|
Biodiversity Data Journal :
Taxonomic Paper
|
|
Corresponding author:
Academic editor: Dimitrios Koureas
Received: 07 Jan 2016 | Accepted: 16 May 2016 | Published: 08 Jun 2016
© 2016 Ramona-Elena Irimia, Marc Gottschling
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Irimia R, Gottschling M (2016) Taxonomic revision of Rochefortia Sw. (Ehretiaceae, Boraginales). Biodiversity Data Journal 4: e7720. https://doi.org/10.3897/BDJ.4.e7720
|
|
Abstract
Background
Rochefortia is a small taxon of woody plants in the Ehretiaceae (Boraginales) exhibiting coriaceous leaves with cystoliths, small whitish flowers and drupaceous fruits containing four pyrenes. It shares the dioecious sex distribution with its sister group Lepidocordia and can be delimited from the latter (and all other Ehretiaceae) by the presence of thorns. Neotropical Rochefortia is distributed over most Caribbean islands, Central America and northern South America. Twenty-eight validly published names (corresponding to twenty-one typified taxa at the species level and below) are available in Rochefortia, but the precise number of species to be accepted has been elusive before this revision.
New information
In the course of the present revision, 353 herbarium collections, comprising approximately 540 Rochefortia specimens, were entried into a BRAHMS data base providing information about protologues and types and retrospective georeferences if possible. Based on the combination of molecular and morphological data we propose to recognise nine species of Rochefortia, namely R. acanthophora, R. bahamensis, R. barloventensis, R. cubensis, R. cuneata, R. lundellii, R. oblongata, R. spinosa and R. stellata (the remaining nineteen validly published names are synonymised under such names). Morphological description of each species and an identification key are provided.
Keywords
asterids; Caribbean; herbarium specimens; morphology; taxonomy
Introduction
Taxonomic history
Rochefortia Sw. was described by the Swede Olof P. Swartz (1760–1818) in 1788 based on plants he collected in Jamaica. The taxon received its name in commemoration of a French author and priest, namely Charles de Rochefort (1605–1683), who lived in the Caribbean for a period of time and wrote the disputed book “Histoire naturelle et morale des Îles Antilles de l\'Amérique ... avec un vocabulaire Caraïbe” (firstly published in 1658).
Twenty-eight validly published names (corresponding to twenty-one typified taxa at the species level and below) are available in Rochefortia, but it has been unclear before this revision how many species are to be accepted. The only morphological revision considering the entirety of Rochefortia is provided by a Master thesis of
During the 20th century, most taxonomic treatment of Rochefortia was performed for restricted geographical regions only (Cuba:
Morphology
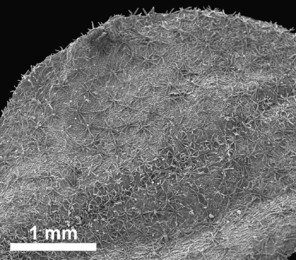
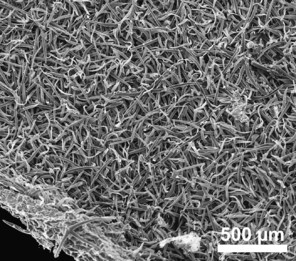
HABIT. Rochefortia comprises evergreen shrubs or trees 1.5 m up to 10.0 m tall (R. spinosa) or even lianas (R. lundellii). In R. lundellii, R. spinosa and to some extent also in R. acanthophora, twigs are arching, and the stem is divided right from the base like in a shrub (herbarium specimen notes). The bark is light grey through brown in colour and its surface smooth or slightly fissured, sometimes peeling in flakes (R. acanthophora). The young twigs and leaves are variously tomentose (R. stellata) through scabrous (R. acanthophora, R. cubensis Britton & P.Wilson, R. oblongata) and sometimes displaying only a few scattered trichomes congregating on both the adaxial and abaxial leaf midrib (R. bahamensis Britton, R. lundellii, R. spinosa).
All species of Rochefortia develop thorns being a unique and therefore diagnostic character in Ehretiaceae (
In Cuban species such as R. cubensis and R. oblongata, short shoot structures are developed (Fig.
Galls. Cuban species occasionally exhibit internally hollow short shoots composed of rudimentary leaves in axils of (modified thorny) bracts or brachyblasts. A. general gall morphology of R. oblongata; B. longisection of gall, R. cubensis, arrow indicate reproductive structures putatively of mites.
LEAVES (Fig.
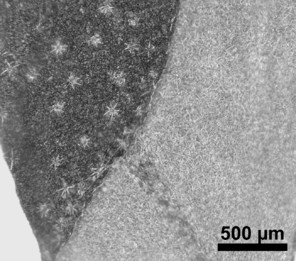
Stellate trichomes with diagnostic importance for Rochefortia stellata. The trait is otherwise rare in the woody borages [present in, e.g., Cordia alliodora (Ruiz & Pav.) Oken]. A.–B. adaxial surface exhibiting scattered trichomes (scanning electron microscopy: SEM); C. stereo-microscopic image of adaxial (dark) and tomentose abaxial surface (light); D.–E. isolated stellate trichomes from adaxial surface (SEM); F. abaxial tomentose surface.
Leaf texture is usually coriaceous and occasionally membranaceous (individuals of R. cuneata). The most common leaf shapes in (woody Boraginales such as) Rochefortia are elliptic and obovate, some individuals in R. bahamensis and R. barloventensis additionally have nearly spherical leaves. In general, plants with larger leaves (i.e., >5.0 cm) assigned to R. cuneata, R. lundellii and R. spinosa are rather easily distinguishable from plants with smaller leaves (i.e., <5.0 cm) present in, for example, R. acanthophora and R. bahamensis. Rochefortia cubensis is the species with the smallest leaves (1.0 cm maximal length), while R. lundellii and R. spinosa are at opposite extremes with leaf lengths up to 12.0 cm. The lamina is always simple, with the margin entire or sometimes revolute (e.g., R. cuneata) and with the tips ciliate. The leaf base is cuneate or rounded, while the apex is rounded through retuse, acute or sometimes cuspidate (R. lundellii and also a few individuals of R. spinosa).
Leaf indument usually comprises at least few scattered trichomes at midribs, but leaves and individuals being completely glabrous are occasionally found in R. bahamensis, R. barloventensis, R. cuneata, R. lundellii and R. spinosa. The presence of a more extensive indument leading to a, for example, hirsute surface of leaves is likewise rare (e.g., R. cubensis, R. stellata). Trichomes are predominantly unicellular and simple, with the exception of R. stellata having characteristic multi-branched and star-shaped trichomes (Fig.
FLOWERS. Generative organs are rarely documented in herbarium specimens, whereas knowledge about flowers and fruits are particularly scarce for Cuban species of Rochefortia. The basic monotelic architecture being thyrsoid shows little variation in Rochefortia, and inflorescences are positioned both axillarily and terminally. The number of flowers in each inflorescence usually varies between 3–10, but can be increased up to 15 (R. lundellii, R. spinosa) or can be reduced to a cluster of 2 and sometimes to a single flower (R. acanthophora, R. cubensis). In inflorescences, bracts are absent in Rochefortia, and flowers are pedicellate through subsessile. The latter trait has taxonomic importance to delimit, for example, R. acanthophora (with sessile flowers) from morphologically similar species (such as R. cubensis with shortly pedicellate flowers).
In Rochefortia, flowers are actinomorphic, tetracyclic, pentamerous and unisexual. Most species have small flowers with corolla diameter of about 0.40–0.50 cm, while R. bahamensis has significantly larger flowers up to 0.70 cm in corolla diameter (♂ individuals). The synsepalous calyx is coriaceous and its aestivation imbricate (plesiomorphic condition in Ehretiaceae:
Female and male flowers differ only with respect to formation of gynoecium and androecium, but not to perianth. Male flowers have very well developed stamens with (in outline) reniform functional anthers as demonstrated by the presence of pollen (visible with the stereo microscope at 60x magnification). Filaments are flattened, which is another unusual character within Ehretiaceae (
Female flowers have smaller, shrivelled anthers lacking pollen (verified in SEM in the course of the present study) on short filaments. The ovary is globose and exhibits the coenocarpous-syncarpous architecture usually developed in Ehretiaceae (
Sex distribution is dioecious, which is a very unusual trait within Ehretiaceae (and argues as synapomorphy for the close relationship between Lepidocordia and Rochefortia:
Both staminate and pistillate flowers occur contemporary in Rochefortia (pooled specimens of all species inspected in this revision).
|
Jan |
Feb |
Mar |
Apr |
May |
Jun |
Jul |
Aug |
Sep |
Oct |
Nov |
Dec |
|
|
♂ |
– |
1 |
3 |
4 |
4 |
3 |
1 |
1 |
– |
1 |
1 |
– |
|
♀ |
2 |
2 |
6 |
3 |
2 |
7 |
3 |
2 |
– |
1 |
3 |
3 |
|
fruit |
5 |
7 |
7 |
4 |
9 |
12 |
12 |
13 |
9 |
7 |
9 |
6 |
Flowers are sometimes fragrant (as noted on herbarium specimens) indicating zoophily like in many other Ehretiaceae. There is a single report about pollinators available from herbarium collections (Zanoni et al. 39552: MO!) stating that flowers are visited by numerous bees (species not indicated).
FRUITS. Fruit is indehiscent and drupaceous, with the shape (sub-)spherical and a diameter of 0.60–0.70 cm. Individuals of R. lundellii from Costa Rica exhibt the largest fruits at all found in Rochefortia with a diameter of 1.00–1.20 cm. The inner architecture of the fruit, and particularly of the endocarp, corresponds (almost indistinguishable) to the four-parted type known from the Ehretia P.Browne I clade (Ehretiaceae;
Distribution and habitat
Most Rochefortia species grow in dry, only seasonally wet climate, relatively close to the sea and at low altitudes. Preferred substrates include rocky and alkaline soils with a calcareous layer, and most of the West Indian species are reported to occur on both limestone and serpentine. Species of Rochefortia are important elements of xeromorphic plant communities that are notedly characteristic in Cuba and include large portions of endemics (
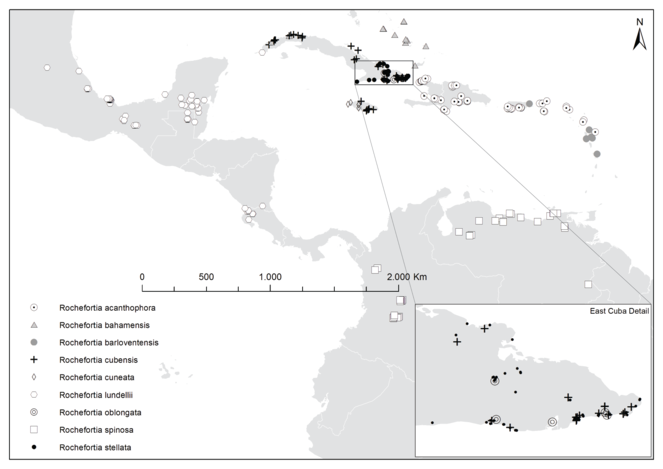
Rochefortia is restricted to Caribbean islands and the adjacent American mainland (Fig.
Materials and methods
Delimitation of Rochefortia species was inconsistent among previous authors (
Species of Rochefortia exhibit a clear biogeographic correlation (Fig.
We adopted a rather broad morphological species concept for Rochefortia and concluded that sympatric populations of different species should not be difficult to distinguish and should not include morphological intermediates. Basically, we started our revision by addressing the question how many species can be distinguished on every separate island in the Caribbean as center of diversity. As a result, we now recognise 9 species in Rochefortia having distributions that are more or less contiguous. However, the island species (e.g., R. bahamensis, R. barloventensis, R. cubensis), but also Central American R. lundellii, exhibit some degree of disjunction in their distributions.
Different from many other taxa of woody borages, species of Rochefortia lack exclusive characters with respect to generative organs. Using such traits for diagnostic purposes is further impeded since they are rarely documented on herbarium specimens: Already
Vegetative traits help to distinguish between species that occur sympatrically, but the degree of homoplasy is high when considered over the entire geographic range. Individual specimens of, for example, R. bahamensis and R. barloventensis can be remarkably similar in their morphology, although no similar morphologies are found within the geographic distance of more than 500 km. The only highly distinctive trait among species of Rochefortia is the presence of stellate trichomes on leaf surface and other plant parts, which is diagnostic for the Cuban endemic R. stellata (
In this revision, we provide comprehensive nomenclatural data about all names published for species of Rochefortia. They are linked to online resources such as Biodiversity Heritage Library (BHL) and Biodiversity Literature Repository (BLR) for bibliography (i.e., protologues) and JSTOR for high quality images of type specimens (although free access is limited). The present printed revision of Rochefortia is also part of an ongoing online project on the dedicated platform for cyber-taxonomy, namely Botanical Research and Herbarium Management System (BRAHMS). We thus made our BRAHMS data base publicly available, which provides access to the information implemented in hundreds of Rochefortia specimens. Searches by taxon, collector, locality name and map area (Fig.
Taxon treatments
Rochefortia
Nomenclature
Rochefortia Sw., Prod.: 53. 1788, Fl. Ind. Occid. 551, pl. XI. 1797. Rochefortia sect. Stellatae G.Klotz, Wiss. Z. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe 29: 470. 1980. Rochefortia ser. Acanthophorae G.Klotz, Revista Jard. Bot. Nac. Univ. Habana 3: 105. 1982.
= Lutrostylis G.Don, Gen. hist. 4: 391. 1838.—TYPE: Lutrostylis spinosa (Jacq.) G.Don, designated here.
Type species
Description
Shrubs or trees, rarely lianas, thorny, short shoot galls present or absent; bark striate, with superficial grooves, longitudinally fissured, grey light to dark brown, with lenticels; indument absent at maturity or puberulent, trichomes simple, sometimes glandular, rarely branched (R. stellata). Leaves fasciculate, rarely alternate or subopposite (R. lundellii, R. spinosa), simple; petiole hirsute through glabrous; blade elliptic through obovate, sometimes orbicular (R. bahamensis), margin entire, texture coriaceous, occasionally membranaceous (R. cuneata), venation brochidodromous, reticulate; indument variously pubescent through hispid, rarely absent, trichomes unicellular, frequently containing cystoliths (also in adjacent subsidiary cells) and causing the typical roughness of leaf surface. Inflorescence thyrsoid, terminal or axillary, branching sympodial, sometimes heavily condensed or flowers solitary, ebracteolate. Buds obovoid, whitish green; flower actinomorphic, unisexual, sometimes fragrant, distinctly pedicellate (R. bahamensis, R. barloventensis, R. lundellii, R. spinosa) to sub-sessile (R. stellata) or almost sessile (R. acanthophora, R. cubensis); calyx persistent, coriaceous, campanulate, aestivation imbricate; corolla sympetalous, predominantly white or rarely yellow orange or greenish, turning brown at later ontogenetic stage, membranaceous, funnel-shaped, aestivation imbricate; stamens 5, rarely 4 or 6, exserted, filaments adnate to corolla tube for 0.10–0.15 cm, flattened, anthers of male flowers with pollen, anthers of female flowers empty and diminutive; ovary of male flowers choricarpous, carpels fused only at the gynobase, stylodia 2, distally with a tuft of dense, grey trichomes, ovary of female flowers coencarpous-syncarpous, with a well developed style consisting of 2 distinct branches or with 2 stylodia, style or stylodia 2–2.5 times longer than ovary and fruit, stigmas capitate, usually well developed. Fruit indehiscent, drupaceous, exocarp brightly red at maturity, later turning brown, exocarp membranaceous, mesocarp fleshy, endocarp ligneous and 4-parted; pyrene smooth through variously ornamented on abaxial surface, enclosing 1 seed, placenta extensive, but not enclosed in sterile chamber of pyrene, embryo curved.
Rochefortia acanthophora
Nomenclature
Rochefortia acanthophora (DC.) Griseb., Fl. Brit. W. I.: 482. 1862. Ehretia acanthophora DC. in A.DC., Prodr. 9: 510. 1845.—TYPE: Caribbean, Dominican Republic. Without precise locality (1821): C.G.L. Bertero s.n. 1821 (sterile) (lectotype, designated here: G-DC18088! isolectotypes: MO-1886637! P fide Lefor 1968); without precise locality at Biajama river (1819): C.G.L. Bertero s.n. 1819 (sterile) (syntype: TO-4998!).
Description
Shrubs up to 4.0 m tall, rarely trees 4.0–7.0 m tall, galls absent; twig indument absent or sericeous, glabrescent at maturity, trichomes simple; bark grey whitish to grey dark or brown, sometimes peeling; thorns 0.5–0.8 cm long, slender, acute, simple, numerous, axillary, alternate, glabrous through variously sericeous. Leaves fasciculate; petiole 0.05–0.2 cm long, slender, hirsute; blade 0.5–1.5 cm long, 0.2–0.3 cm wide, elliptic to obovate, widely obovate or circular (particularly distal immature leaves), coriaceous, primary vein prominent, with scattered trichomes, secondary veins 3–6, tertiary veins arcuate or reticulate; base cuneate, rarely rounded; apex rounded or obcordate, occasionally retuse, emarginated or cleft; adaxial surface hirsute to pubescent, with simple trichomes and cystolith-like structures in subsidiary cells, abaxial surface bright, variously hirsute to glabrous. Inflorescence axillary, flowers in clusters of 2 or rarely 3 or solitary, pedicels up to 0.02-0.03 cm long. Calyx 0.40–0.50 cm long, 0.45–0.50 cm wide, coriaceous, glabrous or with few scattered trichomes outside, sometimes hirsute, glabrous inside with a tuft of trichomes at the distal part of lobes, lobes 0.35–0.40 cm long, 0.40–0.43 cm wide, ovate to broadly ovate, apex acute, margin strigose. Corolla 0.40–0.50 cm long, white, yellowish or pale yellow, rarely green (as in Leonard & Leonard 1579: GH!, Leonard & Leonard 2579: US!, Leonard & Leonard 12579: GH! US!), brownish when dry, membranaceous, glabrous on both surfaces, tube 0.20–0.25 cm long, funnel-shaped, lobes 0.35–0.40 cm long, ovate, rounded, margin slightly ciliate. Anthers of male flower 0.50–0.55 cm long, oblong, filaments 0.45–0.50 cm long, adnate to the corolla tube for further 0.10–0.15 cm, pollen present (SEM), tricolporate; anthers of female flower 0.25–0.30 cm long, oblong, pollen absent, filaments very short, 0.05–0.07 cm long, adnate to the corolla tube for further 0.10–0.12 cm. Ovary of male flower subglobose, 0.10–0.20 cm long, stylodia 2, 0.10–0.15 cm long, distally setose, with grey trichomes, ovules absent; ovary of female flower globose, style 0.35–0.40 cm long, divided, branch 0.30–0.35 cm long, stigmas very well developed, cotyliform. Fruit 0.30–0.45 cm tall, 0.40–0.50 cm wide, globose, green when immature, later orange brown or red to brightly red at maturity, brown when old, mesocarp fleshy; pyrene 0.30–0.40 cm tall, 0.30–0.35 cm wide, 0.35–0.40 cm deep, abaxial surface with reticulate ridges.
Distribution
Coastal habitats and scrublands across Caribbean islands between Haiti in the West and Guadeloupe in the East, with high abundance in the Dominican Republic, Puerto Rico and the Virgin Islands (symbol "⨀" in Fig.
Ecology
Flowering throughout the year (Jan–Dec); fruiting Oct–Mar, May–Aug, but probably contiguously.
Taxon discussion
Traditionally, R. acanthophora has been considered the most widely distributed species of Rochefortia in the Caribbean (
Rochefortia acanthophora is sympatric with only a single other species, namely R. barloventensis, from which it differs in the frequently axillary (versus predominantly terminal) inflorescences comprising few (and not more than 5) flowers, a smaller leaf blade size (0.5–1.5 cm long versus 1.4–3.4 cm long) and a reduced leaf petiole length (0.05–0.2 cm long versus 0.4–1.5 cm long). Anyhow, sympatry between both species is restricted to Guadeloupe (
Morphologically, R. acanthophora is a variable species, particularly with respect to leaf size and shape, and exhibits some overlap to R. cubensis from Cuba and Jamaica (it was occasionally hard to determine the species reliably if geographic origin was unknown). Among the remaining small-leaved species, it differs from R. bahamensis in the more predominantly obovate (versus circular) leaf shapes and from R. stellata in the absence (versus presence) of multi-branched trichomes. Some individuals of R. acanthophora (e.g., Liogier 20494: F!, Leonard & Leonard 12760: US!, Ekman 2154: A! F!) appear as substrate for plants assigned to Tillandsia sp. (Bromeliaceae).
Notes
Representative specimens examined. — HAITI. Port-de-Paix: Baie des Moustiques: trail W to main road, vicinity of Cabaret,
“bois d’ébène”, “ébénier noir”, “galle-galle”, “gratte-galle” in Haiti, “corazón de paloma” (Span., pigeon heart), “ébano” and “trejo” in Dominican Republic, “juso” in Puerto Rico and “greenheart ebony” in US Virgin Islands (
The trunk is employed to make posts for fences (Jiménez 4593: US!), and the wood is reported to be very hard (“bastard lignum vitae”, holly wood: Cook 54: GH!).
Rochefortia bahamensis
Nomenclature
Rochefortia bahamensis Britton, Bull. New York Bot. Gard. 5: 317. 1907. Rochefortia cuneata subsp. bahamensis (Britton) G.Klotz, Revista Jard. Bot. Nac. Univ. Habana 3: 103. 1982.—TYPE: Caribbean, Commonwealth of The Bahamas. San Salvador [Watling’s] Island, scrub lands near Lighthouse (Mar 13, 1907), N.L. Britton & C.F. Millspaugh 6167 (♀ fl) (holotype: NY: 111152! isotype: F-51163!).
Description
Shrubs or small trees up to 1.5–4.5 m tall, galls absent; twig indument puberulent, trichomes simple; bark greyish white to brown, longitudinally fissured, scaly, wood very brittle; thorns 0.3–0.8 cm long, slender, distally somewhat acute, simple, scattered, alternate, axillary or rarely terminal, sericeous. Leaves fasciculate, rarely alternate (Correll 45046: US!, Correll & Wasshausen 46731: US!); petiole 0.5–1.2 cm long, hirsute through pubescent or glabrous; blade 0.7–5.1 cm long, 0.5–2.8 cm wide, obovate, widely obovate or very widely obovate through orbicular (particularly distal immature leaves), coriaceous, primary vein prominent, sometimes with scattered trichomes, secondary veins 3–8, tertiary veins arcuate; base cuneate or rounded; apex rounded, emarginate, obcordate or retuse, rarely cleft; adaxial surface glabrescent, sometimes distally cilliate, bright, with dense cystolith-like structures in epidermal cells, abaxial surface glabrous, occasionally with simple, scattered trichomes, immature leaves sometimes barbellate. Inflorescence axillary, branching sympodial, branches relatively slender, hirsute, pedicels 0.05–0.30 cm long. Calyx 0.35–0.45 cm long, coriaceous, hirsute outside, glabrous inside, lobes 0.35–0.40 cm long, 0.40–0.43 cm wide, divided from the base, ovate to broadly ovate, apex acute, margin strigose, glabrous inside with a tuft of trichomes in upper part of lobes. Corolla 0.50–0.70 cm long, yellow or white-greenish, sometimes fragrant, membranaceous, glabrous on both surfaces, tube 0.20–0.25 cm long, funnel-shaped, lobes 0.50–0.60 cm long, oblong or obtuse, glabrous, rarely with trichomes at margin. Anthers of male flower 0.45–0.50 cm long, oblong, filaments 0.35–0.45 cm long, glabrous, adnate to corolla tube for 0.10–0.12 cm, pollen present, tricolporate; female flower unknown. Ovary of male flower subglobose, 0.15–0.18 cm long, stylodia 2, divided in distal part, branches 0.10–0.12 cm long, hirsute. Fruit 0.40–0.50 cm tall, 0.40–0.60 cm wide, globose, red at maturity; pyrene 0.37–0.40 cm tall, 0.26–0.30 cm wide, 0.12–0.20 cm deep, abaxial surface cutate.
Distribution
Across multiple islands of The Bahamas archipelago (Crooked Island, Great Exuma, Inagua, Long Island, Mayaguana, San Salvador) and putatively also in western Cuba (Pinar del Río, Havana) in scrub lands, rocky coppice hills and thicket edges, at sea level or slightly above (symbol "▲" in Fig.
Ecology
Flowering Feb–Mar, Oct–Dec; fruiting Dec–Jan, Oct.
Taxon discussion
Rochefortia bahamensis is an abundant and morphologically very consistent species in The Bahamas archipelago characterised by the predominantly circular leaf shapes. Overall similarity, however, is great with R. barloventensis from the Lesser Antilles, but molecular data indicate that the two species are only distantly related (
The 3 sterile collections from Cuba are tentatively placed under R. bahamensis and are morphologically somewhat intermediate between R. bahamensis and R. cubensis. With R. bahamensis, the plants share the more membranaceous leaf texture, longer and slenderer petioles, orbicular immature leaves and fewer (maximal 4) leaves clustering in a fascicle. However, they exhibit the dichotomously branched thorn pattern of R. cubensis, and overall leaf size is smaller when compared to the more typical R. bahamensis. Anyhow, molecular data of Hilger & Urquiola 99/20 (B!) indicate that this specimen is distinct from (morphologically also similar, but geographically distant) R. acanthophora and R. cuneata (
Notes
Representative specimens examined. — THE BAHAMAS. Crooked Islands: in coppice along stone wall in hills NE of Cabbage Hill,
unknown.
Rochefortia barloventensis
Nomenclature
Rochefortia barloventensis Irimia & Gottschling, Phytotaxa 236: 63–68, figs 1–2, tab. 1. 2015.—TYPE: Caribbean, Lesser Antilles, Leeward Islands, France. Guadeloupe, Marie Galante: Fréchy district, near Grelin, on wooded hillside, 15º57’N, 61º17’W [retroactively inferred], elev. 65–125 m (May 25, 1960): G.R. Proctor 21020 (♀ fl, fr) (holotype A! isotype: US-1111601!)
Description
Shrubs or small trees 1.5–10.0 m tall, branches spreading, galls absent; indument glabrescent, slightly pubescent when immature, trichomes simple; bark greyish white, longitudinally fissured; thorns 0.5–0.7 cm long, slender, acute, simple, scattered, axillary, alternate, glabrescent. Leaves fasciculate; petiole 0.4–1.5 cm long, glabrescent; blade 1.9–3.4(–6.0) cm long, 1.2–3.0 cm wide, obovate, sometimes circular, coriaceous, primary veins prominent, lateral veins in pairs of 4–5, arching, tertiary veins absent; base cuneate; apex rounded or slightly emarginate; adaxial surface with cystolith-like structures in epidermal cells, mostly glabrous, but sometimes also with scattered, simple, bent trichomes, abaxial surface with scattered trichomes on the midrib, immature leaves sometimes barbellate. Inflorescence axillary and terminal, branching sympodial, branches slender, glabrescent, pedicel 0.20–0.40 cm long. Calyx 0.35–0.45 cm long, coriaceous, hirsute outside, glabrous inside, sometimes with a few clustered trichomes on distal part, lobes 0.30–0.35 cm long, 0.32–0.35 cm wide, obovate, apex rounded to slightly acute, margin strigose. Corolla 0.35–0.40 cm long, yellow through light orange, occasionally greenish (Liogier et al. 32280: MO! US!), membranaceous, glabrous on both surfaces, very rarely with scattered trichomes at distal lobes, tube 0.10–0.20 cm long, funnel-shaped, lobes usually 5 (rarely 4 or 6), 0.30–0.40 cm long, obovate, with 5–6 parallel veins and few glandular trichomes distally. Anthers of male flower 0.30–0.40 cm long, oblong, filaments 0.30–0.33 cm long, adnate to the corolla tube for further 0.05–0.08 cm, pollen present, tricolporate; anthers of female flower 0.12–0.14 cm long, filaments 0.05–0.08 cm long, adnate to corolla tube for further 0.10–0.12 cm, pollen absent. Ovary of male flower subglobose, 0.14–0.20 cm long, stylodia 2, 0.10–0.12 cm long, distally setose, ovules absent; ovary of female flower globose, 0.25–0.30 cm long, style bifid, united for 0.03–0.10 cm at the base, branches 0.25–0.30 cm long, sometimes slightly unequal, stigmas 2, extensively capitate. Fruit 0.50–0.60 cm tall, 0.60–0.70 cm wide, globose, deep orange through vermillion, turning blackish brown at maturity; style accrescent, occasionally persistent; pyrene 0.40–0.43 cm tall, 0.30–0.35 cm wide, 0.20–0.25 cm deep, ovoid, abaxial surface reticulate.
Distribution
Restricted to islands of the Lesser Antilles (Guadeloupe, Marie Galante, Montserrat, Martinique) and eastern Puerto Rico (symbol "●" in Fig.
Ecology
Flowering Apr–May, Jul, Sep–Oct, Dec; fruiting Dec–Jan, May–Jun.
Taxon discussion
Rochefortia barloventensis was discovered as a species new to science in the course of the present revision of Rochefortia (
Notes
Representative specimens examined. — PUERTO RICO. Fajardo: in forest, El Convento, 18°19\'N, 65°37\'W [retroactively inferred], 15 Sep 1981 (fl), Liogier et al. 32280 (MO! US!). — FRANCE. Guadeloupe: Grande–Terre, Porte D’Enfer, 16°29\'N, 61°26\'W [retroactively inferred], 19 Jul 1982 (♀ fl), Barrier 3747 (G! P! P! US!); Îles des Saintes, Terre-de-Bas, 15°51\'N, 61°38\'W [retroactively inferred], 22 Nov 1986 (♀ fl, fr), Sastre 8265 (P! P!); Martinique: Caravelle, Château Dubuc, 14°46\'N, 60°53\'W [retroactively inferred], 9 Apr 1998 (♂ fl), Sastre 9747 (P!).
"bois vert" in Martinique (noted on Jussieu s.n.: P!), "espino" in Puerto Rico (
Rochefortia cubensis
Nomenclature
Rochefortia cubensis Britton & P.Wilson, Mem. Torrey Bot. Club 16: 96. 1920.—TYPE: Caribbean, Republic of Cuba. Havana: E of Playa de Marianao (May 31, 1917): León 7228 (fr) (holotype: NY-111153!).
= Rochefortia oblanceata G.Klotz, Revista Jard. Bot. Nac. Univ. Habana 3: 105. 1982.—TYPE: Caribbean, Republic of Cuba. Pinar del Río: La Palma, Loma Peluda de Cajalbana, elev. 200–300 m (Sep 15, 1970): J. Bisse & H. Lippold [Flora Cuba] 18273 (sterile) (holotype: HAJB isotype: JE-5984!).
Description
Shrubs 1.0–3.0 m tall or small trees up to 6.0 m tall, prickly, branches arching, short shoot galls occasionally present, 0.8–1.0 cm long; indument glabrescent, immature branches finely pubescent, trichomes simple; bark greyish white through grey brown, with longitudinal crevices; thorns 0.3–0.5 cm long, slender, acuminate, dichotomously branched, numerous, axillary, glabrescent. Leaves fasciculate, dark green; petiole nearly sessile or up to 0.2 cm long, glabrescent; blade 0.4–0.9 cm long, 0.3–0.5 cm wide, elliptic, sometimes obovate, coriaceous, primary veins pinnate, secondary veins 5–9, tertiary veins reticulate; base cuneate, rarely round; apex rounded, occasionally emarginated; adaxial surface with distinct cystoliths in epidermal cells, glabrous or sometimes covered with grey, simple, long trichomes (visible by naked eye) emerging from a cystolith-like structure, abaxial surface mostly glabrous, sometimes with scattered trichomes distally and on midrib. Inflorescence axillary or terminal, flowers usually in clusters of 2 or solitary, pedicel 0.02–0.2 cm long. Calyx 0.35–0.40 cm long, 0.30–0.40 cm wide, coriaceous, glabrous or with few scattered trichomes outside, sometimes hirsute, glabrous inside, strigose at tips, lobes 0.30–0.35 cm long, 0.40–0.45 cm wide, divided at the base, ovate to widely ovate, apex slightly acute. Corolla 0.35–0.40 cm long, pale yellow, membranaceous, glabrous on both surfaces, tube 0.20–0.25 cm long, funnel-shaped, lobes 0.30–0.35 cm long, ovate, slightly ciliate at tips. Male flower unknown; anthers of female flower 0.05–0.07 cm long, oblong, filaments 0.07–0.10 cm long, adnate to corolla tube for further 0.05–0.08 cm, pollen absent. Ovary of female flower globose, 0.25–0.32 cm long, stylodia 2, 0.25–0.35 cm long, filiform, glabrous, ovules present, stigmas cotyliform. Fruit 0.20–0.40 cm tall, 0.30–0.50 cm wide, globose; style accrescent, occasionally persistent, pyrene 0.30–0.40 cm tall, 0.23–0.33 cm wide, 0.13–0.20 cm deep, adaxial surface cutate.
Distribution
Cuba and Jamaica (symbol "+" in Fig.
Ecology
Flowering Mar, Jun–Jul; fruiting Mar, Jul–Sep, Dec–Jan.
Taxon discussion
Rochefortia cubensis is a widely distributed and frequently encountered species in Cuba and Jamaica. It is morphologically similar to, but with respect to molecular sequence data distinct from, R. acanthophora occurring on eastward Caribbean islands (
With respect to leaf size and shape,
Rochefortia cubensis is distinctive in the smallest leaves found among species of Rochefortia and has populations on very poor and serpentine substrates, resulting in radically stunted individuals. Such traits resemble Bourreria microphylla Griseb. (likewise Ehretiaceae), and such species are elements of microphyllous plant communities of montane xeromorphic woodland exhibiting large portions of endemics (
Notes
Representative specimens examined. — CUBA. Camagüey: Santayana, in palms barren on serpentine,
“bronce”, “carbonero”, “espuela de caballero” (Span. knight’s spur), sargento (
Rochefortia cuneata
Nomenclature
Rochefortia cuneata Sw., Prod. 54. 1788, Fl. Ind. occid. 552–553. 1797.—TYPE: Caribbean, Jamaica. Without precise locality (between 1784 and 1786): O.P. Swartz s.n. (♀ fl) (lectotype, designated here: UPS V-6577!).
= Rochefortia ovata Sw., Prod. 54. 1788, Fl. Ind. occid. 554. 1797.—TYPE: Caribbean, Jamaica. Without precise locality (between 1784 and 1786): O.P. Swartz s.n. (fl) (lectotype, designated here: UPS V-6578!).
= Rochefortia acrantha Urb., Symb. antill. 5: 479. 1908.—TYPE: Caribbean, Jamaica. Trelawny: Troy, elev. 500–600 m (Dec 6, 1904): W.H. Harris 8821 (♀ fl) (lectotype, designated here: NY-111151! isolectotypes: F-174394! GH-97337! US-655778!); elev. 300–400 m (Nov 21, 1905): W.H. Harris 9073a (♀ fl) (syntype: BM-953141!).
Description
Shrubs up to 2.5 m tall or small trees up to 8.0 m tall, galls absent; indument variously sericeous through glabrescent, trichomes simple; bark grey light through grey dark or brown, superficial grooves present; thorns 0.7–1.0 cm long, slender, acute, simple or rarely branched, scattered, alternate, axillary or rarely terminal, glabrous. Leaves fasciculate, rarely opposite or alternate; petiole 0.3–0.8 cm long, slender, hirsute or sometimes glabrescent; blade 1.5–3.5(–6.6) cm long, 0.5–3.0(–3.5) cm wide, obovate to widely obovate, occasionally orbicular, membranaceous, primary veins prominent, hirsute, secondary veins 4–11, tertiary veins reticulate; base cuneate or rounded; apex rounded, obcordate, sometimes retuse, rarely cleft; adaxial surface brightly glabrescent, with very rare cystoliths-like structures in epidermal cells, ciliate at tips, trichomes emerging from a swelled cystolith cell giving the impression of undulate leaf margin, abaxial surface brightly rugose, with scattered trichomes. Inflorescence axillary or terminal, branches slender, hirsute to glabrescent, pedicel 0.30–0.35 cm long. Calyx 0.35–0.40 cm long, 0.30-0.45 cm wide, coriaceous, hirsute outside, glabrous inside with scattered trichomes at distal blade, lobes 0.30–0.35 cm long, 0.28–0.40 cm wide, divided from the base, obovate, apex rounded. Corolla 0.40–0.55 cm long, membranaceous, glabrous on both surfaces, tube 0.25–0.30 cm long, funnel-shaped, lobes 0.25–0.35 cm long, obovate, slightly cilliate distally. Male flower unknown; anthers of female flower 0.07–0.08 cm long, oblong, filaments 0.03–0.04 cm long, adnate to the corolla tube for further 0.03–0.04 cm, pollen absent. Ovary of female flower globose, 0.25–0.30 cm long, style divided right from the base, branches 0.18–0.25 cm long, stigma cotyliform. Fruit 0.50–0.60 cm tall, 0.50–0.60 cm wide, globose; pyrene 0.45–0.50 cm tall, 0.25–0.30 cm wide, 0.10–0.13 cm deep, abaxial surface cutate.
Distribution
Jamaica, and presumably (with very few collections: Ekman 4145: K!, Leonard & Leonard: 11714 US!) also Haiti (Tortue Island) and Dominican Republic (La Vega province with a single collection: Zanoni 15680: MO!), on limestone soils at sea coast, in wooded and rocky hills (symbol "◊" in Fig.
Ecology
Flowering Mar, Jul, Sep, Nov; fruiting Mar, Jul.
Taxon discussion
Across Caribbean islands, R. cuneata exhibits amongst the largest leaves that have almost comparable size to those of the mainland species R. lundellii and R. spinosa. The species is most abundant in Jamaica, from where it was also discovered in the late 18th century. Three collections outside Jamaica (one from Dominican Republic and two from Haiti) are morphologically similar to R. cuneata, but all attempts failed to verify the determination by DNA sequence data (
Olof P. Swartz’ names were not properly typified before the present revision (
Notes
Representative specimens examined. — JAMAICA. Cockpit: NE of Dolphin Head,
"green ebony" and "bois vert" in Jamaica.
Rochefortia lundellii
Nomenclature
Rochefortia lundellii Camp in Lundell, Contr. Univ. Michigan Herb. 7: 47–48. 1942.—TYPE: Belize. El Cayo: road between Arenal and Valentin (Jun, 1936): C.L. Lundell 6167 (♀ fl, fr) (holotype: NY-335693! isotypes: GH-97203! GH-97336! LL-372669! LL-372670! MICH-1111531! MO-152639! S-04-2384! US-1688323!).
Description
Lianas or shrubs up to 4.5 m tall or trees up to 10.0 m tall, branches arching, galls absent; indument almost glabrescent at maturity, hirsute when young, trichomes simple; bark whitish grey, grey brown or brown dark, with superficial crevices; thorns 0.6–1.5 cm long, robust, acute and slightly curved at tips, simple, scattered (also on older branches), alternate, axillary, hirsute. Leaves fasciculate; petiole 0.3–2.3 cm long, glabrescent; blade (2.0–)5.0–10.5(–13.0) cm long, 1.5–4.5(–6.0) cm wide, obovate, rarely elliptic, coriaceous, primary and secondary veins prominent, secondary veins 6–9, tertiary veins arching; base cuneate; apex acute, retuse, obcordate, sometimes round or cuspidate; adaxial surface bright, with cystolit-like structures in epidermal cells, glabrous, sometimes with scattered, bent trichomes, abaxial surface shiny, papillate, glabrous, sometime barbellate or rarely with trichomes apically. Inflorescence axillary and terminal, branching sympodial, branches slender, hirsute to glabrous, pedicel 0.20–0.50 cm long. Calyx 0.40–0.45 cm long, 0.45–0.49 cm wide, coriaceous, hirsute outside, glabrous inside, lobes 5 or occasionally 4, 0.35–0.40 cm long, 0.35–0.43 cm wide, very widely ovate, apex obtuse, margin strigose. Corolla 0.35–0.60 cm long, yellow, white, dull white or sometimes greenish (Martínez Salas & Álvarez M. 30817: MO!), fragrant or odourless, membranaceous, glabrous on both surfaces, tube 0.10–0.20 cm long, funnel-shaped, lobes 0.30–0.40 cm long, widely ovate, slightly ciliate at tips. Anthers of male flower 0.25–0.38 cm long, filaments 0.10–0.20 cm long, adnate to the corolla tube for further 0.05–0.06 cm, pollen present (SEM); anthers of female flower 0.12–0.19 cm, filaments 0.08–0.10 cm long, adnate to the corolla tube for further 0.04–0.05 cm, pollen absent. Ovary of male flower subglobose, 0.10–0.12 cm long, stylodia 2, 0.05–0.08 cm long, distally strigose, ovules absent; ovary of female flower globose, 0.20–0.25 cm long, style 0.30–0.45 cm long, united in the proximal part for 0.04–0.12 cm, branches 2, 0.20–0.30 cm long, slightly unequal, glabrous, stigmas 2, cotyliform. Fruit 0.50–1.00 cm tall, 0.40–0.90 cm wide, globose, brightly or blackish red at maturity, later becoming orange, widely ovoid or globose; style accrescent; pyrene 0.60–0.80 cm tall, 0.47–0.50 cm wide, 0.30–0.40 cm deep, abaxial surface reticulate, with 2–3 distinct longitudinal ridges.
Distribution
Abundant in Mexico, but also present in adjacent and other countries in Central America (Belize, Costa Rica, Guatemala, Nicaragua), as well as western Cuba (symbol "⬡" in Fig.
Ecology
Flowering Jan, Apr–Aug; fruiting Feb–Mar, May–Dec.
Taxon discussion
The species has a lianescent habit, which is unusual for Rochefortia and for Ehretiaceae as well, and larger stems may bear truly thick and extensive thorns. However, herbarium specimens of R. lundellii are morphologically difficult to distinguish from South American R. spinosa when growth form is not recorded on the label. Consequently, determination has been more or less arbitrary across both species in the past. Molecular data confirm the distinctiveness between both species (
Rochefortia lundellii is a variable species across its different geographical occurrences, particularly regarding size of leaves and thorns, shape of leaf blades and the apex, but also fruit diameter. Specifically, the Mexican population exhibits the largest mature leaves 6.4–11.0(–13.0) cm long, followed shortly by the Costa Rican population 6.2–10.0(–12.5) cm and the Cuban population 4.8–9.0(–11.0) cm. Other slight differences are observed in thorn size, ranging from 0.7–1.5 cm long in Mexican plants over 0.5–1.0 cm long in Costa Rican plants to 0.3–0.7 cm long in Cuban plants. Some Costa Rican specimens consistently have robust thorns that are slightly curved at tips (the trait is shared with some individuals from Mexico, but also from Venezuela assigned to R. spinosa). Leaf apex is usually rounded or emarginated in R. lundellii, but some specimens from Costa Rica and Mexico display a cuspidate apex. Fruit diameter ranges within the general Rochefortia average values except for Costa Rican specimens having the biggest fruits (1.00–1.20 cm diameter) of all species.
Still in the 60ies of the past century,
Notes
Representative specimens examined: — CUBA. Pinar del Río: Peninsula de Guanahacabibes, bw Piñatas and Yagales,
“palo dulce” in Mexico and “carey de costa” in Cuba.
Rochefortia oblongata
Nomenclature
Rochefortia oblongata Urb. & Ekman in Urb., Ark. Bot. 22A.17: 94. 1929.—TYPE: Caribbean, Republic of Cuba. Santiago de Cuba: near Santiago de Cuba, at Río Aguadores (June 14, 1918): E.L. Ekman 9224 (fr) (holotype: S-04-2385! isotypes: G-236086! GH-97339! K-583494!).
Description
Shrubs, short shoot galls occasionally present, 0.7–1.0 cm long; indument glabrous, trichomes simple; bark greyish white, slightly fissured; thorns 0.7–1.1 cm long, relatively robust, acute, simple or dichotomously branched, axillary or terminal, glabrous. Leaves fasciculate; petiole 0.4–1.0 cm long, robust, glabrous; blade 1.1–3.9 cm long, 0.7–1.5 cm wide, oblong-ovate, sometimes elliptic, coriaceous, primary veins very prominent, secondary veins 10–16, tertiary veins absent; base cuneate; apex retuse or obcordate; adaxial surface bright, with numerous cystoliths and simple, bent trichomes emerging from an inflated cystolith basal cell, abaxial surface bright, striate, very rarely with scattered trichomes, midrib and tips with a few trichomes. Inflorescence axillary or terminal, secondarily branched, branches slender, with diffusely scattered trichoms, pedicel 0.50–0.60 cm long. Calyx 0.25–0.30 cm long, 0.28–0.30 cm wide, coriaceous, with scattered trichomes outside, glabrous inside, but with a few scattered trichomes at blade tips, lobes 0.20–0.40 cm long, 0.30–0.35 cm wide, broadly obovate to triangulate, apex obtuse. Flowers at anthesis unknown. Mature fruit unknown; pyrene 0.35-0.40 cm tall, 0.28-0.30 cm wide, 0.20-0.22 cm deep, abaxially completely smooth.
Distribution
Endemic to eastern Cuba (symbol "⌾" in Fig.
Ecology
Fruiting Jun.
Taxon discussion
The species is very similar to Jamaican R. cuneata, but differs in having (at least some) thorns branched and also in leaf traits exhibiting numerous cystoliths and peculiar roughness at touch. Rochefortia oblongata is sympatric with R. cubensis and R. stellata, from which it can reliably be distinguished based on leaves and petioles that are both much longer than in the other species. Moreover, the abaxial surface of pyrenes is entirely smooth (versus reticulate in R. cuneata and all other species of Rochefortia).
The species is endemic to Southern Cuba and is known only from five herbarium collections. According to the Red List of Cuban Plants (
Notes
Representative specimens examined. — CUBA. Guantánamo: Caimanera, W of bay,
none.
Rochefortia spinosa
Nomenclature
Rochefortia spinosa (Jacq.) Urb., Feddes Repert. 13: 472. 1915. Ehretia spinosa Jacq., Enum. syst. pl.: 14. 1760, Select. stirp. amer. hist.: 46, pl. CLXXX 18. 1763. Lutrostylis spinosa (Jacq.) G.Don, Gen. Hist. 4: 391. 1838.—TYPE: [illustration] Republic of Colombia. Bolívar: Cartagena: pl. CLXXX 18 in Jacq., Select. stirp. amer. hist. 1763.
= Ehretia fasciculata Kunth in Humb., Bonpl. & Kunth, Nov. gen. sp. 3: 66. 1818. Crematomia fasciculata (Kunth) Miers, Ann. Mag. Nat. Hist., ser. 4 3: 312–313. 1869. Morelosia fasciculata (Kunth) Kuntze, Revis. gen. Pl. 2: 439. 1891. Bourreria fasciculata (Kunth) Gürke in Engl. & Prantl, Nat. Pflanzenfam. 4 (3a): 87. 1893.—TYPE: Bolivarian Republic of Venezuela. Sucre: Cumaná (Sep, without year), A.J.A. Bonpland & F.W.H.A. von Humboldt 92 (♀ fl, fr) (holotype: P-670679!).
= Rochefortia jacquinii Griseb., nom. corr. (ICN Art. 60.12.), Abh. Königl. Ges. Wiss. Göttingen 7: 253. 1857.—TYPE: Republic of Colombia. Cundinamarca: Tocaima (s.d.): J. Goudot s.n. (♀ fl, fr) (holotype: GOET-395! isotype: K!).
= Rochefortia fasciculata Gürke in Engl. & Prantl, Nat. Pflanzenfam. 4 (3a): 89. 1893.—TYPE: Republic of Colombia. Without precise locality at Valle del río Magdalena, elev. 300–700 m (Jan, 1852): J.J. Triana 3744 (♀ fl, fr) (holotype: COL-112389! photo of holotype: US-110770!).
Description
Trees up to 10.0 m tall or smaller shrubs 2.0–5.0 m tall, crown arching, galls absent; indument glabrescent, young twigs variously pilose through glabrescent; bark grey light to grey brown, longitudinally fissured, wood light; thorns 0.80–1.0 cm long, robust, somewhat acute, sometimes slightly curved at tips, simple, scattered, alternate, glabrous. Leaves fasciculate, occasionally stipulate (2 deciduous stipules, as stated by Lasser 758: US!), dark green, dark pale, sometimes red when older; petiole 0.2–1.5 cm long, slender, hirsute, rarely glabrous; blade 0.9–10.8 cm long, 0.3–4.5 cm wide, obovate, widely obovate, rarely elliptic, coriaceous, primary veins prominent, secondary veins 5–6, tertiary veins reticulate; base cuneate or sometimes rounded, rarely oblique; apex retuse, rounded, obcordate, emarginate, acute, occasionally mucronulate; adaxial surface glabrous or with scattered trichomes (especially on the midrib and at tips), rugose, with cystotliths, abaxial surface glabrous or with stiff trichomes on the midrib and tips. Inflorescence terminal or axillary, secondarily branched, branches slender, glabrescent, pedicels 0.30–0.40 long. Calyx 0.18–0.25 cm long, coriaceous, hirsute outside, glabrous inside, lobes 0.15–0.18 cm long, 0.10–0.20 cm wide, divided from the base, triangulate, apex slightly acute, margin strigose. Corolla 0.35–0.40 cm long, white, yellowish, orange, rarely green (Ferrari 706: F!), tube funnel-shaped, 0.18–0.20 cm long, lobes 0.20–0.25 cm long, ovate, glabrous on both sides, slightly ciliate at tips. Anthers of male flower 0.15–0.30 cm long, filaments 0.20–0.23 cm long, adnate to the corolla tube for 0.10–0.12 cm, pollen present; anthers of female flower 0.08–0.10 cm long, filaments 0.06–0.10 cm long, adnate to the corolla tube for 0.02–0.03 cm, pollen absent. Ovary of male flower subglobose, stylodia 2, branches 0.07–0.10 cm long, glabrous, with strigose, grey trichomes at tips; ovary of female flower globose, 0.12–0.14 cm long, stylodia 2, 0.18–0.22 cm long, slightly unequal, 2 well developed stigmas, cotyliform. Fruit 0.50–0.80 cm tall, 0.60–0.80 cm wide, globose, green when immature, yellowish reddish through red and dark purple during maturation; style accrescent, not persistent; pyrene 0.40–0.60 cm tall, 0.30–0.40 cm wide, 0.15–0.30 cm deep, with various ridges on the abaxial surface.
Distribution
Northern South America (symbol "□" in Fig.
Ecology
Flowering Jan, Mar–May, Jul, Sep, Nov; fruiting Feb–Dec.
Taxon discussion
Rochefortia spinosa is among the species with the largest leaf size after R. lundellii. Specimens across different geographical areas are relatively homogenous displaying a low variability of morphological traits (e.g., few collections have leave lengths shorter than 5.5 cm). The species is not sympatric with any other species but morphologically, it resembles R. lundellii. It can be distinguished from the latter by the presence of 2 stylodia and generally by the less extensive developed thorns on stems. In the past, R. spinosa was one of the most confusing Rochefortia species and was believed being distributed across multiple islands of the Caribbean. However, molecular data (
Notes
Representative specimens examined. — COLOMBIA. Bolívar: Cartagena, Tierra Bomba,
“macarao”, “pega paloma” and “tachuelo” in Venezuela and “cruceto macho” and “tatacun o revienta puerco” in Colombia.
In Venezuela, the species is occasionally cultivated and forms hedgerows together with guamacho (probably Pereskia guamacho F.A.C.Weber: Cactaceae, as noted on Ferrari & Bunting 713: F!).
Rochefortia stellata
Nomenclature
Rochefortia stellata Britton & P.Wilson, Mem. Torrey Bot. Club 16: 96. 1920.—TYPE: Caribbean, Republic of Cuba. Santiago de Cuba: Santiago bay, vicinity of El Morro, coastal thicket, Ensenada Cabanita (Mar, 1912), N.L. Britton, E.G. Britton & J.F. Cowell 12634 (♀ fl, fr) (holotype: NY-111155! isotypes: NY-111156! F-492560! US-1047763!).
= Rochefortia stellata subsp. maisiensis G.Klotz, Wiss. Z. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe 29: 471. 1980.—TYPE: Caribbean, Republic of Cuba. Guantánamo: Maisí, Mesa del Chivo (Dec 30, 1959): E.E. Liogier [Alain] & L. Figueiras 7061 (fl) (holotype: HAJB).
= Rochefortia victoriniana G.Klotz, Wiss. Z. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe 29: 471–472. 1980.—TYPE: Caribbean, Republic of Cuba. Guantánamo: N of Baitiquiri (May, 1968): J. Bisse & E. Köhler [Flora Cuba] 7865 (sterile) (holotype: HAJB isotype: JE-5999!).
= Rochefortia septentrionalis G.Klotz, Wiss. Z. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe 29: 472, fig. 9. 1980.—TYPE: Caribbean, Republic of Cuba. Holguín: Sierra de Nipe, Mayarí Abajo, Loma de la Bandera (Jun, 1967): J. Bisse & L. Rojas [Flora Cuba] 3983 (fr) (holotype: HAJB isotype: JE-5990!).
= Rochefortia septentrionalis var. cristalensis G.Klotz, Wiss. Z. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe 29: 472, fig. 10. 1980.—TYPE: Caribbean, Republic of Cuba. Holguín: Sierra Cristal, loma Saca la Lengua (Apr, 1968): J. Bisse & E. Köhler [Flora Cuba] 6923 (fr) (holotype: HAJB isotype: JE-5998!).
= Rochefortia septentrionalis var. obovata G.Klotz, Wiss. Z. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe 29: 472. 1980.—TYPE: Caribbean, Republic of Cuba. Holguín: Sierra de Nipe, Charrasco de la Bandera, elev. 400 m (Apr 19, 1960): E.E. Liogier [Alain] & A.J.B. Acuña Galé 7802 (sterile) (holotype: SV).
= Rochefortia holguinensis G.Klotz, Wiss. Z. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe 29: 472–473. 1980.—TYPE: Caribbean, Republic of Cuba. Las Tunas: Puerto Padre (May 27, 1931): M. Curbelo s.n. 1931 (♀ fl, fr) (holotype: SV).
Description
Shrubs or small trees 3.0–5.0 m tall, galls absent; indument tomentose, especially on young organs, trichomes simple or stellate; bark grey whitish to light brown, longitudinally fissured; 0.7–1.3 cm long, slender, acute, successively branched at 2 or 3 levels, numerous, alternate or terminally of twigs, variously tomentose through glabrescent. Leaves fasciculate; petiole 0.4–0.6 cm long, robust, covered with a dense layer of stellate trichomes; blade 0.6–3.1 cm long, 0.4–2.1 cm wide, elliptic, widely elliptic or sometimes widely ovate, coriaceous, primary veins prominent, secondary veins 5–8, tertiary veins arcuate; base rounded or cuneate; apex rounded, retuse, obcordate, rarely mucronulate; adaxial surface bright, with cystoliths, rugose to almost glabrous or with a few scattered trichomes on the midrib, cilliate at tips, or with long (visible by the naked eye), simple, curved trichomes emerging from an inflated cystolith cell, abaxial surface densely tomentose, occasionally almost glabrous. Inflorescence axillary, flowers in clusters of 2 to 8, pedicels 0.10-0.15 cm long. Calyx 0.20–0.25 cm long, coriaceous, densely stellate outside, ciliate at tips, sometimes with simple trichomes scattered inside towards the distal part, lobes 0.18–0.22 cm long, 0.13–0.15 cm wide, shape triangular, apex acute, occasionally tridentate. Flowers at anthesis unknown. Fruit 0.40–0.50 cm tall, 0.40–0.50 cm wide; style 0.18–0.20 cm long, globose, divided in the proximal part, branches 2, 0.08–0.10 cm long, smaller than fruit, persistent (also the stigmas); pyrene 0.35–0.40 cm tall, 0.18–0.20 cm wide, 0.12–0.13 cm deep, abaxial surface with 5-6 longitudianal ridges.
Distribution
Rochefortia stellata (symbol "•" in Fig.
Ecology
Flowering Mar–Aug, Nov–Dec; fruiting: Apr–Aug.
Taxon discussion
Rochefortia stellata is the most distinctive species and can be easily distinguished from all other Rochefortia species, because of the consistent presence of multi-branched (i.e., stellate) trichomes (Fig.
Within his Rochefortia sect. Stellatae,
Some individuals of R. stellata (e.g., Bucher s.n.: F! Bisse & Köhler 8300: JE!) appear as substrate for plants assigned to Tillandsia sp. (Bromeliaceae).
Notes
Representative specimens examined. — CUBA. Granma: Cabo Cruz,
“espino de costa” in Holguín and “carey de costa” in Santiago de Cuba.
Identification keys
|
Key to the species of Rochefortia |
||
| 1 | Abaxial leaf surface with stellate (i.e., multi-branched) trichomes. | R. stellata |
| – | Abaxial leaf surface with simple trichomes or glabrous. | 2 |
| 2 | Thorns (at least some) branched; plants from Cuba. | 3 |
| – | Thorns simple, plants from elsewhere (if from Cuba, then mature leaf length <3.0 cm except R. lundellii). | 4 |
| 3 | Mature leaf length < 3.0 cm; flowers sub-sessile; pyrenes abaxially ornamented. | R. cubensis |
| – | Mature leaf length > 3.0 cm; flowers distinctly pedicellate; pyrenes abaxially smooth. | R. oblongata |
| 4 | Flowers sessile, 1–2 at an individual node. | R. acanthophora |
| – | Flowers distinctly pedicellate. | 5 |
| 5 | Mature leaf length > 8.0 cm. | 6 |
| – | Mature leaf length < 6.0 cm. | 7 |
| 6 | Lianas from Central America and westernmost Cuba; style divided in proximal half. | R. lundellii |
| – | Trees and shrubs from elsewhere; 2 stylodia. | R. spinosa |
| 7 | Leaves (occasionally > 6.0 cm) predominantly membranaceous, mainly obovate. | R. cuneata |
| – | Leaves predominantly coriaceous, very widely obovate to orbicular. | 8 |
| 8 | Plants from The Bahamas and western Cuba, with dense cystolith-like structures adaxially. | R. bahamensis |
| – | Plants from the Leeward Islands and Eastern Puerto Rico, adaxial surface mostly glabrous. | R. barloventensis |
Discussion
Alphabethic list of names today assigned to Rochefortia (names to be accepted are in bold face)
Bourreria fasciculata (Kunth) Gürke, = Rochefortia spinosa (Jacq.) Urb.
Crematomia fasciculata (Kunth) Miers, = Rochefortia spinosa (Jacq.) Urb.
Ehretia acanthophora DC., ≡ Rochefortia acanthophora (DC.) Griseb.
Ehretia fasciculata Kunth, = Rochefortia spinosa (Jacq.) Urb. (
Ehretia spinosa Jacq., ≡ Rochefortia spinosa (Jacq.) Urb.
Lutrostylis spinosa (Jacq.) G.Don, ≡ Rochefortia spinosa (Jacq.) Urb. (
Morelosia fasciculata (Kunth) Kuntze, = Rochefortia spinosa (Jacq.) Urb.
Rochefortia acanthophora (DC.) Griseb.
Rochefortia acrantha Urb., = Rochefortia cuneata Sw., syn. nov.
Rochefortia bahamensis Britton
Rochefortia barloventensis Irimia & Gottschling
Rochefortia cubensis Britton & P.Wilson
Rochefortia cuneata Sw.
Rochefortia cuneata subsp. bahamensis (Britton) G.Klotz, ≡ Rochefortia bahamensis Britton
Rochefortia fasciculata Gürke, = Rochefortia spinosa (Jacq.) Urb. (
Rochefortia holguinensis G.Klotz, = Rochefortia stellata Britton & P.Wilson, syn. nov.
Rochefortia jacquinii Griseb., = Rochefortia spinosa (Jacq.) Urb. (
Rochefortia lundellii Camp
Rochefortia oblanceata G.Klotz, = Rochefortia cubensis Britton & P.Wilson, syn. nov.
Rochefortia oblongata Urb. & Ekman
Rochefortia ovata Sw., = Rochefortia cuneata Sw., syn. nov.
Rochefortia septentrionalis G.Klotz, = Rochefortia stellata Britton & P.Wilson, syn. nov.
Rochefortia septentrionalis var. cristalensis G.Klotz, = Rochefortia stellata Britton & P.Wilson, syn. nov.
Rochefortia septentrionalis var. obovata G.Klotz, = Rochefortia stellata Britton & P.Wilson, syn. nov.
Rochefortia spinosa (Jacq.) Urb.
Rochefortia stellata Britton & P.Wilson
Rochefortia stellata subsp. maisiensis G.Klotz, = Rochefortia stellata Britton & P.Wilson, syn. nov.
Rochefortia victoriniana G.Klotz, = Rochefortia stellata Britton & P.Wilson, syn. nov.
Excluded names (validly published names are in bold face)
Desmophyla aliena Raf., not validly published (ICN Art. 52.1.), Sylva tellur.: 43. 1838.—TYPE: Bolivarian Republic of Venezuela. Sucre: Cumaná (Sep, without year), A.J.A. Bonpland & F.W.H.A. von Humboldt 92 (♀ fl, fr) (holotype: P-670679!), ≡ Rochefortia spinosa (Jacq.) Griseb.
Diplostylis H.Karst. & Triana (ICN Art. 60.8.), not validly published (ICN Art. 53.1., non ≡ Diplostylis Sond. = Adenocline Turcz., Euphorbiaceae), in Triana, Nuev. jen. esp.: 25–26. 1854.—TYPE: Diplostylis fasciculata H.Karst. & Triana.
Diplostylis fasciculata H.Karst. & Triana, not validly published (ICN Art. 35.1.), Linnaea 28: 433. 1857.—TYPE: see Rochefortia fasciculata Gürke, = Rochefortia spinosa (Jacq.) Urb.
Lutrostylis inermis G.Don, not validly published (ICN Art. 52.1.), Gen. hist. 4: 391. 1838.—TYPE: Bolivarian Republic of Venezuela. Sucre: Cumaná (Sep, without year), A.J.A. Bonpland & F.W.H.A. von Humboldt 92 (♀ fl, fr) (holotype: P-670679!), ≡ Rochefortia spinosa (Jacq.) Griseb.
Lutrostylis montevidensis (Spreng.) G.Don, Gen. hist. 4: 391. 1838. Ehretia montevidensis Spreng., Syst. veg. 1: 647. 1825.—TYPE: Oriental Republic of Uruguay. Montevideo: Montevideo (without date), F. Sellow s.n. (sterile) (holotype: †B), = Citharexylum barbinerve (Spreng.) Moldenke (Verbenaceae).
Rochefortia acanthophora forma microphylla Griseb., not validly published (ICN Art. 38.1.a), Cat. pl. Cub. 210. 1866.—TYPE: Caribbean, Republic of Cuba. Guantánamo: Mt Toro (May 1, [1861]): Ch. Wright [725] 3126 (sterile) (holotype: GOET-394! isotype: GH-97338!), = Rochefortia cubensis Britton & P.Wilson. Further material collected by Ch. Wright (NY-111150! G-442332! GH-97338! P-3876535! YU-65231!) should not be considered isotype material (
Rochefortia brasiliensis Hoffm. in Willd. ex Schult., Syst. Veg. 6: 210. 1820.—TYPE: Federative Republic of Brazil. Without precise locality (1801): J.C. von Hoffmannsegg s.n. 1801 (fl) (holotype: B-W5463! isotype: HAL-135753), identity unclear, but Rochefortia does not occur in Brazil. Moreover, the Berlin specimen exhibits a number of bisexual flowers excluding it from Rochefortia (and Lepidocordia as well).
Rochefortia transversalis G.Klotz, not validly published (ICN Arts 10.1., 36.1., 38.1.a), Wiss. Z. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe 28: 644, 646, 647. 1979.
Acknowledgements
We thank the curators of the herbaria B, F, G, GH, K, M, MO, P and US for providing us with material and/or information, Hermann Manitz (Jena) for literature and access to herbarium JE, Stan Huzarewicz (Storrs) for providing a *.pdf file of M.W. Lefor\'s thesis, Mia Ehn (Stockholm), Laura Guglielmone (Turin) and Mats Hjertson (Uppsala) for sending images of (putative) type material, Lucian Ionuț Roşu (Iaşi) for assistance with the distribution map and Denis Filer (Oxford) for his support on the BRAHMS online system. Hartmut H. Hilger (Berlin) and another anonymous reviewer provided helpful comments to this revision that are greatfully acknowledged here.
References
- Adams CD (1972) 166. Boraginaceae. Flowering Plants of Jamaica.University of the West Indies,Mona (Jamaica),616–626pp.
- Bentham G, Hooker JD (1876) Ordo CXII. Boragineæ. Sistens dicotyledonum gamopetalarum ordines XLV: Caprifoliaceas—Plantagineas.2.Reeve, Williams & Norgate,London,832–841pp.
- Lista roja de la flora vascular cubana.Jardín Botánico Atlántico,Gijón,86pp.
- Phytogeography and vegetation ecology of Cuba.Akadémiai Kiadó,Budapest,858pp.
- Britton N, Millspaugh CF (1920) Family 5. Ehretiaceae Schrad. The Bahama flora.Hafner,New York,357–360pp.
- Objetos de conservación de la flora y la vegetación del paisaje natural protegido Estrella-Aguadores, Santiago de Cuba, Cuba.Foresta Veracruzana15:7‑14.
- Don G (1838) Order CLXVII. Cordiaceae. Corollifloræ. A general history of the dichlamydeous plants 4.Rivington & Rivington,London,374–393pp.
- A list of the Dicotyledoneae of Belize.Rhodora83:161‑236.
- Floral ontogeny in Bourreria (Ehretiaceae, Boraginales).Flora199:409‑423. https://doi.org/10.1078/0367-2530-00169
- Phylogenetic analysis and character evolution of Ehretia and Bourreria (Ehretiaceae, Boraginales) and their allies based on ITS1 sequences.Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie123:249‑268.
- Gottschling M, Hilger HH, Weigend M (2016) Ehretiaceae Mart. In: Kadereit JW, Bittrich V (Eds) Flowering plants. eudicots, The families and genera of vascular plants.14.Springer,Berlin,165–178pp.
- Generative ontogeny in Tiquilia (Ehretiaceae: Boraginales) and phylogenetic implications.Biological Journal of the Linnean Society112:520‑534.
- Molecular delimitations in the Ehretiaceae (Boraginales).Molecular Phylogenetics and Evolution72:1‑6.
- Gürke M (1893) Borraginaceae. In: Engler A, Prantl K (Eds) Die natürlichen Pflanzenfamilien.4 (3a).Engelmann,Leipzig,71–131pp.
- Charles Wright in Cuba, 1856-1867.Chadwyck-Healey,Alexandria, VA,90pp.
- Howard RA (1989) Boraginaceae. Flora of the Lesser Antilles 6.Arnold Arboretum, Harvard University,Jamaica Plain, Massachusetts,188–211pp.
- A new species of Rochefortia (Ehretiaceae, Boraginales) from the Lesser Antilles.Phytotaxa236:62‑70. https://doi.org/10.11646/phytotaxa.236.1.5
- Strong biogeographic signal in the phylogenetic relationships of Rochefortia Sw. (Ehretiaceae, Boraginales).Plant Systematics and Evolution301:1509‑1516. https://doi.org/10.1007/s00606-014-1162-1
- Selectarum stirpium americanarum historia, pl.author,Vienna,264pp.
- Studies in the Boraginaceae, XVIII. Boraginaceae of the southern West Indies.Journal of the Arnold Arboretum30:111‑138. https://doi.org/10.5962/bhl.part.18048
- Die Boraginaceen-Gattung Rochefortia Swartz in Cuba.Wiss. Z. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe28:643‑647.
- Zur intraspezifischen Varianz und ihrer Widerspiegelung in taxonomischen Kategorien.Wiss. Z. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe29:453‑479.
- Systematische Gliederung der Gattung Rochefortia Swartz.Revista del Jardín Botánico Nacional, Universidad de la Habana3(2):99‑110.
- Lefor MW (1968) A revision of the genus Rochefortia Sw. (Boraginaceae).Univ. of Connecticut, Mansfield,62pp.
- Liogier de Sereys Allut EE (1994) Familia 182. - Boraginaceae. La Flora de la Española.VI.52 Serie Científica 27.Taller,San Pedros de Macorís,121–173pp.
- Liogier de Sereys Allut EE (1995) Family 124. - Boraginaceae. Descriptive flora of Puerto Rico and adjacent islands.Universidad de Puerto Rico,San Juan,298–319pp.
- Liogier de Sereys Allut EE, Martorell LF (1999) Boraginaceae. Flora of Puerto Rico and adjacent islands. A systematic synopsis.Universidad de Puerto Rico,San Juan, Puerto Rico,382pp.
- Arboles de Puerto Rico y las Islas Vírgenes.2.U.S. Dep. Agric.,1177pp.
- A revision of the New World species of Ehretia (Boraginaceae).Annals of the Missouri Botanical Garden76:1050‑1076. https://doi.org/10.2307/2399691
- Dioecy and a reevaluation of Lepidocordia and Antrophora (Boraginaceae: Ehretioideae).American Journal of Botany77:543‑551. https://doi.org/10.2307/2444389
- Buck Island Reef National Monument, U.S. VirginIslands, Vegetation Mapping Project, 2009.U.S. Department of the Interior,Fort Collins, USA–CO,459pp.
- Le fruit de quelques Ehrétiées.Bull. Soc. Bot. Fr.86:325‑332. https://doi.org/10.1080/00378941.1939.10834190
- Sauget y Barbis [Léon] JS, Liogier de Sereys Allut [Alain] EE (1957) Familia 3. — Boraginaceae. Flora de Cuba.4.La Habana,252–278pp.
- Flora of Guatemala. Part IX.Fieldiana24:1‑236.
- Nova genera & species plantarum seu Prodromus descriptionum vegetabilium maximam partem incognitorum quac sub itinere in Indiam occidentalem annis 1783-87.Swederij,Stockholm,152pp. https://doi.org/10.5962/bhl.title.4400
- Flora Indiae Occidentalis aucta atque illustrata; sive, Descriptiones plantarum in prodromo recensitarum.Erlangae, Germany,640pp. https://doi.org/10.5962/bhl.title.6152
- Nuevos jeneros i especies.Imprenta del Neo-Granadino,Bogotá,28pp.
- Urban I (1908) Borraginaceae. Symbolae antillanae seu fundamenta florae indiae occidentalis.Bornträger,Leipzig,474–483pp.
- Verdcourt B (1991) Boraginaceae. In: Polhill RM (Ed.) Flora of tropical East Africa.Balkema,Rotterdam,1–77pp.