geographic distribution pattern and microbiotope preferences.
Keywords
Myriapoda, taxonomy, Utrish Nature Reserve, soil fauna
Introduction
The myriapod fauna of some parts of Russia still remains poorly known. This holds particularly true for the Caucasus, where most large-scale investigations of the group were performed a long time ago (
The Abrau Peninsula is located between the cities of Novorossiysk and Anapa, Krasnodar Region. Its 9065 ha area is occupied by the Utrish State Nature Reserve. The climate of the Abrau Peninsula is sub-Mediterranean with cool rainy winters without a stable snow cover and with hot dry summers. The mean annual precipitation is 500 mm, the mean July and February temperatures are 23.7°C and 2.7°C, respectively (
The abundance of millipedes and centipedes at the Abrau Peninsula averages from 784 to150 ind./m2, or ca. 65% and 10% of all soil macroinvertebrates (
The goal of this paper is to summarize and expand the current knowledge on the composition and the distribution of Chilopoda and Diplopoda in the Abrau Peninsula, which would help to improve the conservation policies in the Utrish Nature Reserve and facilitate further studies on these groups in the region.
Materials and methods
The data presented in this paper are primarily based on the field studies of the myriapods collected by the authors (a description of the sampling sites is provided in Table
|
No. |
Habitat |
Coordinates and elevation (m a.s.l.) |
|
{1} |
Quercus petraea, Fagus orientalis, Carpinus caucasica forest |
|
|
{2} |
Q. petraea, F. orientalis, C. caucasica forest |
|
|
{3} |
Quercus pubescens – C. caucasica forest |
|
|
{4} |
Q. pubescens – C. caucasica forest |
|
|
{5} |
Mixed Q. pubescens, C. caucasica, Juniperus excelsa forest with Achnatherum bromoides, Physospermum cornubiense, Dictamnus albus |
|
|
{6} |
Q. pubescens forest with Carpinus orientalis and steppe Graminaea species. |
|
|
{7} |
Steppe meadow with Vitex agnus-castus, Q. pubescens and C. orientalis |
|
|
{8} |
Q. pubescens – C. caucasica forest near the Lake Sukhoy Liman |
|
|
{9} |
Mixed Q. pubescens C. caucasica, Fraxinus excelsior forest |
|
|
{10} |
Tilia begoniifolia – Q. petraea forest with the oriental hornbeam and Acer laetum |
|
|
{11} |
F. orientalis forest |
|
|
{12} |
Q. pubescens – C. orientalis forest with Cotinus coggygria |
|
|
{13} |
Q. pubescens – C. orientalis forest with Juniperus oxycedrus, J. excelsa, Ruscus ponticus and Graminaea. |
|
|
{14} |
Marine station RAS; mixed Q. pubescens, C. orientalis, Pistacia mutica forest with J. excelsa and fruit-trees (Prunus avium, P. armeniaca, Ficus carica, Morus sp.). |
|
|
{15} |
F. orientalis forest with C. caucasica |
|
|
{16} |
T. begoniifolia forest with F. orientalis and C. caucasica |
|
|
{17} |
F. orientalis forest with Q. petraea |
|
|
{18} |
Q. petraea forest with A. laetum |
|
|
{19} |
C. caucasica forest with Q. petraea |
|
Photos of selected habitats in the Abrau Peninsula.
b: P. mutica - Juniperus shrubland on the dry slope
c: Q. petraea, F. orientalis, C. caucasica forest
d: T.Yu. Lushnikova and K.B. Gongalsky collecting invertebrates near a stream in F. orientalis - C. caucasica forest
Sampling. The method of myriapod sampling differed from site to site, since the samples were collected by several authors in different years. In general sampling followed on of the four basic procedures described below.
- "Hand" – animals were collected by hand on sampling sites with the average size of 400 m2 by way of sorting the leaf litter, upper soil layers and woody debris.
- "Sample" – minimum 4 replicates of soil samples (25x25 cm square and 15 cm deep) were collected, then sorted by hand and sifted.
- "Corer" – 25 soil cores of 20 cm in diameter, and a depth of 15 cm were collected with the cylindrical soil corer. Samples were delivered to the laboratory in cool boxes and processed within 2-3 days, or sorted by hand immediately.
- "Whipping" – whipping the branches of trees and shrubs.
The short quoted procedure names were used in the list below to specify the method of material collection for each species.
All specimens were preserved in 70% ethanol and stored in the collection of the Zoological Museum of Lomonosov Moscow State University or in personal collections of I.I. Semenyuk, I.H. Tuf and D.I. Korobushkin.
Data analysis. To evaluate similarity of myriapod taxonomic composition in different microbiotopes with respect to the species' geographical distribution pattern, we applied Single Linkage Clustering analysis (Jaccard similarity index) with the presence/absence data standardization (
An annotated checklist of Chilopoda and Diplopoda species in the Abrau Peninsula
Class Chilopoda
Order Scutigeromorpha
Family Scutigeridae
Scutigera coleoptrata
-
country: Russia; stateProvince:Krasnodar; locality:{14}; verbatimCoordinates:; eventDate:
44°42'21'' N, 37°28'15'' E ; 1606/2009; individualCount:2; recordedBy:AAP; Sampling: hand; sample -
country: Russia; stateProvince:Krasnodar; locality:{14}; verbatimCoordinates:; eventDate:
44°42'21'' N, 37°28'15'' E ; 1606-15-14; individualCount:10; recordedBy:DIK; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506-20-12; individualCount:2; recordedBy:DIK; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{14}; verbatimCoordinates:; eventDate:
44°42'21'' N, 37°28'15'' E ; 1606/2013; individualCount:1; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:P. mutica- Juniperus shrubland; verbatimCoordinates:; eventDate:
44°42'2'' N, 37°28'15'' E ; 1606-16-13; individualCount:1; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:P. mutica- Juniperus shrubland on the dry slope; verbatimCoordinates:; eventDate:
44°42'48'' N, 37°27'58'' E ; 12606-18-13; individualCount:1; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:Abrau city; eventDate:06-20-13; individualCount:2; recordedBy:IHT; Sampling: hand
-
country: Russia; stateProvince:Krasnodar; locality:{3}; verbatimCoordinates:; eventDate:
44°42'51'' N, 37°28'45'' E ; 4706/2013; individualCount:3; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample
S. coleoptrata is an indigenous species in the Mediterranean region, which is largely introduced by human activities throughout Europe, Asia, North America and South America (
Order Lithobiomorpha
Family Lithobiidae
Harpolithobius spinipes
-
country: Russia; stateProvince:Krasnodar; locality:{1}; verbatimCoordinates:; eventDate:
44°45'15'' N, 37°29'53'' E ; 19506/2013; individualCount:1; recordedBy:KBG, DIK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:1; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:1; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample
This species is widespread in the Caucasus (
Lithobius (Monotarsobius) curtipes
-
country: Russia; stateProvince:Krasnodar; locality:{5}; verbatimCoordinates:; eventDate:
44°42'38'' N, 37°27'31'' E ; 1006/2013; individualCount:2; recordedBy:KBG, DIK; Sampling: sample -
country: Russia; stateProvince:Krasnodar; locality:{6}; verbatimCoordinates:; eventDate:
44°42'34'' N, 37°27'25'' E ; 606/2013; individualCount:3; recordedBy:KBG, DIK; Sampling: sample
The most common and abundant species in the European part of Russia (
Lithobius (Monotarsobius) ferganensis
-
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506-20-12; individualCount:3; recordedBy:DMK; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{14}; verbatimCoordinates:; eventDate:
44°42'21'' N, 37°28'15'' E ; 1606/2013; individualCount:1; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:6; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{13}; verbatimCoordinates:; eventDate:
44°42'39''N, 37°28'37'' E ; 3106-18-13; individualCount:4; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:P. mutica - Juniperus shrubland on the dry slope; verbatimCoordinates:; eventDate:
44°42'48'' N, 37°27'58'' E ; 12606-18-13; individualCount:2; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{8}; verbatimCoordinates:; eventDate:
44°45'14'' N, 37°27'26'' E ; 30806-19-13; individualCount:5; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:Abrau city; eventDate:06-20-13; individualCount:1; recordedBy:IHT; Sampling: hand
-
country: Russia; stateProvince:Krasnodar; locality:{1}; verbatimCoordinates:; eventDate:
44°45'15'' N, 37°29'53'' E ; 19506/2013; individualCount:5; recordedBy:KBG, DIK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:6; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{3}; verbatimCoordinates:; eventDate:
44°42'51'' N, 37°28'45'' E ; 4706/2013; individualCount:4; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{3}; verbatimCoordinates:; eventDate:
44°42'51'' N, 37°28'45'' E ; 4706/2013; individualCount:6; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{11}; verbatimCoordinates:; eventDate:
44°42'56''N, 37°28'50'' E ; 5406-15-13; individualCount:9; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{12}; verbatimCoordinates:; eventDate:
44°42'48''N, 37°28'39'' E ; 5006-16-13; individualCount:4; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{13}; verbatimCoordinates:; eventDate:
44°42'39''N, 37°28'37'' E ; 3106-17-13; individualCount:7; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506/2010; individualCount:6; recordedBy:TYL; Sampling: Corer -
country: Russia; stateProvince:Krasnodar; locality:A. laetum - F. excelsior forest with Q. petraea; eventDate:06/2010; individualCount:6; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:C. caucasica forest with F. excelsior, Q. petraea and T. begoniifolia; eventDate:06/2010; individualCount:1; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Q. pubescens - C. orientalis forest; eventDate:06/2010; individualCount:2; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:T. begoniifolia - Q. petraea forest; eventDate:06/2010; individualCount:2; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Tangle of Paliurus spina-christi; eventDate:06/2010; individualCount:1; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:C. caucasica-F. orientalis forest; verbatimCoordinates:; eventDate:
44°43'41'' N, 37°29'25'' E ; 18806-11-10; individualCount:1; recordedBy:TYL; Sampling: Corer -
country: Russia; stateProvince:Krasnodar; locality:C. caucasica-F. orientalis forest; eventDate:06-11-10; individualCount:1; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:C. orientalis - Q. pubescens forest with T. begoniifolia and F. excelsior; eventDate:06-13-10; individualCount:6; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Q. pubescens - Pinus pityusa forest; eventDate:06-13-10; individualCount:2; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Open area north of the lake Sukhoy Liman; eventDate:06-14-10; individualCount:4; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Q. petraea, F. orientalis forest with T. begoniifolia; eventDate:06-16-10; individualCount:1; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Q. petraea - F. excelsior forest; eventDate:06-17-10; individualCount:5; recordedBy:TYL; Sampling: Corer
This species is widespread in Central Asia, the Caucasus and the Crimea reaching the Chinese Karakoram in the east, and Greece and Romania in the west (
Lithobius forficatus
-
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506-20-13; individualCount:1; recordedBy:DMK; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:3; recordedBy:KBG, DIK; Sampling: sample -
country: Russia; stateProvince:Krasnodar; locality:{1}; verbatimCoordinates:; eventDate:
44°45'15'' N, 37°29'53'' E ; 19506/2013; individualCount:3; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample
L. forficatus is an eurytopic species, showing a pan-Holarctic distribution pattern, widely distributed from Great Britain to Turkey and Georgia, the eastern boundary of its range reaching the Ural Mountains (
Lithobius mutabilis
-
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506-20-12; individualCount:7; recordedBy:DMK; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506-14-13; individualCount:2; recordedBy:DMK; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{14}; verbatimCoordinates:; eventDate:
44°42'21'' N, 37°28'15'' E ; 1606/2013; individualCount:2; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:2; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{8}; verbatimCoordinates:; eventDate:
44°45'14'' N, 37°27'26'' E ; 30806-19-13; individualCount:3; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:open areas north of the lake Sukhoy Liman; eventDate:06-14-10; individualCount:1; recordedBy:KBG, DIK; Sampling: sample
-
country: Russia; stateProvince:Krasnodar; locality:{1}; verbatimCoordinates:; eventDate:
44°45'15'' N, 37°29'53'' E ; 19506/2013; individualCount:1; recordedBy:KBG, DIK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:C. caucasica - F. orientalis forest; verbatimCoordinates:; eventDate:
44°43'41'' N, 37°29'25'' E ; 18806-11-10; individualCount:2; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:4; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{3}; verbatimCoordinates:; eventDate:
44°42'51'' N, 37°28'45'' E ; 4706/2013; individualCount:1; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{3}; verbatimCoordinates:; eventDate:
44°42'51'' N, 37°28'45'' E ; 4706/2013; individualCount:5; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{11}; verbatimCoordinates:; eventDate:
44°42'56''N, 37°28'50'' E ; 5406-15-13; individualCount:10; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{11}; verbatimCoordinates:; eventDate:
44°42'56''N, 37°28'50'' E ; 5406-15-13; individualCount:10; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{12}; verbatimCoordinates:; eventDate:
44°42'48''N, 37°28'39'' E ; 5006-16-13; individualCount:2; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{13}; verbatimCoordinates:; eventDate:
44°42'39''N, 37°28'37'' E ; 3106-17-13; individualCount:6; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{13}; verbatimCoordinates:; eventDate:
44°42'39''N, 37°28'37'' E ; 3106-18-13; individualCount:11; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506/2011; individualCount:1; recordedBy:TYL; Sampling: Corer -
country: Russia; stateProvince:Krasnodar; locality:A. laetum - F. excelsior forest with Q. petraea; eventDate:06/2011; individualCount:2; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:T. begoniifolia - Q. petraea forest; eventDate:06/2011; individualCount:2; recordedBy:TYL; Sampling: Corer
This species is known from mesophilous woodlands (e.g. beech and fir-beech forests) in Central Europe, Italy, Bulgaria, Greece, the southern part of Russia and Ukraine, including the Crimea (
Lithobius peregrinus
-
country: Russia; stateProvince:Krasnodar; locality:{4}; verbatimCoordinates:; eventDate:
44°42'50'' N, 37°27'01'' E ; 806/2013; individualCount:5; recordedBy:DIK, AAP, IHT; Sampling: hand; sample -
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506-20-13; individualCount:8; recordedBy:DMK; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{13}; verbatimCoordinates:; eventDate:
44°42'39''N, 37°28'37'' E ; 3106/2013; individualCount:2; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{14}; verbatimCoordinates:; eventDate:
44°42'21'' N, 37°28'15'' E ; 1606/2013; individualCount:3; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:4; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:P. mutica - Juniperus shrubland on the dry slope; verbatimCoordinates:; eventDate:
44°42'48'' N, 37°27'58'' E ; 12606-18-13; individualCount:2; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{8}; verbatimCoordinates:; eventDate:
44°45'14'' N, 37°27'26'' E ; 30806-19-13; individualCount:4; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{5}; verbatimCoordinates:; eventDate:
44°42'38'' N, 37°27'31'' E ; 1006/2009; individualCount:6; recordedBy:KBG, DIK; Sampling: sample -
country: Russia; stateProvince:Krasnodar; locality:{8}; verbatimCoordinates:; eventDate:
44°45'14'' N, 37°27'26'' E ; 30806/2009; individualCount:7; recordedBy:KBG, DIK; Sampling: sample -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:5; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{3}; verbatimCoordinates:; eventDate:
44°42'51'' N, 37°28'45'' E ; 4706/2013; individualCount:1; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{13}; verbatimCoordinates:; eventDate:
44°42'39''N, 37°28'37'' E ; 3106-07-13; individualCount:3; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{11}; verbatimCoordinates:; eventDate:
44°42'56''N, 37°28'50'' E ; 5406-15-13; individualCount:2; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:C. caucasica - F. orientalis forest; eventDate:06-11-10; individualCount:1; recordedBy:TYL; Sampling: Corer
L. peregrinus displays a mostly Mediterranean distribution pattern. The species is known from Italy, Croatia, Montenegro, Serbia, Macedonia, Albania, Greece, Bulgaria, Russian and Georgian sectors of Caucasus. It is also introduced into Great Britain, Panama, Bermuda Islands, and South Africa (
Order Scolopendromorpha
Family Cryptopidae
Cryptops anomalans
-
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506-14-13; individualCount:2; recordedBy:DMK; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506-14-13; individualCount:3; recordedBy:DMK; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{1}; verbatimCoordinates:; eventDate:
44°45'15'' N, 37°29'53'' E ; 19506/2013; individualCount:5; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{13}; verbatimCoordinates:; eventDate:
44°42'39''N, 37°28'37'' E ; 3106/2013; individualCount:2; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:5; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{8}; verbatimCoordinates:; eventDate:
44°45'14'' N, 37°27'26'' E ; 30806/2013; individualCount:6; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:P. mutica - Juniperus shrubland on the dry slope; verbatimCoordinates:; eventDate:
44°42'48'' N, 37°27'58'' E ; 12606-18-13; individualCount:2; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{3}; verbatimCoordinates:; eventDate:
44°42'51'' N, 37°28'45'' E ; 4706/2013; individualCount:4; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{3}; verbatimCoordinates:; eventDate:
44°42'51'' N, 37°28'45'' E ; 4706/2013; individualCount:1; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{11}; verbatimCoordinates:; eventDate:
44°42'56''N, 37°28'50'' E ; 5406-15-13; individualCount:5; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{12}; verbatimCoordinates:; eventDate:
44°42'48''N, 37°28'39'' E ; 5006-16-13; individualCount:3; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{13}; verbatimCoordinates:; eventDate:
44°42'39''N, 37°28'37'' E ; 3106-17-13; individualCount:2; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:Q. pubescens - C. orientalis forest; verbatimCoordinates:; eventDate:
44°43'41'' N, 37°29'25'' E ; 18806-08-10; individualCount:2; recordedBy:TYL; Sampling: Corer -
country: Russia; stateProvince:Krasnodar; locality:C. caucasica - F. orientalis forest; eventDate:06-11-10; individualCount:4; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Q. petraea, F. orientalis forest with T. begoniifolia; eventDate:06-16-10; individualCount:2; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506/2011; individualCount:1; recordedBy:TYL; Sampling: Corer -
country: Russia; stateProvince:Krasnodar; locality:A. laetum - F. excelsior forest with Q. petraea; eventDate:06/2011; individualCount:1; recordedBy:TYL; Sampling: Corer
This South European species is distributed from Spain to Turkey and southern Ukraine (Askania Nova Biosphere Reserve). It is especially common in the Crimea Peninsula (
Cryptops hortensis
-
country: Russia; stateProvince:Krasnodar; locality:{1}; verbatimCoordinates:; eventDate:
44°45'15'' N, 37°29'53'' E ; 19506/2013; individualCount:18; recordedBy:KBG, DIK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:18; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample
This Centralasiatic-European species ranges from Great Britain and Iceland in the north to Morocco and Turkey in the south and Uzbekistan and Tajikistan in the East. The species has also been introduced into North America, some Atlantic and Pacific islands (
Family Scolopendridae
Scolopendra cingulata
-
country: Russia; stateProvince:Krasnodar; locality:{14}; verbatimCoordinates:; eventDate:
44°42'21'' N, 37°28'15'' E ; 1606/2011; individualCount:2; recordedBy:AAP; Sampling: hand; sample -
country: Russia; stateProvince:Krasnodar; locality:{6}; verbatimCoordinates:; eventDate:
44°42'34'' N, 37°27'25'' E ; 606/2009; individualCount:4; recordedBy:KBG, DIK; Sampling: sample -
country: Russia; stateProvince:Krasnodar; locality:{7}; verbatimCoordinates:44°42'29'', 37°27'28'' E; 2; eventDate:06/2009; individualCount:4; recordedBy:KBG, DIK; Sampling: sample
-
country: Russia; stateProvince:Krasnodar; locality:{9}; verbatimCoordinates:; eventDate:
44°41'44'' N, 37°29'06'' E ; 906/2009; individualCount:4; recordedBy:KBG, DIK; Sampling: sample -
country: Russia; stateProvince:Krasnodar; locality:{3}; verbatimCoordinates:; eventDate:
44°42'51'' N, 37°28'45'' E ; 4706/2008; individualCount:1; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:Q. pubescens - C. orientalis; verbatimCoordinates:; eventDate:
44°43'41'' N, 37°29'25'' E ; 18806-11-10; individualCount:1; recordedBy:TYL; Sampling: Corer -
country: Russia; stateProvince:Krasnodar; locality:Juniperus - Quercus shrubland; verbatimCoordinates:; eventDate:
44°43'14'' N, 37°29'11'' E ; 10706-17-10; individualCount:1; recordedBy:TYL; Sampling: Corer
This species is widely distributed in the Mediterranean. It is common in Crimea and the Caucasus, known from Iran, Turkey and Middle Asia (
Order Geophilomorpha
Family Dignathodontidae
Henia (Meinertia) taurica
-
country: Russia; stateProvince:Krasnodar; locality:{4}; verbatimCoordinates:; eventDate:
44°42'50'' N, 37°27'01'' E ; 806/2013; individualCount:1; recordedBy:DIK, AAP, IHT; Sampling: hand; sample -
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506-14-13; individualCount:3; recordedBy:DMK; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506-14-13; individualCount:2; recordedBy:DMK; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{13}; verbatimCoordinates:; eventDate:
44°42'39''N, 37°28'37'' E ; 3106/2013; individualCount:1; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{8}; verbatimCoordinates:; eventDate:
44°45'14'' N, 37°27'26'' E ; 30806-19-13; individualCount:2; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{1}; verbatimCoordinates:; eventDate:
44°45'15'' N, 37°29'53'' E ; 19506/2013; individualCount:1; recordedBy:KBG, DIK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:2; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{13}; verbatimCoordinates:; eventDate:
44°42'39''N, 37°28'37'' E ; 3106-17-13; individualCount:3; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506/2011; individualCount:3; recordedBy:TYL; Sampling: Corer -
country: Russia; stateProvince:Krasnodar; locality:A. laetum - F. excelsior forest with Q. petraea; eventDate:06/2011; individualCount:4; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:T. begoniifolia-Q. petraea forest; eventDate:06/2011; individualCount:2; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Q. petraea, F. orientalis forest with T. begoniifolia; eventDate:06-16-13; individualCount:1; recordedBy:TYL; Sampling: Corer
This species was originally described by Seliwanoff in the Crimea Peninsula (
Family Geophilidae
Clinopodes caucasicus
-
country: Russia; stateProvince:Krasnodar; locality:{11}; verbatimCoordinates:; eventDate:
44°42'56''N, 37°28'50'' E ; 5406-15-13; individualCount:2; recordedBy:DMK; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506-20-13; individualCount:3; recordedBy:DMK; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:2; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{8}; verbatimCoordinates:; eventDate:
44°45'14'' N, 37°27'26'' E ; 30806-19-13; individualCount:3; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{1}; verbatimCoordinates:; eventDate:
44°45'15'' N, 37°29'53'' E ; 19506/2013; individualCount:5; recordedBy:KBG, DIK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:1; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506/2011; individualCount:4; recordedBy:TYL; Sampling: Corer -
country: Russia; stateProvince:Krasnodar; locality:C. orientalis -Q. pubescens forest with T. begoniifolia and F. excelsior; eventDate:06/2011; individualCount:1; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Q. pubescens - J. excelsa forest; eventDate:06/2011; individualCount:1; recordedBy:TYL; Sampling: Corer
C. caucasicus is a species with the Caucasian and eastern Anatolian distribution (
Clinopodes escherichii
-
country: Russia; stateProvince:Krasnodar; locality:{4}; verbatimCoordinates:; eventDate:
44°42'50'' N, 37°27'01'' E ; 806/2013; individualCount:15; recordedBy:DIK, AAP, IHT; Sampling: hand; sample -
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506-20-12; individualCount:9; recordedBy:DMK; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:1; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{13}; verbatimCoordinates:; eventDate:
44°42'39''N, 37°28'37'' E ; 3106-18-13; individualCount:4; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:P. mutica - Juniperus shrubland on the dry slope; verbatimCoordinates:; eventDate:
44°42'48'' N, 37°27'58'' E ; 12606-18-13; individualCount:3; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{8}; verbatimCoordinates:; eventDate:
44°45'14'' N, 37°27'26'' E ; 30806-19-13; individualCount:2; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{5}; verbatimCoordinates:; eventDate:
44°42'38'' N, 37°27'31'' E ; 1006/2009; individualCount:2; recordedBy:KBG, DIK; Sampling: sample -
country: Russia; stateProvince:Krasnodar; locality:{7}; verbatimCoordinates:44°42'29'', 37°27'28'' E; 2; eventDate:06/2009; individualCount:1; recordedBy:KBG, DIK; Sampling: sample
-
country: Russia; stateProvince:Krasnodar; locality:{1}; verbatimCoordinates:; eventDate:
44°45'15'' N, 37°29'53'' E ; 19506/2013; individualCount:4; recordedBy:KBG, DIK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{11}; verbatimCoordinates:; eventDate:
44°42'56''N, 37°28'50'' E ; 5406-15-12; individualCount:2; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{12}; verbatimCoordinates:; eventDate:
44°42'48''N, 37°28'39'' E ; 5006-16-12; individualCount:3; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{13}; verbatimCoordinates:; eventDate:
44°42'39''N, 37°28'37'' E ; 3106-17-12; individualCount:3; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{3}; verbatimCoordinates:; eventDate:
44°42'51'' N, 37°28'45'' E ; 4706/2013; individualCount:7; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:Q. pubescens - C. orientalis forest; eventDate:06-08-10; individualCount:4; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:C. orientalis forest with F. excelsior, Q. pubescens and T. begoniifolia; eventDate:06-09-10; individualCount:1; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Q. pubescens - C. orientalis forest; verbatimCoordinates:44°43'41 N, 37°29'25'' E; eventDate:06-09-10; individualCount:1; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:C. orientalis - Q. pubescens forest with T. begoniifolia and F. excelsior; eventDate:06-13-10; individualCount:2; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Q. pubescens - Pinus pityusa forest; eventDate:06-13-10; individualCount:1; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Juniperus - Quercus shrubland, with C. orientalis; eventDate:06-14-10; individualCount:2; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Woodless locality north of the Lake Sukhoy Liman; eventDate:06-14-10; individualCount:2; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Q. petraea - F. excelsior forest; eventDate:06-17-10; individualCount:5; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Q. pubescens-C. orientalis forest; eventDate:06-17-10; individualCount:2; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Tangle of P. spina-christi; eventDate:06-18-10; individualCount:1; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506/2011; individualCount:2; recordedBy:TYL; Sampling: Corer -
country: Russia; stateProvince:Krasnodar; locality:A. laetum - F. excelsior forest with Q. petraea; eventDate:06/2011; individualCount:12; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:F. orientalis forest with C. caucasica; eventDate:06/2011; individualCount:2; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Q. pubescens - C. orientalis forest; eventDate:06/2011; individualCount:2; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:T. begoniifolia - Q. petraea forest; eventDate:06/2011; individualCount:7; recordedBy:TYL; Sampling: Corer
This species inhabits soil and litter layers of various forests in Russia and Ukraine surrounding the Black Sea. It is also known to occur in the Balkans and extends to the Carpathians in the north. Several records are known from some Aegean islands and Anatolia (
Diphyonyx conjungens
-
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506-14-11; individualCount:3; recordedBy:DMK; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506-20-12; individualCount:2; recordedBy:DMK; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{14}; verbatimCoordinates:; eventDate:
44°42'21'' N, 37°28'15'' E ; 1606/2013; individualCount:2; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{1}; verbatimCoordinates:; eventDate:
44°45'15'' N, 37°29'53'' E ; 19506/2013; individualCount:5; recordedBy:KBG, DIK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:1; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:C. orientalis - Q. pubescens forest with T. begoniifolia and F. excelsior; eventDate:06-13-10; individualCount:1; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506/2011; individualCount:2; recordedBy:TYL; Sampling: Corer -
country: Russia; stateProvince:Krasnodar; locality:F. orientalis forest with C. caucasica; eventDate:06/2011; individualCount:2; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:T. begoniifolia - Q. petraea forest; eventDate:06/2011; individualCount:2; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Q. petraea, F. orientalis forest with T. begoniifolia; eventDate:06-18-13; individualCount:1; recordedBy:TYL; Sampling: Corer
D. conjungens is found in the Crimea, and also recorded in the Balkan Peninsula, throughout the entire Anatolia from the western coast and southern Sporades islands to the easternmost part of Western Armenia, northwards to the Pontic mountains and southwards to the Tauric mountains (
Geophilus cf. oligopus
-
country: Russia; stateProvince:Krasnodar; locality:Juniperus-Quercus shrubland, with C. orientalis; eventDate:06-16-10; individualCount:2; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506/2011; individualCount:4; recordedBy:TYL; Sampling: Corer -
country: Russia; stateProvince:Krasnodar; locality:A. laetum - F. excelsior forest with Q. petraea; eventDate:06/2011; individualCount:4; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:F. orientalis forest with C. caucasica; eventDate:06/2011; individualCount:3; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:P. mutica - Juniperus shrubland; verbatimCoordinates:; eventDate:
44°42'38'' N, 37°27'55'' E ; 5106/2011; individualCount:3; recordedBy:TYL; Sampling: Corer -
country: Russia; stateProvince:Krasnodar; locality:Q. pubescens - C. orientalis forest; eventDate:06/2011; individualCount:1; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:T. begoniifolia - Q. petraea forest; eventDate:06/2011; individualCount:8; recordedBy:TYL; Sampling: Corer
In habitus, the studied material strongly resembles G. oligopus, although our localities are situated far away from the currently known range of the species. Until now, G. oligopus has mostly been known from the Alpine-Dinaric area, being also recorded in the Carpathians (
Pachymerium ferrugineum
-
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:3; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{8}; verbatimCoordinates:; eventDate:
44°45'14'' N, 37°27'26'' E ; 30806-19-13; individualCount:1; recordedBy:IHT; Sampling: hand -
country: Russia; stateProvince:Krasnodar; locality:{1}; verbatimCoordinates:; eventDate:
44°45'15'' N, 37°29'53'' E ; 19506/2013; individualCount:6; recordedBy:KBG, DIK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306/2013; individualCount:4; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{3}; verbatimCoordinates:; eventDate:
44°42'51'' N, 37°28'45'' E ; 4706/2013; individualCount:5; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{11}; verbatimCoordinates:; eventDate:
44°42'56''N, 37°28'50'' E ; 5406-15-13; individualCount:1; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:C. caucasica - F. orientalis forest; eventDate:06-11-10; individualCount:1; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Q. petraea, F. orientalis forest with T. begoniifolia; eventDate:06-16-10; individualCount:3; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506/2011; individualCount:2; recordedBy:TYL; Sampling: Corer
P. ferrugineum is widely distributed through most parts of the Palaearctic (
Family Schendylidae
Schendyla nemorensis
-
country: Russia; stateProvince:Krasnodar; locality:Q. petraea, F. orientalis forest with T. begoniifolia; eventDate:06/2011; individualCount:3; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:Q. pubescens - J. excelsa forest; eventDate:06/2011; individualCount:2; recordedBy:TYL; Sampling: Corer
A common soil-dwelling European species, distributed from the Great Britain to Macaronesia and through the Mediterranean region to Rostov region of Russia (
Class Diplopoda
Order Polyxenida
Family Polyxenidae
Propolyxenus aegeus
-
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506-14-13; individualCount:7; recordedBy:IHT; Sampling: hand, whipping -
country: Russia; stateProvince:Krasnodar; locality:{2}; verbatimCoordinates:; eventDate:
44°44'13'' N, 37°28'46'' E ; 15306-15-13; individualCount:3; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample, whipping
P. trivittatus is known from Israel, western Turkey and Greece; P. aegeus is found in Rhodes (
Family Lophoproctidae
Lophoproctus coecus
-
country: Russia; stateProvince:Krasnodar; locality:{17}; verbatimCoordinates:; eventDate:
44°43'46''N, 37°29'13'' E ; 11606/2011; individualCount:11; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{1}; verbatimCoordinates:; eventDate:
44°45'15'' N, 37°29'53'' E ; 19506/2013; individualCount:25; recordedBy:KBG, DIK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{11}; verbatimCoordinates:; eventDate:
44°42'56''N, 37°28'50'' E ; 5406/2013; individualCount:17; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:{13}; verbatimCoordinates:; eventDate:
44°42'39''N, 37°28'37'' E ; 3106/2013; individualCount:3; recordedBy:KBG, DIK, DMK, AAP, IHT; Sampling: hand, sample -
country: Russia; stateProvince:Krasnodar; locality:Q. pubescens-Pinus pityusa forest; eventDate:06-13-10; individualCount:7; recordedBy:TYL; Sampling: Corer
-
country: Russia; stateProvince:Krasnodar; locality:{10}; verbatimCoordinates:; eventDate:
44°43'31''N, 37°29'04'' E ; 8506/2011; individualCount:21; recordedBy:TYL; Sampling: Corer -
country: Russia; stateProvince:Krasnodar; locality:F. orientalis forest with C. caucasica; eventDate:06/2011; individualCount:2; recordedBy:TYL; Sampling: Corer
L. coecus is widespread throughout Europe, particularly in Eastern Europe with its distribution extending to Central Asia (
Order Glomerida
Family Glomeridae
Hyleoglomeris awchasica
-
country: Russia; stateProvince:Krasnodar; locality:{16}; verbatimCoordinates:; eventDate:
44°44'27''N, 37°29'53'' E ; 29506/2011; individualCount:7; recordedBy:IIS; Sampling: Hand
This species is known from Colchis, Georgian and Russian parts of the western Caucasus (
Trachysphaera costata
-
country: Russia; stateProvince:Krasnodar; locality:{16}; verbatimCoordinates:; eventDate:
44°44'27''N, 37°29'53'' E ; 29506/2011; individualCount:20; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{18}; verbatimCoordinates:; eventDate:
44°44'02''N, 37°29'32'' E ; 17206/2011; individualCount:12; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{19}; verbatimCoordinates:; eventDate:
44°44'10''N, 37°28'47'' E ; 14906/2011; individualCount:13; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{15}; verbatimCoordinates:; eventDate:
44°45'02''N, 37°30'05'' E ; 27306/2013; individualCount:23; recordedBy:IIS; Sampling: Hand
T. costata is known from parthenogenetic populations in Central and Eastern Europe, as well as in some areas in the Caucasus, while bisexual populations are restricted to southern Romania, the Balkans, Anatolia, Israel, most of the Caucasus, and Crimea (
Order Polyzoniida
Family Hirudisomatidae
Hirudisoma roseum
-
country: Russia; stateProvince:Krasnodar; locality:{15}; verbatimCoordinates:; eventDate:
44°45'02''N, 37°30'05'' E ; 27306/2011; individualCount:6; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{16}; verbatimCoordinates:; eventDate:
44°44'27''N, 37°29'53'' E ; 29506/2011; individualCount:2; recordedBy:IIS; Sampling: Hand
The species distribution covers southern Russia, Abkhazia, Georgia, north-western Azerbaijan and eastern Turkey (
Order Julida
Family Blaniulidae
Nopoiulus kochii
-
country: Russia; stateProvince:Krasnodar; locality:{15} under bark of fallen branches; verbatimCoordinates:; eventDate:
44°45'02''N, 37°30'05'' E ; 27306/2011; individualCount:4; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{17}; verbatimCoordinates:; eventDate:
44°43'46''N, 37°29'13'' E ; 11606/2011; individualCount:7; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{18}; verbatimCoordinates:; eventDate:
44°44'02''N, 37°29'32'' E ; 17206/2011; individualCount:9; recordedBy:IIS; Sampling: Hand
N. kochii is recorded widely across the continent. It has been introduced to New Zealand, North and South America (
Family Nemasomatidae
Nemasoma caucasicum
-
country: Russia; stateProvince:Krasnodar; locality:{15}; verbatimCoordinates:; eventDate:
44°45'02''N, 37°30'05'' E ; 27306/2011; individualCount:3; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{16}; verbatimCoordinates:; eventDate:
44°44'27''N, 37°29'53'' E ; 29506/2011; individualCount:3; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{17}; verbatimCoordinates:; eventDate:
44°43'46''N, 37°29'13'' E ; 11606/2011; individualCount:1; recordedBy:IIS; Sampling: Hand
The species is known from Turkey, Armenia, Azerbaidjan, Georgia and southern Russia (
Family Julidae
Chaetoleptophyllum cf. flexum
-
country: Russia; stateProvince:Krasnodar; locality:{15}; verbatimCoordinates:; eventDate:
44°45'02''N, 37°30'05'' E ; 27306/2011; individualCount:4; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{17}; verbatimCoordinates:; eventDate:
44°43'46''N, 37°29'13'' E ; 11606/2011; individualCount:10; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{18}; verbatimCoordinates:; eventDate:
44°44'02''N, 37°29'32'' E ; 17206/2011; individualCount:1; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{19}; verbatimCoordinates:; eventDate:
44°44'10''N, 37°28'47'' E ; 14906/2011; individualCount:1; recordedBy:IIS; Sampling: Hand
Identification of the above species was difficult as only females and juveniles were found. C. flexum is a common and abundant species in the Krasnodar region (Chumachenko, in litt.), it was also reported from Georgia, Stavropol region and the montane parts of the Republic of Adygea, Russia (
Cylindroiulus sp.
-
country: Russia; stateProvince:Krasnodar; locality:{15}; verbatimCoordinates:; eventDate:
44°45'02''N, 37°30'05'' E ; 27306/2011; individualCount:15; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{16}; verbatimCoordinates:; eventDate:
44°44'27''N, 37°29'53'' E ; 29506/2011; individualCount:15; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{17}; verbatimCoordinates:; eventDate:
44°43'46''N, 37°29'13'' E ; 11606/2011; individualCount:1; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{18}; verbatimCoordinates:; eventDate:
44°44'02''N, 37°29'32'' E ; 17206/2011; individualCount:13; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{19}; verbatimCoordinates:; eventDate:
44°44'10''N, 37°28'47'' E ; 14906/2011; individualCount:16; recordedBy:IIS; Sampling: Hand
This is most likely a new species that requires description. All specimens were collected from the leaf litter and under barks of fallen trees in all types of broad-leaf forests.
Julus colchicus
-
country: Russia; stateProvince:Krasnodar; locality:{15}; verbatimCoordinates:; eventDate:
44°45'02''N, 37°30'05'' E ; 27306/2011; individualCount:12; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{16}; verbatimCoordinates:; eventDate:
44°44'27''N, 37°29'53'' E ; 29506/2011; individualCount:2; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{17}; verbatimCoordinates:; eventDate:
44°43'46''N, 37°29'13'' E ; 11606/2011; individualCount:3; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{18}; verbatimCoordinates:; eventDate:
44°44'02''N, 37°29'32'' E ; 17206/2011; individualCount:4; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{19}; verbatimCoordinates:; eventDate:
44°44'10''N, 37°28'47'' E ; 14906/2011; individualCount:3; recordedBy:IIS; Sampling: Hand
J. colchicus is known from Turkey, Georgia, Abkhazia and the southern regions of Russia (
Megaphyllum rossicum
-
country: Russia; stateProvince:Krasnodar; locality:{17}; verbatimCoordinates:; eventDate:
44°43'46''N, 37°29'13'' E ; 11606/2011; individualCount:1; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{19}; verbatimCoordinates:; eventDate:
44°44'10''N, 37°28'47'' E ; 14906/2011; individualCount:2; recordedBy:IIS; Sampling: Hand
M. rossicum is known from the south and center of European Russia, Crimea and the Caucasus, reaching Ural Mountains in the East (
Pachyiulus krivolutskyi
-
country: Russia; stateProvince:Krasnodar; locality:{15}; verbatimCoordinates:; eventDate:
44°45'02''N, 37°30'05'' E ; 27306/2011; individualCount:8; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{16}; verbatimCoordinates:; eventDate:
44°44'27''N, 37°29'53'' E ; 29506/2011; individualCount:3; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{17}; verbatimCoordinates:; eventDate:
44°43'46''N, 37°29'13'' E ; 11606/2011; individualCount:2; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{18}; verbatimCoordinates:; eventDate:
44°44'02''N, 37°29'32'' E ; 17206/2011; individualCount:1; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{19}; verbatimCoordinates:; eventDate:
44°44'10''N, 37°28'47'' E ; 14906/2011; individualCount:1; recordedBy:IIS; Sampling: Hand
P. krivolutskyi is mostly known from the Caucasus (
Order Chordeumatida
Family Anthroleucosomatidae
gen. sp. undetermined
-
country: Russia; stateProvince:Krasnodar; locality:{15}; verbatimCoordinates:; eventDate:
44°45'02''N, 37°30'05'' E ; 27306/2011; individualCount:1; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{16}; verbatimCoordinates:; eventDate:
44°44'27''N, 37°29'53'' E ; 29506/2011; individualCount:5; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{17}; verbatimCoordinates:; eventDate:
44°43'46''N, 37°29'13'' E ; 11606/2011; individualCount:3; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{18}; verbatimCoordinates:; eventDate:
44°44'02''N, 37°29'32'' E ; 17206/2011; individualCount:2; recordedBy:IIS; Sampling: Hand
This is most likely a new, yet undescribed species. The specimens were found in broad-leaf forests, under the bark of fallen trees and branches, rarely in leaf litter.
Order Polydesmida
Family Polydesmidae
Brachydesmus furcatus
-
country: Russia; stateProvince:Krasnodar; locality:{16}; verbatimCoordinates:; eventDate:
44°44'27''N, 37°29'53'' E ; 29506/2011; individualCount:4; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{17}; verbatimCoordinates:; eventDate:
44°43'46''N, 37°29'13'' E ; 11606/2011; individualCount:2; recordedBy:IIS; Sampling: Hand
B. furcatus was described in the Northern Caucasus (
Brachydesmus kalischewskyi
-
country: Russia; stateProvince:Krasnodar; locality:{15}; verbatimCoordinates:; eventDate:
44°45'02''N, 37°30'05'' E ; 27306/2011; individualCount:2; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{17}; verbatimCoordinates:; eventDate:
44°43'46''N, 37°29'13'' E ; 11606/2011; individualCount:5; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{18}; verbatimCoordinates:; eventDate:
44°44'02''N, 37°29'32'' E ; 17206/2011; individualCount:3; recordedBy:IIS; Sampling: Hand
The species is known from the western part of Turkey, Adsharia and Abkhasia (
Polydesmus muralewiczi
-
country: Russia; stateProvince:Krasnodar; locality:{15}; verbatimCoordinates:; eventDate:
44°45'02''N, 37°30'05'' E ; 27306/2011; individualCount:13; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{16}; verbatimCoordinates:; eventDate:
44°44'27''N, 37°29'53'' E ; 29506/2011; individualCount:19; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{17}; verbatimCoordinates:; eventDate:
44°43'46''N, 37°29'13'' E ; 11606/2011; individualCount:16; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{18}; verbatimCoordinates:; eventDate:
44°44'02''N, 37°29'32'' E ; 17206/2011; individualCount:9; recordedBy:IIS; Sampling: Hand -
country: Russia; stateProvince:Krasnodar; locality:{19}; verbatimCoordinates:; eventDate:
44°44'10''N, 37°28'47'' E ; 14906/2011; individualCount:26; recordedBy:IIS; Sampling: Hand
The species is widespread in the Caucasus (Stavropol and Krasnodar regions of Russia and Western Georgia), where it occurs in a wide range of habitats (
Analysis
In total, in the Abrau Peninsula 33 species of Chilopoda and Diplopoda were collected. The centipede fauna is represented by typical Caucasian (e.g. H. spinipes, C. caucasicus) as well as Mediterranean elements (D. conjungens, S. cingulata) and widespread Palearctic species (e.g. L. curtipes, P. ferrugineum). The millipede fauna consists mainly of Caucasian (e.g. B. furcatus, H. roseum, P. krivolutskyi) and some Euromediterranean species (M. rossicum, T. costata). The highest species diversity of Diplopoda is noted in the mountainous deciduous forests (all 16 species). However, in xerophytic forests presence of typically Mediterranean species is also notable (P. aegeus / P. trivittatus).
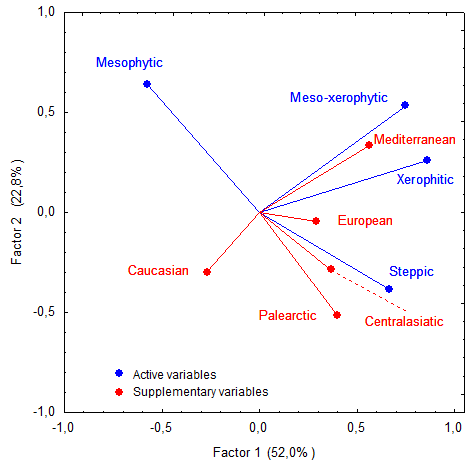
The PCA (Fig.
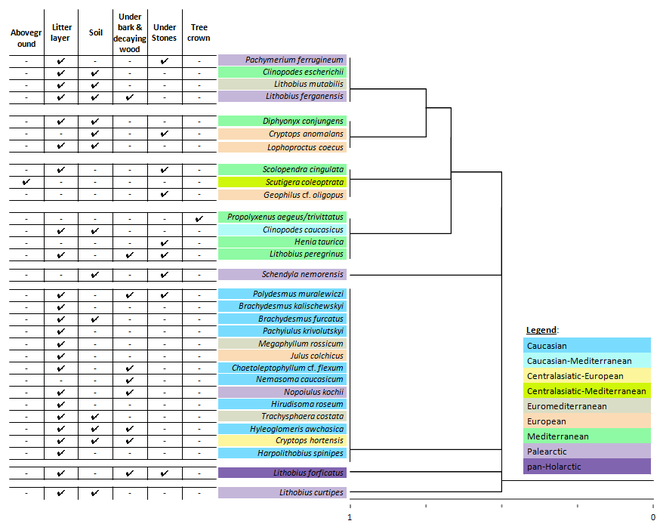
The cluster analysis revealed that habitat preferences of myriapods within the set of sampling plots (Table
Cluster analysis of similarity (Jaccard) of myriapod species distribution patterns across sampling plots in the Abrau Peninsula with respect to their distribution type and microbiotope preferences. The geographical distribution pattern of each species has been marked using color-key. The table to the left of the cluster tree demonstrates microbiotopes, in which the respective species were found.
The top two clusters in Fig.
Our results suggest that the Abrau Peninsula is identified as a unique vegetation formation (
Acknowledgements
We would like to thank S.I. Golovatch, K.B. Gongalsky, K.S. Onufrieva, M. Short, K.S. Speranskaya and A.S. Zaytsev for their support. T.Y. Lushnikova, D.M. Kuznetsova, K.B. Gongalsky and A.A. Panchenkov assisted during the collecting process. We would also like to thank V.S. Rudovsky for the photographs. The authors are grateful to L.M. Mukhametov, the Director of the Utrish Marine Biological Station RAS and O.N. Bykhalova, the Deputy Director of the Utrish State Nature Reserve. We greatly appreciate the improvements to the manuscript suggested by L. Danyi, P. Stoev, B. Vagalinsky and M. Zapparoli. The study was performed with the financial support of the Russian Science Foundation (project # 14-14-00894).
References
- Interactive agricultural ecological atlas of Russia and neighboring countries. Economic plants and their diseases, pests and weeds. http://www.agroatlas.ru
- Nationalnyckeln till Sveriges flora och fauna. Mångfotingar Myriapoda.SLU,Uppsala,351pp.
- Chilopoda Geophilomorpha of Europe: a revised list of species, with taxonomic and nomenclatorial notes.Zootaxa3770:1‑136. https://doi.org/10.11646/zootaxa.3770.1.1
- The centipede genus Clinopodes C. L. Koch, 1847 (Chilopoda, Geophilomorpha, Geophilidae): reassessment of species diversity and distribution, with a new species from the Maritime Alps (France).Zoosystema33:175‑205. https://doi.org/10.5252/z2011n2a3
- Geophilomorph centipedes of Latvia (Chilopoda, Geophilomorpha).Latvijas entomologs42:5‑17.
- Morphology, taxonomy and distribution of Diphyonyx gen. n., a lineage of geophilid centipedes with unusually shaped claws (Chilopoda: Geophilidae).Eur. J. Entomol.105(2):343‑354. https://doi.org/10.14411/eje.2008.041
- Die Erdläufer (Chilopoda, Geophilida) des Wiener Stadtgebietes.Verh. Zool.-Bot. Gesellsch. Österr.133:107‑132.
- Geophilus oligopus (Attems, 1895) a species new to the fauna of Romania and to the whole of the Carpathian Mountains.Schubartiana2:39‑48.
- Lithobius (Monotarsobius) franciscorum sp. nov., a new lithobiid species from the Altai, with a key to the Central Asian species of the subgenus (Chilopoda: Lithobiomorpha).Zootaxa3182:16‑28.
- On some Lithobiomorpha from the mountains of Kirghizia and Kazakhstan (Chilopoda).Arthrop. Sel.6:117‑121.
- The millipedes of Turkey (Diplopoda).Steenstrupia29(2):175‑198.
- Fauna Europaea. Myriapoda. Fauna Europaea, version 1.2. http://www.faunaeur.org
- Cluster analysis.Chichester,Chichester, U.K.,330pp. https://doi.org/10.1002/9780470977811
- Two new genera of cave-dwelling millipedes (Diplopoda), with remarks on the millipede fauna of West Caucasian caves.Int. J. Speleol.14:39‑50. https://doi.org/10.5038/1827-806x.14.1.5
- Golovatch SI (1992) Some patterns in the distribution and origin of the millipede fauna of the Russian Plain.Berichte Naturwissenchaftlich-Medizinischen Verein in Innsbruck, supplementum 10, Proceedings of the 8 International Congress of Myriapodology,373-383pp.
- On three remarkable millipedes (Diplopoda) from the Crimea, Ukraine.Int. J. Myriap.1:97‑110. https://doi.org/10.1163/187525408x316767
- The millipede genus Trachysphaera Heller, 1858 in the Ukraine (Diplopoda: Glomeridae).Arthropoda selecta19(1):1‑5.
- Colobognatha millipedes in the Caucasus (Diplopoda: Polyzoniida, Platydesmida, Siphonocryptida).Zootaxa3972(2):250‑266. https://doi.org/10.11646/zootaxa.3972.2.6
- Review of the millipede genus Hyleoglomeris Verhoeff, 1910 (Diplopoda, Glomerida, Glomeridae), with descriptions of new species from caves in Southeast Asia.Zoosystema28(4):887‑915.
- Do boundaries of soil animal and plant communities coincide? A case study of a Mediterranean forest in Russia.Eur. J. Soil Biol.44(4):355‑363. https://doi.org/10.1016/j.ejsobi.2008.04.004
- Stratification and dynamics of bait-lamina perforation in three forest soils along a North-South gradient in Russia.Appl. Soil Ecol.25(2):111‑122. https://doi.org/10.1016/j.apsoil.2003.09.001
- Genetic diversity and the phylogeography of parthenogenesis: comparing bisexual and thelytokous populations of Nemasoma varicorne (Diplopoda: Nemasomatidae) in Denmark.Hereditas136:184‑194. https://doi.org/10.1034/j.1601-5223.2002.1360302.x
- Principal Component Analysis.Springer,NY,487pp.
- Role of allochthonous carbon in the energy of terrestrial invertebrate communities at different distances from the Black sea and a freshwater lake (isotopic evidence).Russian Journal of Ecology45(3):223‑230. https://doi.org/10.1134/s1067413614030060
- Isotopic niche (δ13С and δ15N values) of soil macrofauna in temperate forests.Rapid Communications in Mass Spectrometry28:1303‑1311. https://doi.org/10.1002/rcm.6903
- Über die Diplopoden des Kaukasusgebietes.Göteborgs Kungliga Vetenskaps- och Vitterhets-Samhälles Handlinger, femte följdenSer. B, 5(1):1‑196.
- The millipedes of Albania: recent data, new taxa; systematical, nomenclatural and faunistical review (Myriapoda, Diplopoda).Zoosystema19:255‑292.
- The millipedes (Diplopoda) of the Asian part of Russia.Pensoft,Bulgaria,293pp.
- Zur Myriapodenfauna des Kaukasus.Zool. Anz.31:329‑351.
- Übersicht über die Chilopodenfauna des Kaukasus. II.Zool. Anz.69:27‑44.
- Palmén E (1988) Myriapoda of Eastern Scandinavia. In: Krivolutzkij DA (Ed.) Soil Biology of Northern Europe.Nauka,Moscow,16-40pp. [InRussian].
- The order Siphonophorida – A taxonomist’s nightmare? Lessons from a Brazilian collection.Soil Organisms81:543‑556.
- Notes to identification of Russian Myriapoda.Horae Societatis Entomologicae Rossicae18:69‑121. [InRussian].
- Contribution to the vascular plant flora of the Utrish area, a relic sub-Mediterranean ecosystem of the Russian Black Sea Coast.Willdenowia37(2):451‑463. https://doi.org/10.3372/wi.37.37207
- Shear WA (2011) Class Diplopoda de Blainville in Gervais, 1844. In: Zhang Z- (Ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness.Zootaxa 3148,159-164pp.
- New records of Lophoproctus coecus Pocock, 1894 (Diplopoda, Polyxenida, Lophoproctidae) extend the range of the genus Lophoproctus.ZooKeys510:209‑222. https://doi.org/10.3897/zookeys.510.8668
- Revision of the genus Propolyxenus Silvestri with description of a new species.Int. J. Myriap.3:1‑17. https://doi.org/10.1163/187525410X12578602960263
- Biodiversity of soil microarthropods: the filtering of species.Biodiversity & Conservation5(2):251‑260. https://doi.org/10.1007/bf00055834
- Pachymerium ferrugineum (C.L. Koch, 1835) - two distinct forms in Crete?Bull. Brit. Myriap. Isopod Gr.19:57‑61.
- Centipedes (Chilopoda) from Greece in the collection of the National Museum of Natural History, Sofia.Hist. nat. bulg.16:81‑88.
- Определитель многоножек-костянок СССР. [Identification book of the lithobiomorph centipedes of the USSR (Chilopoda: Lithobiomorpha)].Nauka,Moscow,212pp. [InRussian].
- The scolopendromorph centipedes (Chilopoda, Scolopendromorpha).Nauka,Moscow,103pp. [InRussian].
- Zalesskaja NT, Titova LP, Golovatch SI (1982) The fauna of Myriapoda of Moscow Region. In: Gilyarov MS (Ed.) Soil invertebrates of Moscow Region.Nauka,Moscow,179-200pp. [InRussian].
- Note su tassonomia, corologia ed ecologia di Lithobius peregrinus Latzel, 1880 (Chilopoda: Lithobiomorpha).Ann. Naturhist. Mus. Wien93:161‑179.
- Catalogue of the centipedes from Greece (Chilopoda).Fragmenta entomologica34:56‑168.
- An annotated catalogue of the epigeic and cave centipedes (Chilopoda) of Sardinia.Zootaxa2318:56‑168.
- The centipedes (Chilopoda) of Corsica: catalogue of species with faunistic, zoogeographical and ecological remarks.International Journal of Myriapodology7:15‑68. https://doi.org/10.3897/ijm.7.3110
- Preliminary data on the millipedes (Diplopoda) from the Stavropol Territory, northern Caucasus, Russia.Arthrop. Sel.23(4):347‑354.