|
Biodiversity Data Journal : Taxonomic paper
|
|
Corresponding author: Guanyang Zhang (gyz151@gmail.com)
Academic editor: Pavel Stoev
Received: 17 Feb 2016 | Accepted: 16 Jun 2016 | Published: 08 Jul 2016
© 2016 Guanyang Zhang, Elwood R Hart, Christiane Weirauch.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Zhang G, Hart E, Weirauch C (2016) A taxonomic monograph of the assassin bug genus ZelusFabricius (Hemiptera: Reduviidae): 71 species based on 10,000 specimens. Biodiversity Data Journal 4: e8150. doi: 10.3897/BDJ.4.e8150
|

|
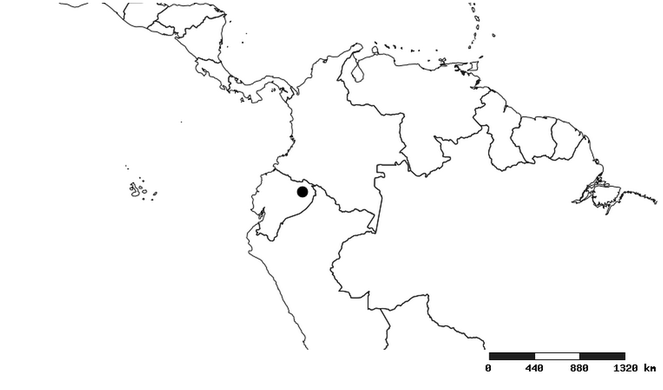
The New World assassin bug genus Zelus Fabricius, 1803 (Insecta: Hemiptera: Heteroptera: Reduviidae: Harpactorinae: Harpactorini) is revised based on more than 10,000 specimens. Seventy-one species are recognized and twenty-four described as new: Zelus aithaleos sp. n., Zelus amblycephalus sp. n., Zelus antiguensis sp. n., Zelus auralanus sp. n., Zelus bahiaensis sp. n., Zelus banksi sp. n., Zelus casii sp. n., Zelus championi sp. n., Zelus cordazulus sp. n., Zelus fuliginatus sp. n., Zelus gilboventris sp. n., Zelus gracilipes sp. n., Zelus grandoculus sp. n., Zelus kartaboides sp. n., Zelus lewisi sp. n., Zelus panamensis sp. n., Zelus paracephalus sp. n., Zelus rosulentus sp. n., Zelus russulumus sp. n., Zelus spatulosus sp. n., Zelus truxali sp. n., Zelus umbraculoides sp. n., Zelus umbraculus sp. n., and Zelus xouthos sp. n. Five species, Zelus araneiformis Haviland, 1931, Zelus gradarius Bergroth, 1905, Zelus modestus (Stål, 1862), Zelus subfasciatus Stål, 1860 and Zelus vittaticeps Stål, 1866, are removed from Zelus and placed incertae sedis within Harpactorini. Nine new synonyms are recognized (senior synonym in parentheses): Zelus atripes Champion, 1898 syn. nov. (=Zelus conjungens [Stål, 1860]), Zelus dispar Fabricius, 1803 syn. nov. (=Zelus pedestris Fabricius, 1803), Zelus formosus Haviland, 1931 syn. nov. (=Zelus laticornis Herrich-Schaeffer, 1853), Zelus obscuridorsis (Stål, 1860) syn. nov. (=Zelus pedestris), Zelus pallidinervus Haviland, 1931 syn. nov. (=Zelus kartabensis Haviland, 1931), Zelus personatus Berg, 1879 syn. nov. (=Zelus versicolor Herrich-Schaeffer, 1848), Zelus trimaculatus Champion, 1898 syn. nov. (=Zelus means Fabricius, 1803), Zelus trimaculicollis (Stål, 1855) syn. nov. (=Zelus means), and Zelus tristis Haviland, 1931 syn. nov. (=Zelus laticornis). Zelus conjungens (Stål, 1860) stat. rev. Is resurrected from junior synonymy with zealous armillatus (Lepeletier & Seville, 1825). Zelus ambulans Stål, 1862 stat. rev. and Zelus cognatus (Costa, 1862) stat. rev. are resurrected from synonymy with Zelus exsanguis Stål, 1862. Iquitozelus Bérenger syn. nov. is synonymized with Zelus and its only species transferred to Zelus, hence resulting in a new combination, Zelus couturieri (Bérenger, 2003) comb. nov. Lectotypes, paralectotypes or neotypes are designated for a number of species. Habitus images, illustrations of male genitalia, distribution maps and measurements are provided for nearly all species. The three previously recognized subgenera of Zelus are found to be based upon superficial characters and these divisions do not reflect natural groupings. Using sets of characters, especially those of the male genitalia, eleven species groups are proposed. It is also hypothesized that Zelus is closely related to three other New World genera: Atopozelus Elkins, Ischnoclopius Stål and an undescribed genus "Hartzelus" [manuscript name]. Zelus is endemic to the New World, occurring naturally in the Caribbean and all but one of the continental countries, with introductions to Pacific islands, Europe and Chile.
Harpactorinae, Heteroptera, natural enemy, Nearctic, Neotropical, new species, Reduviidae, synonymy, systematics, taxonomic revision, Zelus
Introduction
Zelus Fabricius, 1803 is one of the largest reduviid genera (
In the current study seventy-one species are treated and twenty-four described as new. Five species are removed from Zelus and placed incertae sedis within Harpactorini. Nine new synonyms are recognized. Three species are resurrected. Iquitozelus Bérenger is synonymized with Zelus. Habitus images, illustrations of male genitalia, distribution maps, and identification keys are provided. This work evaluates and maintains most of the manuscript new species names proposed in
Review of taxonomic history
The taxonomic history of Zelus is complex and the generic limit of Zelus has undergone constant fluctuations. The first species of Zelus, Z. longipes (Linnaeus), was described by Linnaeus in the 12th edition of Systema Naturae (
A series of works by Stål greatly changed the generic limits of Zelus (
However, the subgeneric groups were raised to the generic rank by
Uhler listed a species of Diplodus from one of the U. S. Geological Survey expeditions (
The generic and subgeneric definitions of Stål were also used by
In his study of the Heteroptera of eastern North America,
In his Ph.D. dissertation
Recent taxonomic activities on Zelus spp. are scant.
Materials and methods
Specimens, databasing and georeferencing
During the course of this study, 10,626 specimens were examined and databased. Among those, 4,833 are males, 5,626 are females and the remainders are immatures or with sex undetermined (usually because of missing abdomen). Specimen loans were kindly provided by museums or collections listed in
List of museums/collections
| Acronym | Museum/Collection | Manager/Curator |
| AMNH | American Museum of Natural History, New York, USA | Ruth Salas/Randall T. Schuh |
| BMNH | Natural History Museum, London, UK | Mick Webb |
| BPBM | Bernice Pauahi Bishop Museum, Honolulu, USA | |
| CAS | California Academy of Sciences, San Francisco, USA | Norman D. Penny |
| CUIC | Cornell University Insect Collection, Ithaca, USA | Rick Hoebeke/James Liebherr |
| FMNH | Field Museum of Natural History, Chicago, USA | James Boone |
| FSCA | Florida State Collection of Arthropods, Florida Department of Agriculture, Gainesville, USA | Susan Halbert |
| ICN | Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Bogotá, Colombia | Carlos Sarmiento |
| IEXA | Instituto de Ecologia, Xalapa, México | Luis Cervantes |
| INBIO | Instituto Nacional de Biodiversidad, San José, Costa Rica | James Lewis |
| KU | Snow Entomological Museum, University of Kansas, Lawrence, USA | Zachary H. Falin |
| LACM | Natural History Museum of Los Angeles County, California, USA | Weiping Xie/Brian Brown |
| MEFLG | Museo Entomológico Francisco Luis Gallego, Medellín, Colombia | John Albeiro Quiroz |
| NHMW | Natural History Museum of Vienna, Vienna, Austria | Herbert Zettel |
| NHRS | Sweden Museum of Natural History, Stockholm, Sweden | Gunvi Lindberg |
| RMNH | Nationaal Natuurhistorisch Museum (formerly Rijksmuseum van Natuurlijke Historie), Leiden, Netherlands | Yvonne van Nierop |
| TAMU | Texas A&M University Insect Collection, College Station, USA | Edward G. Riley |
| UCB | Essig Museum of Entomology, University of California, Berkeley, USA | Cheryl Barr |
| UCD | Bohart Museum of Entomology, University of California, Davis, USA | Steve Heydon |
| UCR | Entomology Research Museum, University of California, Riverside, USA | Douglas Yanega |
| UMSP | University of Minnesota Insect Collection, St. Paul, USA | Philip J. Clausen |
| UNAB | Museo Entomológico, Facultad de Agronomía, Universidad Nacional de Colombia, Bogotá, Colombia | Francisco Serna |
| UNAM | Universidad Autonoma de México, Instituto de Biología, México | Harry Brailovsky |
| USNM | United States National Museum of Natural History, Washington DC, USA | Michele Touchet/Thomas Henry |
| ZMAN | Zoological Museum Amsterdam, Amsterdam, Netherlands | Willem Hogenes |
| ZMUC | Copenhagen University Zoological Museum, Copenhagen, Denmark | Henrick Enghoff |
Type specimens
Information of type specimens of described, valid species, when available, were reported in the 'Materials' section of each species, as holotype, lectotype or neotype.
Distribution and mapping
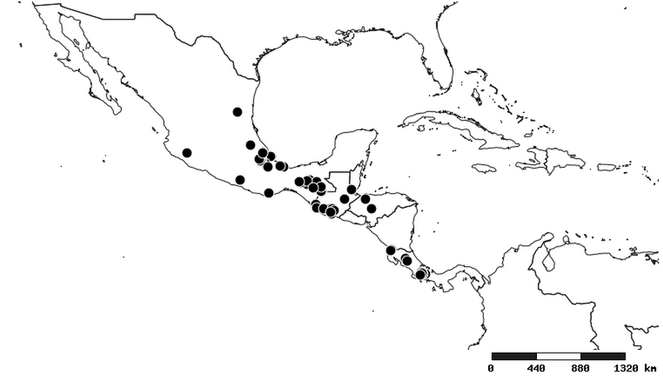
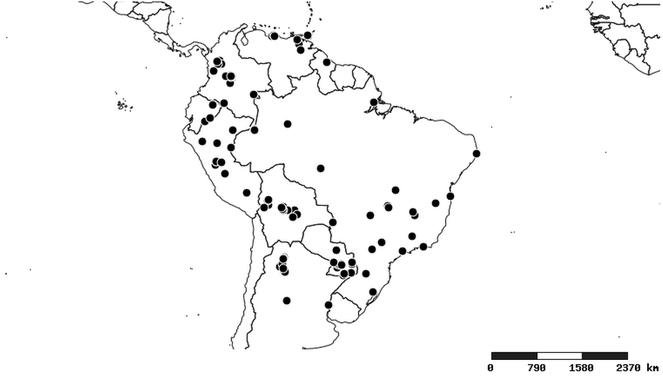
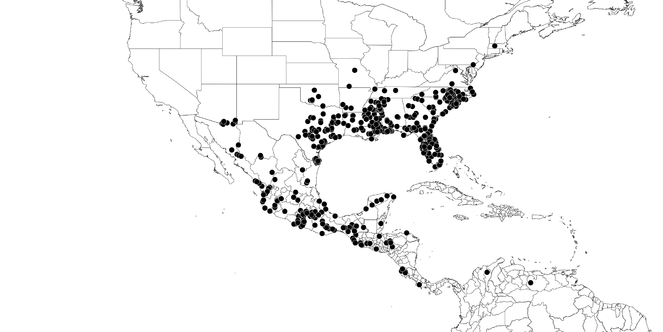
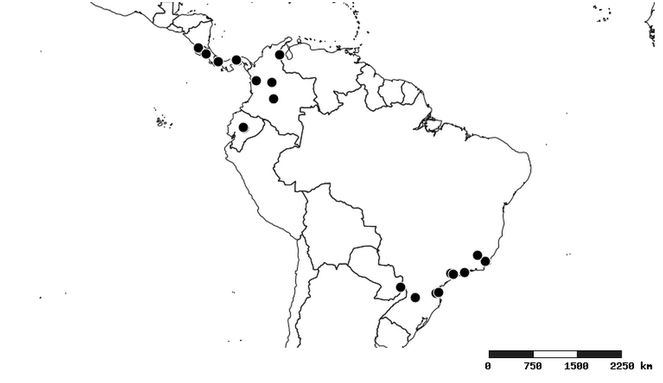
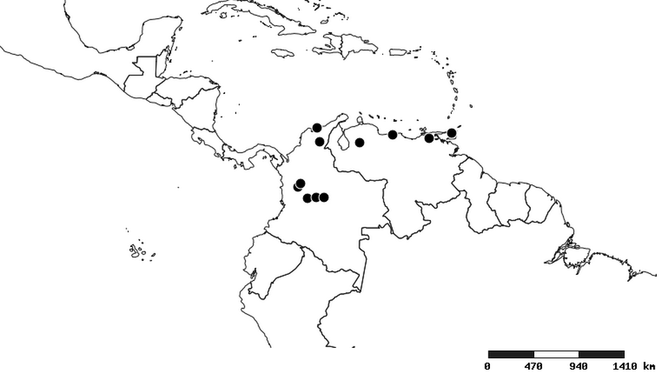
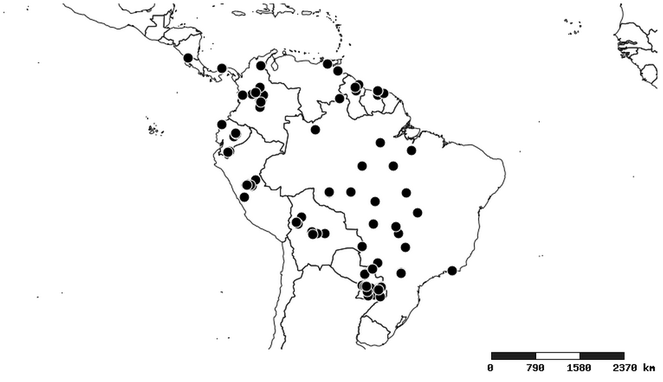
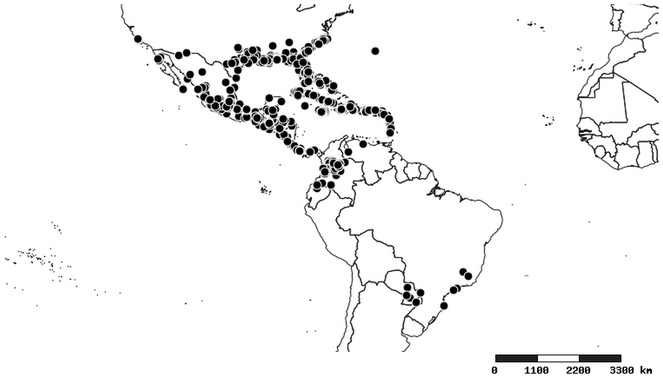
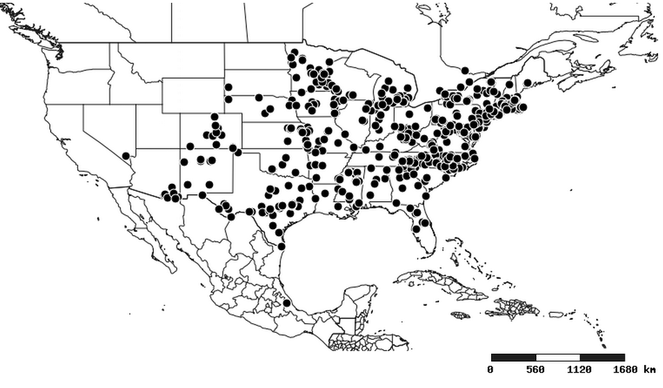
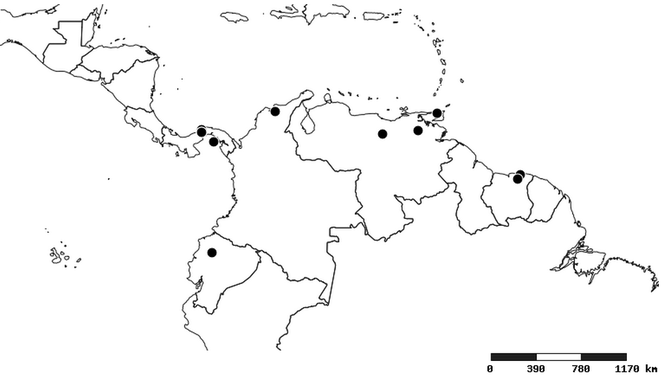
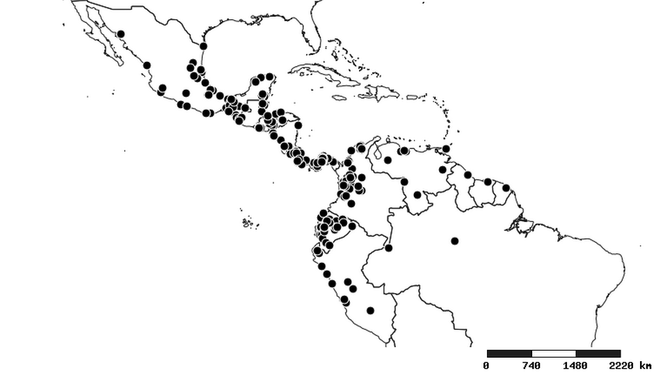
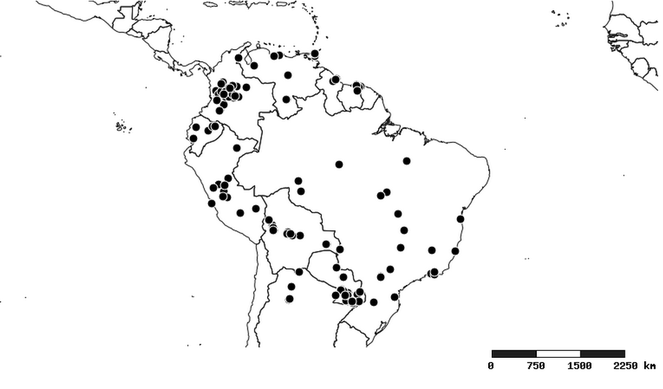
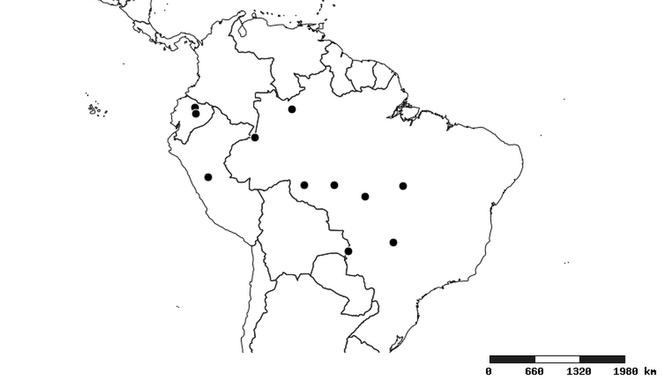
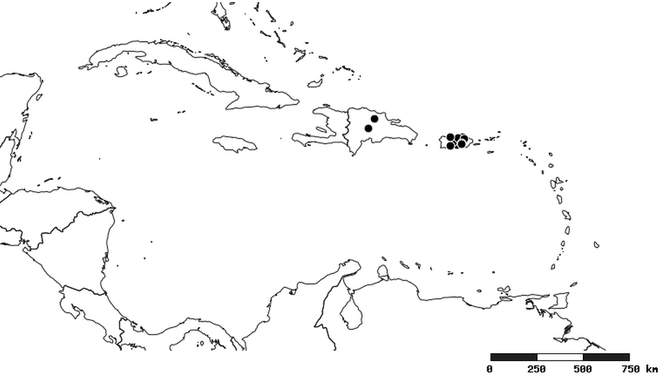
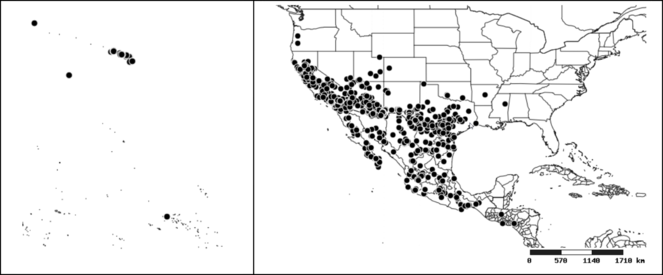
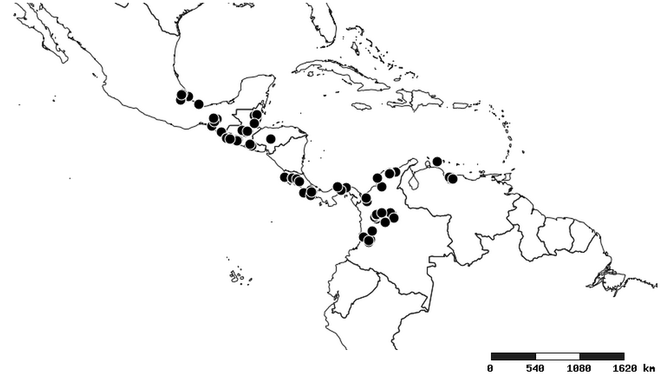
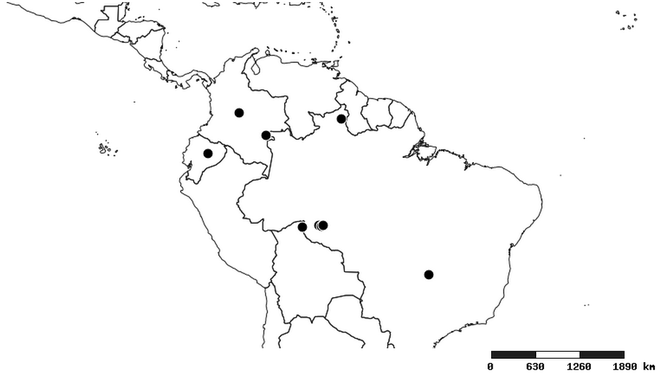
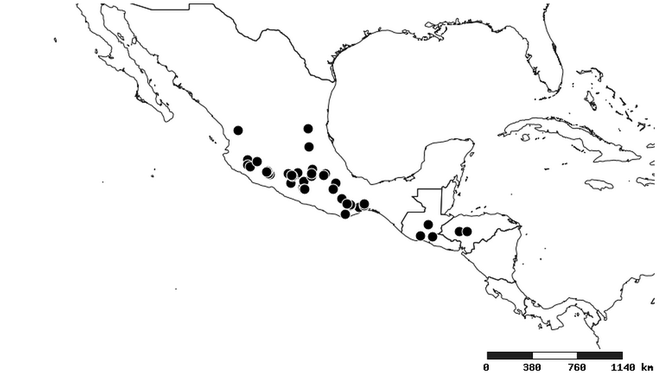
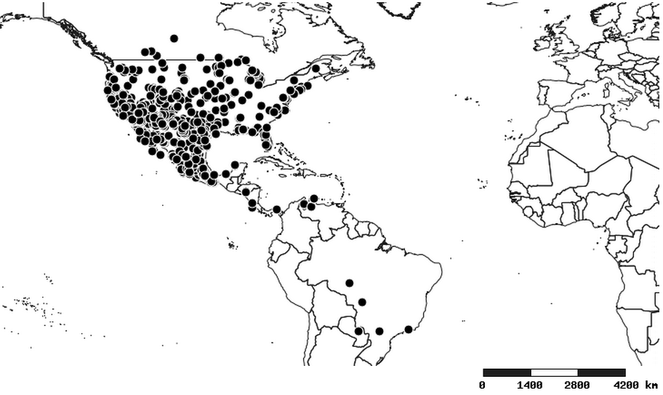
Distributions were based on specimen records captured in the current study. We have gathered the largest samples ever known of all species, which represent the best available knowledge of the distributions of the species of Zelus. Maps were created using the Simple Mapper tool through the PBI website (http://research.amnh.org/pbi/maps) based on the geo-referenced locality data. Because accuracy and error of geo-referencing are highly variable, distribution records shown on the maps are at best indicative. Besides, ambiguous localities and other localities provided only at the country or state level were not geo-referenced and thus not reflected on the distribution maps. It is, therefore, advisable to look up actual specimen data for locality information (
Morphological methods – dissection, observation, imaging and measurement
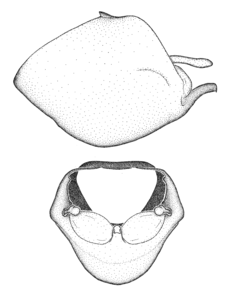
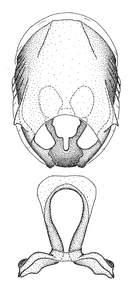
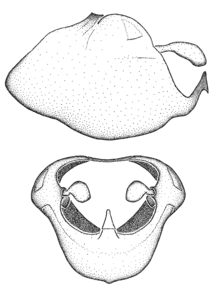
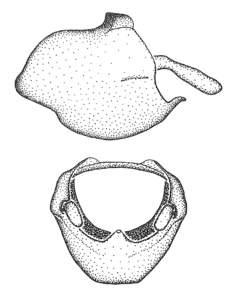
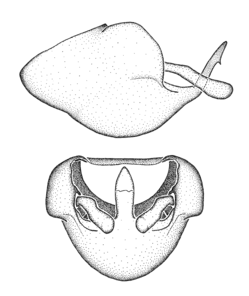
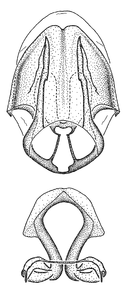
Dissection. Male genitalia, including the eighth abdominal segment and pygophore with phallus enclosed, were removed, cleared in heated 10% potassium hydroxide (KOH) solution for 5–10 minutes, washed in distilled water, and stored in glycerol. To remove the genitalia, a specimen was softened by soaking the abdomen in water. This was achieved by stationing a pinned specimen on play clay with its abdomen pointing down and immersed in water, while the rest of the specimen was not submerged. This method avoided soaking the whole specimen or removing the entire abdomen. A 'genitalia hook' was made by melting the tip of a glass Pasteur pipette with a minuten insect pin inserted and fixed into it. The pygophore was carefully removed by holding the softened abdomen with forceps on one hand, and inserting the genitalia hook into the membranous connection between the seventh and eighth segments, breaking that membrane and pulling off the pygophore, a series of actions performed by the other hand.
Observation. Observations were made using a Nikon stereo dissecting microscope SMZ1500, illuminated by a Nikon NI-150 High Intensity Illuminator. Initial observations of morphological characters were made based on typically a small number of specimens (one to five) and intraspecific variations were subsequently examined based on a larger selection of geographical representation. Genitalia were observed in glycerol. Structures in this medium may look different from their dry state, especially for soft cuticles. For example, the apices of the parameres of Z. cervicalis, Zelus luridus Stål, 1862 and many other species usually appear shriveled in dry specimens, but are fully expanded in glycerol or 70% ethanol. The orientation of the dissected structures shown in illustrations does not necessarily reflect their natural condition.
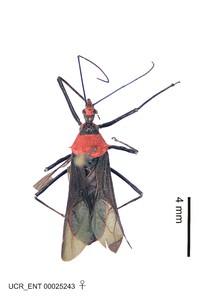
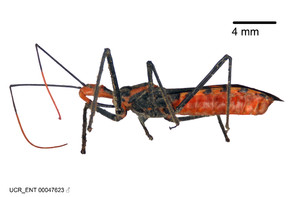
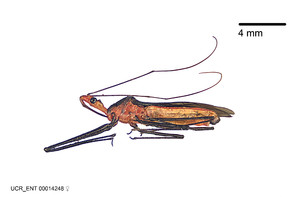
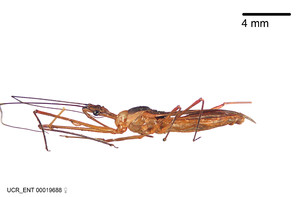
Imaging and illustration. Dorsal and lateral habitus images of specimens were taken with a Microptics-USA system (now Visionary Digital, http://www.duninc.com/index.html) with a K2 lens and CF-2 or CF-4 objectives connected to a Canon EOS 1D digital SRL. Images were edited in Photoshop (CS3/CS4) to adjust levels and sharpness. Image background was removed and replaced using CorelDRAW or Photoshop. For most species illustrations of male genitalia were adapted from
Measurement. Measurements were made on a dissecting scope equipped with a two-axes movable stage (Mitutoyo Corp.), with the aid of two digital micrometers (Boeckeler®), which were connected to a Microcode II RS-232 digital readout (Boeckeler®). Most measurements were done in dorsal view, but various orientations were necessary for measuring appendages. Typically, five to ten specimens were measured for each species, but the number may be fewer for species without enough properly preserved specimens. All measurement values reported here are in mm, unless otherwise stated. In
Length measurements
- Total length: length of body from clypeus to apex of hemelytron
- Clyp-Abd: Clypeus-abdomen (length from clypeus to apex of abdomen)
- Head (length of head from clypeus to collar of anterior pronotal lobe)
- AntOc: Anteocular (length of anteocular region of head, from clypeus to anterior margins of eyes)
- PostOc: Postocular (length of postocular region of head, from posterior margins of eyes to collar of anterior pronotal lobe)
- AntPron: Anterior pronotal lobe (length from collar to transverse sulcus of pronotum)
- PostPron: Posterior pronotal lobe (length from transverse sulcus of pronotum to posterior margin of posterior pronotal lobe)
- Scut: Scutellum (only exposed part measured, from posterior margin of pronotum to apex of scutellum)
- Scap: Scape
- Ped: Pedicel
- Antn3: Antennal segment 3/Basiflagellomere (the basiflagellomere tends to be curled and in that case two or several consecutive measurements were taken and their sum was used)
- Antn4: Antennal segment 4/Distiflagellomere
- Profem: Profemur
- Protib: Protibia
- Mesofem: Mesofemur
- Mesotib: Mesotibia
- Metafem: Metafemur
- Metatib: Metatibia
- Lb1: 1st visible labial segment (this is actually homologous to the second labial segment in other heteropteran insects, and Lb2 and Lb3 are homologous to the third and fourth segments. See
Weirauch 2008b - Lb2: 2nd visible labial segment
- Lb3: 3rd visible labial segment
Width measurements
- Head (width from outer margin of one eye to that of the other)
- InterOcDi: Interocular distance (width from inner margin of one eye to that of the other)
- AntPron: Anterior pronotal lobe (width across the widest part)
- PosPron: Posterior pronotal lobe (width between humeral angles, not including processes)
- Abd: Abdomen (measured at the widest part of the abdomen)
- Profem: Profemur (measured at median point)
- Mesofem: Mesofemur (measured at median point)
- Metafem: Metafemur (measured at median point)
Descriptive taxonomy
Description. Observations were recorded with the software DEscriptive Language for TAxonomy (DELTA) (
Description of intraspecific variations. Intraspecific variations were described and indicated by terms or phrases as the following: sometimes, occasionally and in some specimens. When variations in coloration can be roughly delimited to several patterns, they were described and the frequency of the patterns sometimes mentioned as well. Intraspecific variations in male genitalic structures were usually not described or documented unless they are important for species diagnosis and identification, which is usually the case only for closely related species.
Association of males and females. For the majority of species, males and females show limited sexual dimorphism in size and coloration, and could be readily associated based on external morphology, corroborated by collecting data. However, sexual dimorphism is pronounced in a number of species. Males and females differ drastically in size, body configuration and coloration. Association of sexes for these species was based mainly on locality data and series of specimens of both sexes. Observations of mating reported in the literature were also consulted and used as corroborative evidence whenever available.
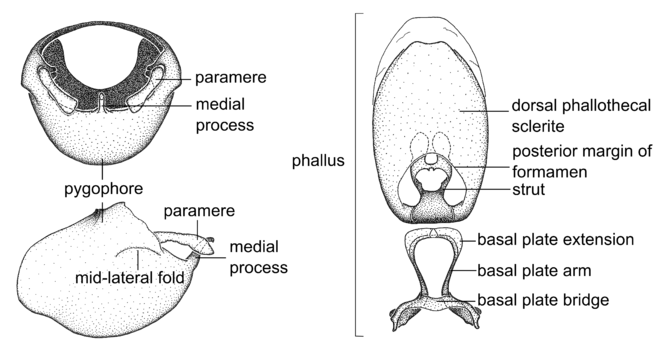
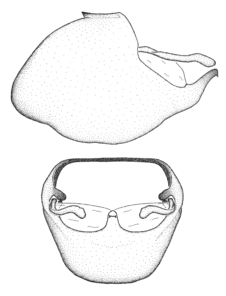
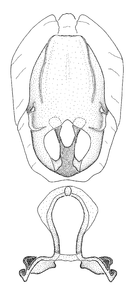
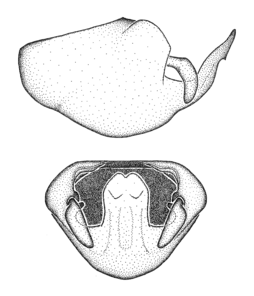
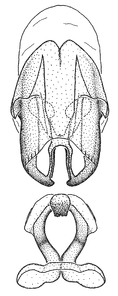
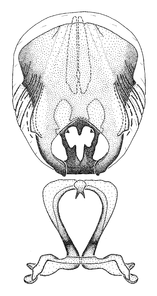
Terminology and abbreviation. External morphology and genitalic terminology followed
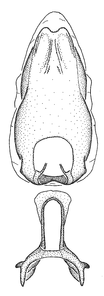
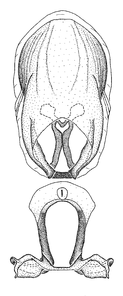
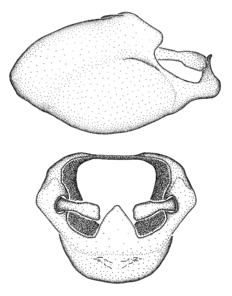
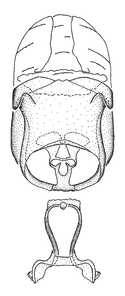
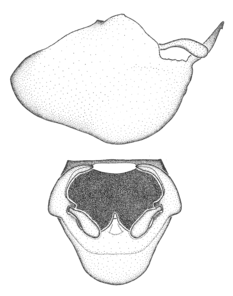
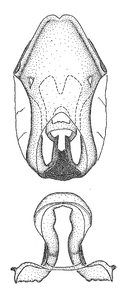
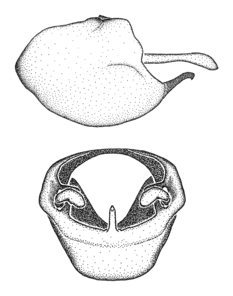
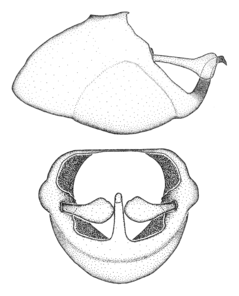
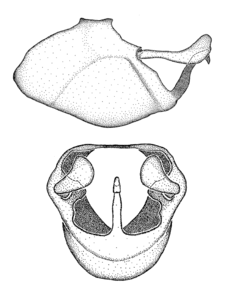
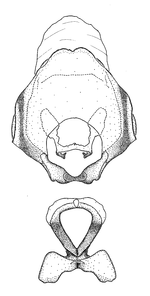
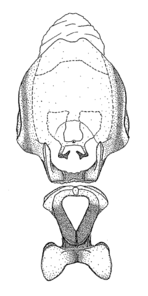
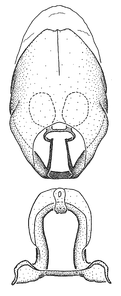
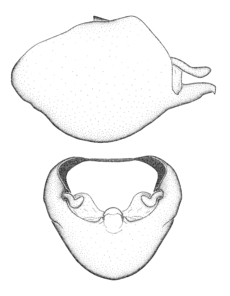
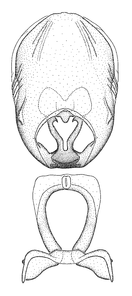
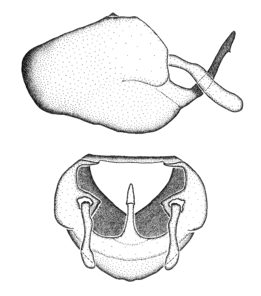
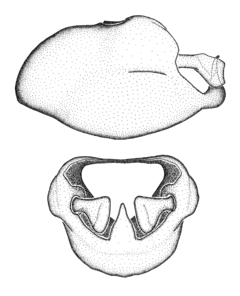
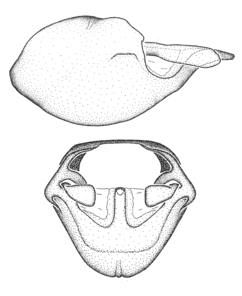
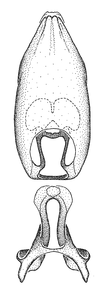
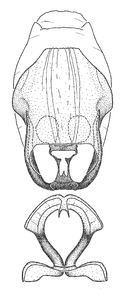
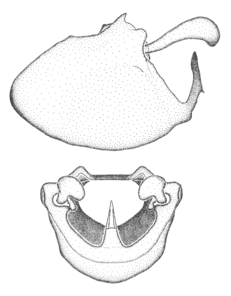
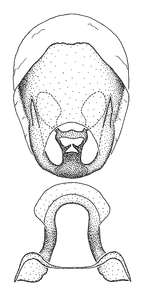
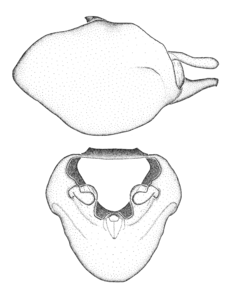
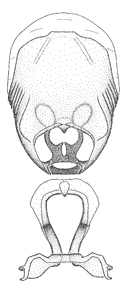
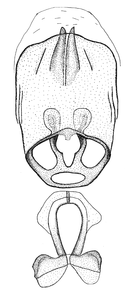
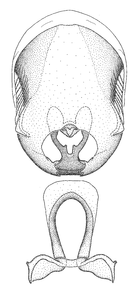
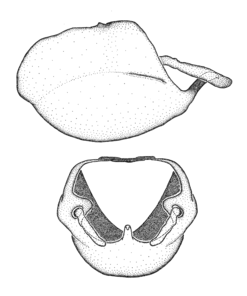
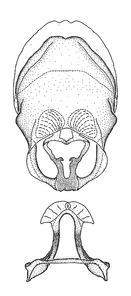
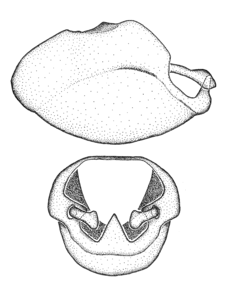
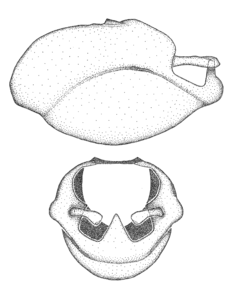
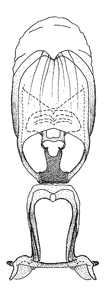
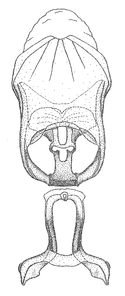
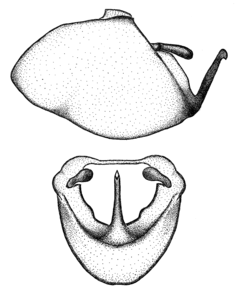
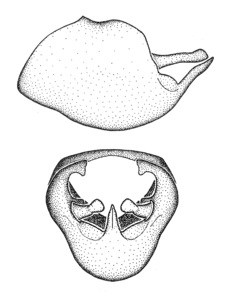
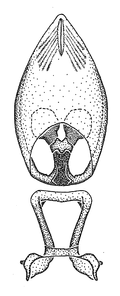
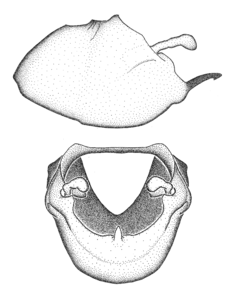
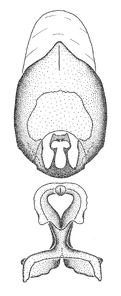
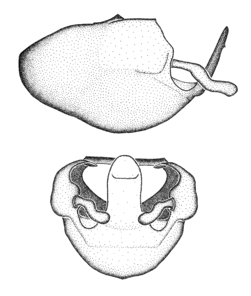
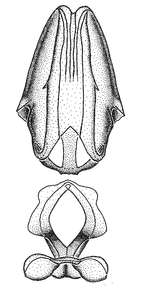
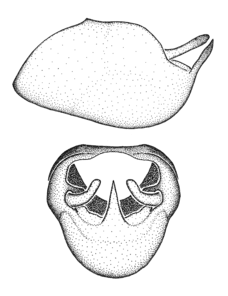
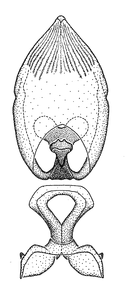
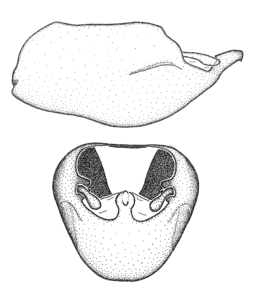
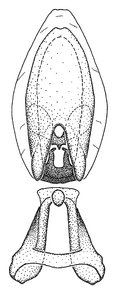
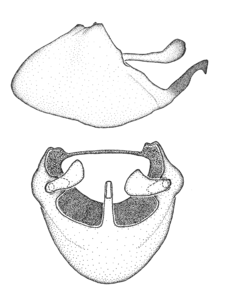
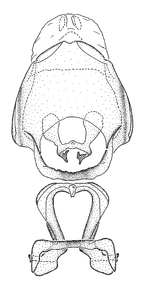
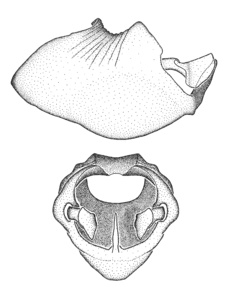
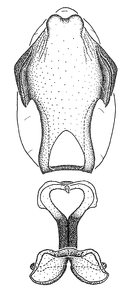
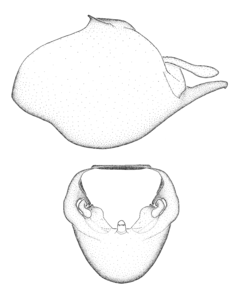
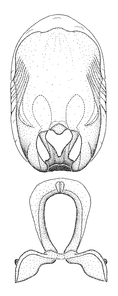
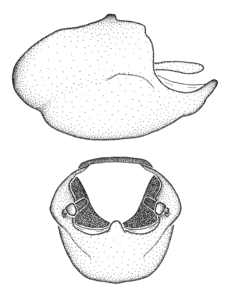
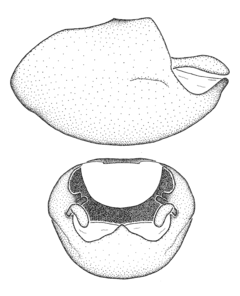
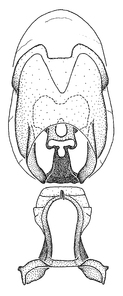
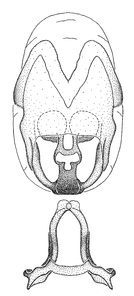
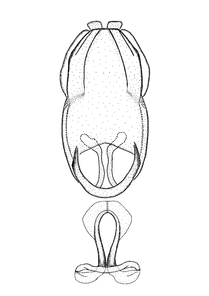
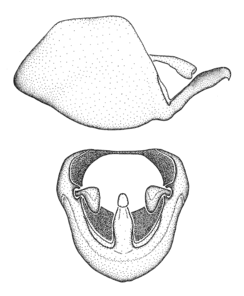
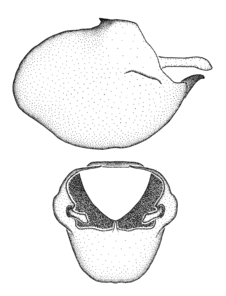
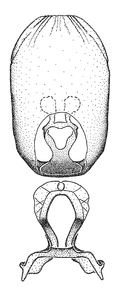
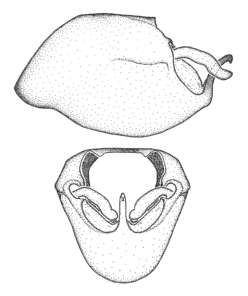
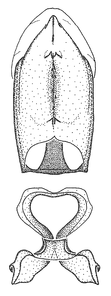
Male genitalic structure terms (Zelus errans Fabricius, 1803 is shown in the illustrations)
Nomenclature (Annotated synonymic list). The synonymic list comprises abbreviated synonymies and included those names that previously appeared in the taxonomic literature or have affected the taxonomy of the species. Citation to ecological, agricultural or other non-taxonomic literature was presented when appropriate, but not meant to be exhaustive. Historical taxonomic publications were briefly annotated to indicate kind of taxonomic information or nomenclatural acts such as .orig descr., checklist, cat., note, fig. and key. When a species name is followed by the original author and year, there is no colon (:) separating the name and the author. A species name followed by a colon indicates that the author of the work is not the author of the name.
Electronic publication
This publication is registered in ZooBank. In accordance with the 2012 Amendment to the International Code of Zoological Nomenclature regarding electronically published works (
Taxon treatments
Zelus
Nomenclature
Zelus Fabricius, 1803, p. 281, orig. descr.; Latreille, 1804, p. 260, list; Latreille, 1807, p. 129, list; Latreille, 1810, p. 433, type desig.; Lepeletier and Serville, 1825, p.815, list and descr.; Laporte, 1832, p. 9, type desig.; Burmeister, 1835, p. 225, descr.; Brulle, 1836, p. 316-317, descr.; Blanchard, 1840, p. 100, descr. and note; Blanchard, 1845, p. 433, 438, list and note; Herrich-Schaeffer, 1848, p. 88, descr. and note; Kolenati, 1857, p. 458-459, descr.; Stål, 1861, p. 148, descr.; Stål, 1862, p. 449-454, key and subgeneric descr. (with subgenus Zelus); Carpenter and Westwood, 1863, p. 565, note; Mayr, 1866, p. 138, list; Stål, 1866, p. 296, list; Stål, 1868, p. 107, restriction of definition; Stål, 1872, p. 69, 88, key and cat. (with subgenus Zelus); Walker, 1873, VII., p. 49, key, VIII., p. 131-136, cat.; Berg, 1879, p. 150, list (with subgenus Zelus); Uhler, 1886, p. 24, checklist; Provancher,1887, p. 179, note; Lethierry and Severin, 1896, p. 151, cat.; Champion, 1898, p. 251, cat. and note; Kirkaldy, 1900a, p. 263, type verification; Kirkaldy, 1900b, p. 242, syn.; Kirkaldy, 1902, p. 149, note; Fracker, 1913, p. 223, 238-240, key and note (with subgenus Zelus); Van Duzee, 1916, p. 30, checklist (with subgenus Zelus); Van Duzee, 1917, p. 258-259, cat. (with subgenus Zelus); Blatchley, 1926, p. 567-568, key, descr. and note (with subgenus Zelus); Readio, 1927, p. 167, 168-169, key, descr. and note; Zimmerman, 1948, p. 137, note; Wygodzinsky, 1949a, p. 48, checklist; Fracker and Usinger, 1949, p. 277, key to nymphs; Alayo, 1967, p. 5, 35, list, key and note; Hart, 1986, key to North American species; Hart, 1987, key to Caribbean species; Maldonado, 1990, p. 325-332, cat.
Reduvius Fabricius, 1775 (type by subsequent desig., Cimex personatus Linnaeus, 1758), Lepeletier and Serville, 1825 (in part), p. 272, descr.; Perty, 1834 (in part), p. 173, list of species.
Arilus Hahn, 1831 (type by subsequent designation, Cimex carinatus Forster, 1771); Burmeister, 1835 (in part), p. 227-228, descr.; Herrich-Schaeffer, 1848 (in part), p. 33-35, descr.
Euagoras Burmeister, 1835 (type by subsequent designation, E. stollii Burmeister, 1835) (in part), p. 226, descr.; Amyot and Serville, 1843 (in part), p. 368, descr. (as Evagoras); Herrich-Schaeffer, 1848 (in part), p. 43-44, descr.; Stål, 1855 (in part), p. 189, list (as Eccagoras); Stål, 1861, p. 148, (in part) junior syn. of Zelus Fabr.; Mayr, 1866, p. 139, list; Walker, 1873, p. 49, 117, key and cat.; Provancher, 1887, p. 182, descr. (as Evagoras); Kirkaldy, 1900b, p. 242, junior syn. of Zelus Fabr.; Kirkaldy, 1903, p. 215-216, note.
Diplodus Amyot and Serville, 1843, p. 370, descr.; Burmeister, 1853, p. 91, list (included in Euagoras Burm.); Stål, 1860, p. 74, list; Stål, 1862, p. 450, descr. (as subgenus of Zelus); Stål, 1866, p. 296, key; Stål, 1872, p. 90, list (as subgenus of Zelus); Walker, 1873, VII., p. 49, VIII., p. 123, key and cat. (as Diploda); Berg, 1879, p. 151, list (as subgenus of Zelus); Uhler, 1886, p. 24, checklist; Provancher, 1887, p. 179, key and descr.; Kirkaldy, 1903, p. 232, note; Fracker, 1913, p. 239, 240, key and list (as subgenus of Zelus).
Darbanus Amyot and Serville, 1843 (type by monotypy, D. nigrolineatus); Provancher, 1872, p. 106, species descr.; Uhler, 1886, p. 24, checklist; Provancher, 1887, p. 179, 181, key and note; Van Duzee, 1912, p. 324; Fracker, 1913, p. 241, note.
Pindus Stål, 1862, p. 454, orig. descr. (as subgenus of Zelus); Stål, 1866, p. 296, key (as genus); Stål, 1872, p. 92, list and cat., as subgenus of Zelus); Walker, 1873, VII., p. 66, list and cat. (as genus); Berg, 1879, p. 150, list (as subgenus of Zelus) ; Thierry and Severin, 1896, p. 151, cat.; Fracker, 1913, p. 223, 240, key and list; Van Duzee, 1916, p. 30, checklist; Van Duzee, 1917, p. 261, cat.; Blatchley, 1926, p. 569, key.
Diplacodus Kirkaldy, 1900b, p. 242, new name for Diplodus A. and S. (preocc.).
Diplocodus Van Duzee, 1916, p. 30, checklist (new name for Diplacodus Kirkaldy, preocc.); Van Duzee, 1917, p. 260, cat.; Blatchley, 1926, p. 569, key.
Iquitozelus Bérenger, 2003, p. 23, orig. descr., syn. nov. (current study).
Type species
Description
Male: Small to large, total length 8-25 mm (
Female: Larger than male. Coloration usually similar to that of male and more variable in some species, but may differ between sexes dramatically in certain species. Eye and ocellus smaller than in male in some species. Basiflagellomere not swollen and about equal diameter as or smaller than pedicel. Lateral process on humeral angle, if present, usually more produced and longer than in male. Mesofemur slightly swollen in many species. Lateral margins of abdomen expanded in some species.
Diagnosis
This genus is distinguished from other genera of the New World Harpactorini by the cylindrical head, the length of the head being at least 1.9X its width; the unarmed antenniferous tubercles; the second labial segment being at least 1.3x the length of the first segment; the long scape and basiflagellomere that are subequal in length and the short pedicel and distiflagellomere; the generally unarmed (i.e. no tubercles or spines) disc of the posterior pronotal lobe (except in Zelus tetracanthus Stål, 1862, Zelus lewisi sp. n. and Zelus minutus Hart, 1987); the humeral angle with or without process, and if present, usually not prominently projected; the legs with sundew setae and sticky glands (
Distribution
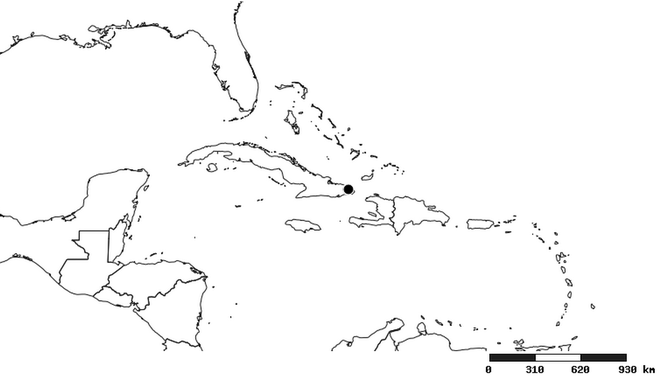
Native to (except for Chile) and throughout the New World, including the Caribbean, with highest diversity in the Neotropics. One species (Z. renardii) has been introduced to Hawaii, the Polynesian islands, Jamaica, Philippines, Spain, Greece and Chile.
Biology
We provide a non-exhaustive account of the biology of various species of this genus. As with other harpactorines, species of Zelus generally do not show associations with or preferences of host plants, probably due to their generalist habits. However, two recent studies have found two species of Zelus that have both nymphs and adults occurring in the same plant species in relatively large quantities. In
Several species of Zelus are possibly mimics of various other insects. Zelus errans Fabricius, 1803, Zelus vespiformis Hart, 1987 and to some extent Zelus vagans Fabricius, 1803 and Zelus gracilipes sp. n. have wing and body color patterns similar to many braconid wasps, an intriguing form of mimicry seen also in a number of other Neotropical harpactorine genera. Zelus vagans shows areas of black and orange colors, however, the posterior pronotal lobe is medially dark and laterally orange. Zelus gracilipes also shows a uniformly orange posterior pronotal lobe, but the hemelytron is uniformly dark and lacks the banding pattern typical to a wasp mimic. Zelus nigromaculatus Champion, 1899 has an appearance similar to that of a typical vespid, the only species in this genus with that kind of color pattern. Zelus laticornis (Herrich-Schaeffer, 1853), Zelus grassans Stål, 1862 and Zelus ruficeps Stål, 1862 have red and dark markings on abdomens and orange or reddish dorsal surfaces, a pattern found in many species of pyrrhocorids (e.g., Dysdercus spp.) and coreids (e.g. Hypselonotus spp.). Interestingly, in Z. laticornis, it is only the females showing this coloration. Certain color forms of Z. longipes are possibly mimics of the milkweed bug, Oncopeltus fasciatus (Dallas).
Taxon discussion
The generic limit of Zelus is now relatively well defined and the genus can be separated from all other but one genera of New World Harpactorini based on characters discussed in the diagnosis. Based on a molecular phylogeny,
The genus that we are uncertain about its relationship with Zelus is Pronozelus Forero, erected by
Except for several pairs or complexes of closely related species, identification of males can be almost always unambiguously performed based on exposed genitalic structures such as paramere and medial process, further corroborated with phallic structures, external morphology and coloration. Identification of females of many species, where females appear to be as distinct as males, is straightforward based on coloration and external morphology. However, identification can be difficult for closely related species, where females are indistinguishable based on external morphology. In these cases, association of males and females and identification of females were primarily based on collecting event information. Sexual dimorphism presents another special challenge. While most species show limited sexual dimorphism that does not go beyond minor size and coloration differences, some species exhibit pronounced differences between the sexes (see Material and Methods for discussion of association of male and female specimens). Based on the observation that species in closely related genera do not exhibit strong sexual dimorphism, we here hypothesize that pronounced sexual dimorphism is a derived condition within Zelus.
Species groups
We find here that previous subgeneric groups are based on superficial resemblance and these are not adopted. Instead, we recognize eleven species groups in the current study, based primarily on characters of the male genitalia, but also on non-genitalic external morphology if those characters can be applied to both sexes. Several species for which only females are known are therefore not assigned to a species group. Although the groupings proposed here are not based on a cladistic analysis, they show a degree of congruence with the relationships recovered in the phylogenetic analysis based on molecular data in
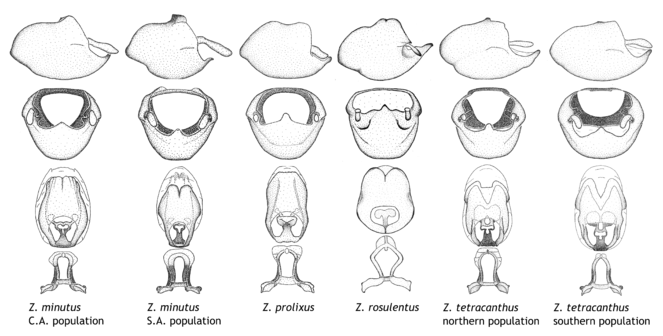
1. Zelus tetracanthus species group.
Zelus minutus Hart, 1987, Zelus prolixus Stål, 1860, Zelus rosulentus sp. n. and Zelus tetracanthus Stål, 1862.
Members of this group have a rather broad, indistinct medial process, the base of which is nearly continuous with or inseparable from the ventral rim of the pygophore. We speculate that this character represents a plesiomorphic condition as it is seen in several other genera of the New World Harpactorini and thus the condition of the medial process does not necessarily support the monophyly of this group. Zelus tetracanthus and Z. minutus also both have tubercles on the disc of the posterior pronotal lobe, which are more pronounced in the former. Comparative views of male genitalia are shown in
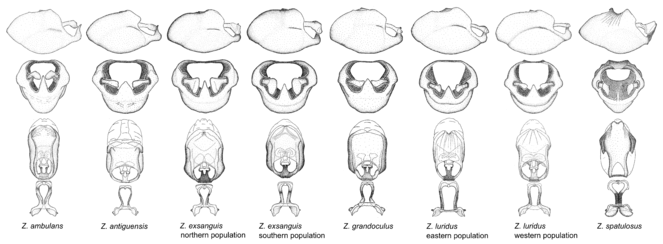
2. Zelus luridus species group.
Zelus ambulans Stål, 1862, Zelus antiguensis sp. n., Zelus exsanguis Stål, 1862, Zelus grandoculus sp. n., Zelus luridus Stål, 1862 and Zelus spatulosus sp. n.
This is a group of species with primarily a North American distribution, with some species extending to northern Central America. The males show an apically expanded paramere and a triangular medial process that has a protrusion at the base but lacks any apical modifications. Notably, Z. spatulosus has a slender medial process, deviating greatly from the remainders of the group. It is placed in this group mainly because of the apically expanded paramere and the uniform coloration. Zelus ambulans and Z. exsanguis have the humeral angle elevated to about same level of and nearly continuous with the disc of the posterior pronotal lobe, a condition rarely seen in the genus. The coloration is quite homogenous among members of this genus, most of which have a uniform greenish (in live specimens) or dull brownish (in preserved specimens) habitus, with only Z. ambulans showing variable patterns or banding on the pronotum or legs. Comparative views of male genitalia are shown in
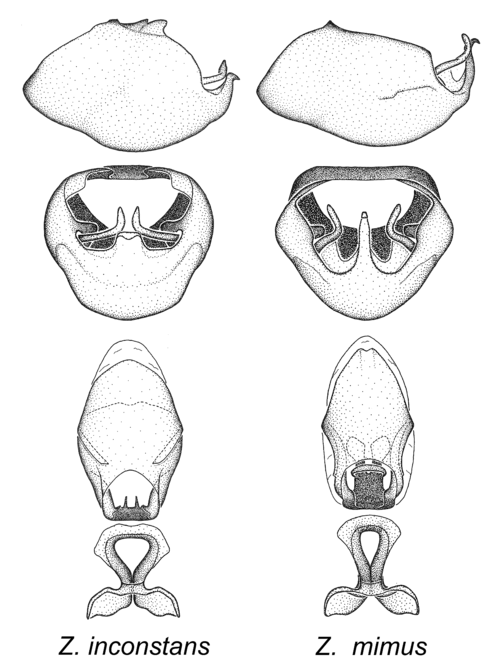
3. Zelus mimus species group
Zelus inconstans Champion, 1898 and Zelus mimus Stål 1862.
Members of this group, consisting of only two species, exhibit a highly unique paramere and a medial process of the pygophore. The paramere is slender and apically curved dorsad at an angle of nearly ninety degrees. The medial process, as is especially evident in Z. inconstans, possesses a simple posterior liplike fold at the apex; its lateral margins are subparallel and not broadened significantly at the base. Both have a semi-cylindrical dorsal phallothecal sclerite which is modified by a fold running obliquely toward the base from the middle of the lateral margins. Both species, being quite small, exhibit the usual reduction of setal tracts common to nearly all small species. Comparative views of male genitalia are shown in
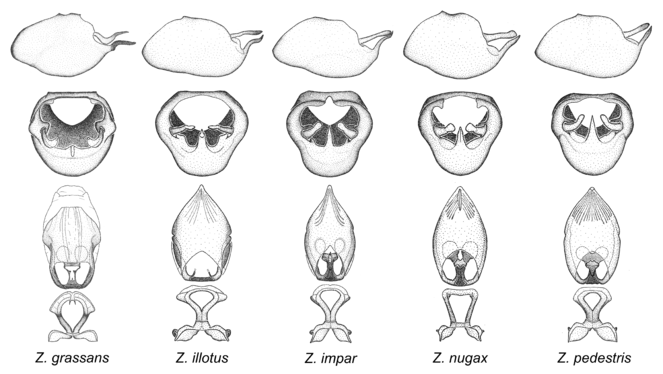
4. Zelus nugax species group.
Zelus grassans Stål, 1862, Zelus illotus Berg, 1879, Zelus impar Kuhlgatz, 1902, Zelus nugax Stål, 1862 and Zelus pedestris Fabricius, 1803.
This is a group of smallish species with quite variable distributional ranges. The defining characters include a slender, laterally compressed medial process that is curved or recurved, and an acute apex of the dorsal phallothecal sclerite (except in Z. grassans). Zelus nugax has one of the widest distribution ranges in this genus, ranging from much of Mexico to northern South America. Zelus grassans is found primarily in Central America and the remaining two species mainly in northern South America. Comparative views of male genitalia are shown in
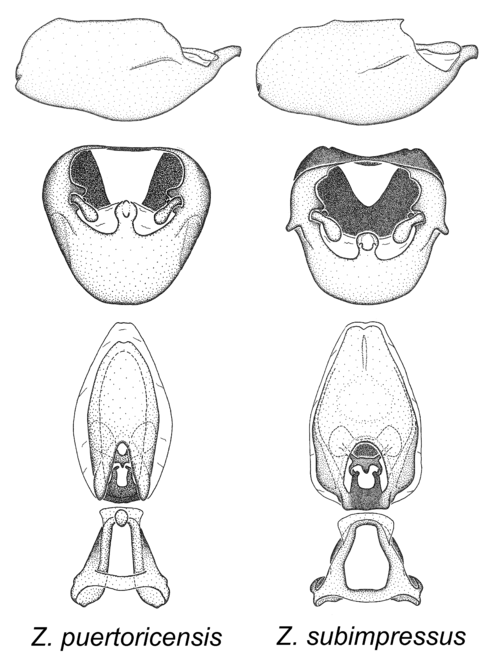
5. Zelus puertoricensis species group.
Zelus bruneri De Zayas, 1960, Zelus puertoricensis Hart, 1987, Zelus subimpressus Stål, 1872 and Zelus zayasi Bruner and Barber, 1937.
Members of this group are restricted to the Caribbean. They can be easily recognized by the rather slender body form. The posteriorly directed, robust medial process with a somewhat blunt apical protrusion is also distinctive of this group. The basal plate arms are widely separate and diverging and these features are rare in other species in the genus. They show resemblance to species of the Zelus renardii species group, especially to Z. cervicalis. Zelus bruneri was not physically examined, but the rather slender body form as seen in the original illustration places it within this group. Comparative views of male genitalia are shown in
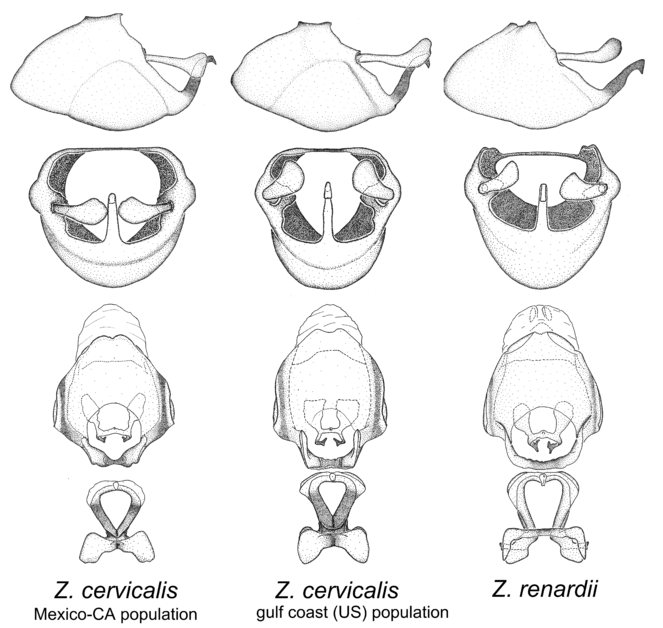
6. Zelus renardii species group.
Zelus cervicalis Stål, 1872 and Zelus renardii Kolenati, 1856.
The two members of this group are very likely sister species since they share a number of unique characters: the apex of the medial process is greatly bent ventrad and hooklike, the lateral margin of the dorsal phallothecal sclerite is recurved dorsad and the basal part of the strut is absent. Both species are mainly distributed in North and Central America, but Z. cervicalis extends to northern South America. Comparative views of male genitalia are shown in
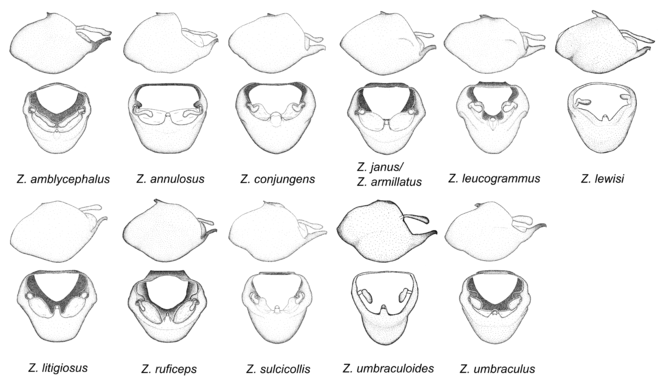
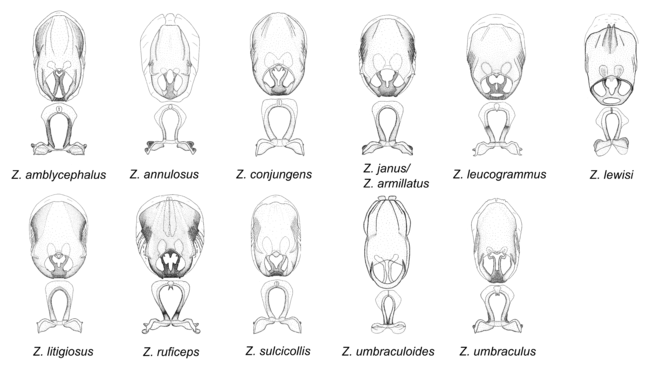
7. Zelus armillatus species group.
Zelus amblycephalus sp. n., Zelus annulosus (Stål, 1866), Zelus armillatus (Lepeletier & Serville, 1825), Zelus conjungens (Stål, 1860), Zelus janus Stål, 1862, Zelus leucogrammus (Perty, 1833), Zelus lewisi sp. n., Zelus litigiosus Stål, 1862, Zelus ruficeps Stål, 1862, Zelus sulcicollis Champion, 1899, Zelus umbraculoides sp. n. and Zelus umbraculus sp. n.
This is one of the two largest groups in the genus (the other being the Zelus panamensis species group). Species in this group are generally robust and large-sized (15-25 mm), and some are among the largest in the genus. The most distinctive character is that of the medial process, which has the apex slightly projected into two minute small lateral prongs or processes. This condition is different from that in several species groups listed below, where the apex of the medial process is hook-like and more strongly projected. The lateral spine of the humeral angle tends to be pronounced and somewhat broadened into a dentate effect. The pygophore is large, rounded, and somewhat shortened relative to the total length of the individual. The dorsal phallothecal sclerite having dorsolateral expansions or projections close to the basal arm is also unique to some species of this group. This condition, however, is not seen in Z. amblycephalus, Z. umbraculus, or Z. umbraculoides, which appear to be divergent from the remainders of the group, but the features of the medial process unambiguously place them in this species group. Comparative views of male genitalia are shown in
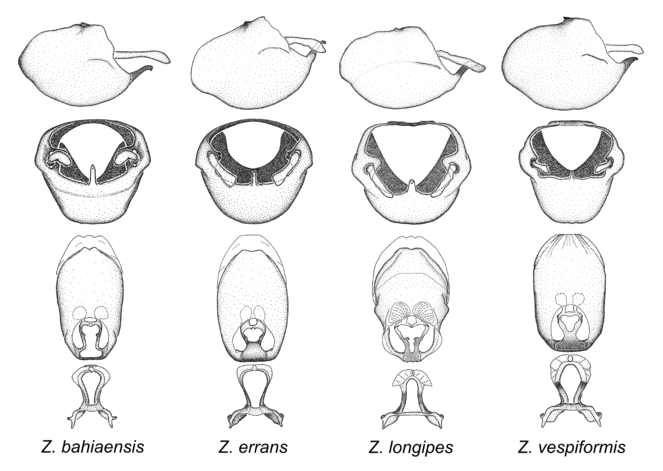
8. Zelus longipes species group.
Zelus bahiaensis sp. n., Zelus errans Fabricius, 1803, Zelus longipes (Linnaeus, 1767), 1803 and Zelus vespiformis Hart, 1987.
This and the next species group (Zelus vagans species group) possess dense, spine-like setae on the head and pronotum, and a rounded, unarmed humeral angle, both characters rather unique in Zelus and probably synapomorphies uniting the two groups. The former character is possibly homoplastic as it is also seen in two species in the Zelus armillatus species group. The medial process is slender and cylindrical and this condition is among the most extreme in the genus. It is semi-erect and posteriorly directed. The paramere exceeds the apex of the medial process. The dorsal phallothecal sclerite has subparallel margins and lacks obvious modifications or ornamentations (except for small lateral folds in Z. longipes). Some individuals of Z. errans and Z. vespiformis appear to be wasp mimics. Comparative views of male genitalia are shown in
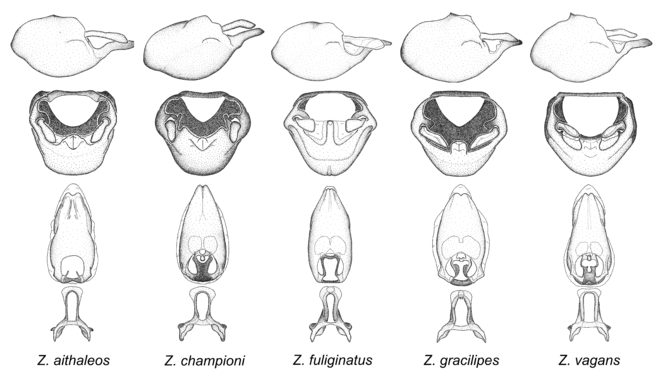
9. Zelus vagans species group.
Zelus aithaleos, Zelus championi sp. n., Zelus fuliginatus sp. n., Zelus gracilipes sp. n. and Zelus vagans Fabricius, 1803.
Species of the Zelus vagans group share two characters also present in the preceding group (Zelus longipes species group): spinelike setae and rounded humeral angle. However, they differ in the structure of the male genitalia in significant ways. The medial process shapes like a somewhat laterally flattened cone. It is relatively broad at base, narrowing toward the apex, and is laterally compressed. The medial process is posteriorly directly, nearly horizontal. The paramere is removed from or barely reaching apex of the medial process. Furthermore, the phallus is elongated and slightly constricted toward the apex (not conspicuous in Z. gracilipes). Zelus vagans and Z. gracilipes also resemble wasps to some extent, but both not as perfectly as seen in Z. errans and Z. vespiformis. Comparative views of male genitalia are shown in
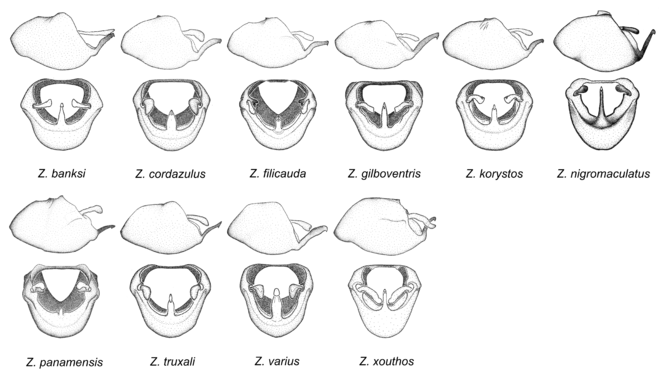
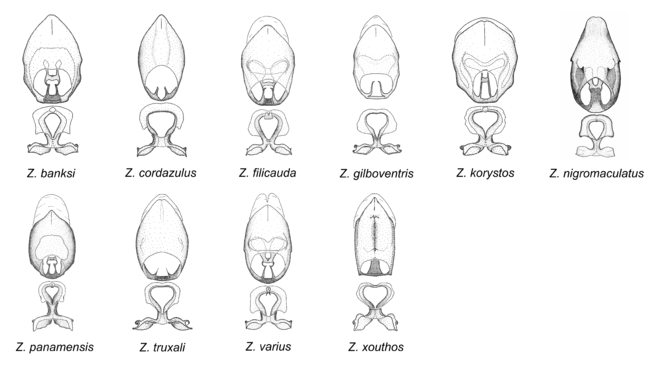
10. Zelus panamensis species group.
Zelus banksi sp. n., Zelus cordazulus sp. n., Zelus filicauda Bergroth, 1893, Zelus gilboventris sp. n., Zelus korystos Hart, 1986, Zelus nigromaculatus Champion, 1899, Zelus panamensis sp. n., Zelus truxali sp. n., Zelus varius (Herrich-Schaeffer, 1853) and Zelus xouthos sp. n.
This is another large group with ten species. Interestingly, most (seven) are new species. It is characterized by having an acute apical modification usually in the shape of a hook on the medial process and the conspicuous medial carination of the apical part of the dorsal phallothecal sclerite. The condition of the apical modification of the medial process differs from that in the Zelus armillatus species group in that it is much more prominent, usually acute and sometimes extending further ventrally. Rugulosity of the posterior pronotal lobe is highly pronounced relative to the other groups. Sexual dimorphism is pronounced in some species in this group (e.g., Z. gilboventris and Z. truxali). Most species in this group are concentrated in southern Central America and northern South America. Comparative views of male genitalia and habitus images are in
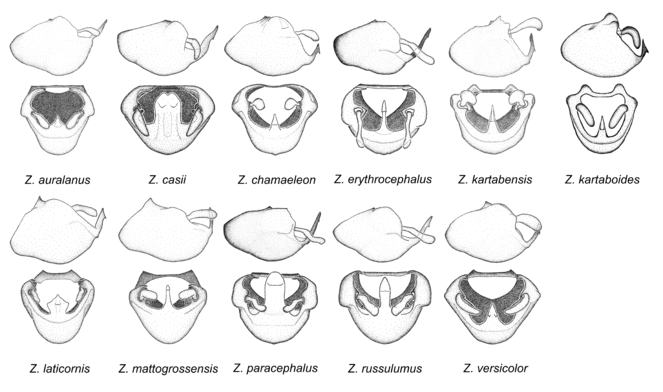
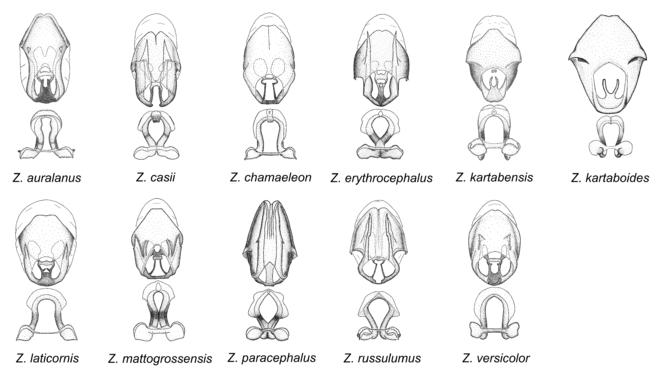
11. Zelus erythrocephalus species group.
Zelus auralanus sp. n., Zelus casii sp. n., Zelus chamaeleon Stål, 1872, Zelus erythrocephalus Fabricius, 1803, Zelus kartabenoides sp. n., Zelus kartabensis Haviland, 1931, Zelus laticornis (Herrich-Schaeffer, 1853), Zelus mattogrossensis Wygodzinsky, 1947, Zelus paracephalus sp. n., Zelus russulumus sp. n. and Zelus versicolor (Herrich-Schaeffer, 1848).
Two diagnostic characters identify members of this group. The medial process possesses a broad ridge-like projection or carina that initiates from the apex and extends ventrally or is removed from apex. The second feature is the apically oriented lateral sharp processes or projections on the dorsal phallothecal sclerite. These are not to be confused with the lateral expansion seen in the Zelus armillatus species group, where the direction of the expansion is laterad. In Z. auralanus and Z. versicolor, this process is short and somewhat dorsally directed, rather than apically directed. Three species, Z. kartabenoides, Z. kartabensis and Z. chamaeleon lack this structure. Their placement in this group is primarily based on the configuration of the medial process and the absence of characters of other groups. Also, the longitudinal ridge-like elevation or hook on the medial process is similar to the condition in another species, Z. laticornis, although the latter has a short modification. In this species group the parameres are usually somewhat bulbous and curved medially with moderate to long erect setae on the apical 1/2. The medial process is broadened at base, and usually anteroposteriorly compressed. Furthermore, the basal plate of the phallus is strongly curved in some members of this species group. Pronounced sexual dimorphism is seen in some species of this group. Notably, three species, Z. erythrocephalus, Z. paracephalus and Z. russulumus have purple, blue or greenish iridescence on the membrane of the hemelytron. Species of this group show a predominant southern South American distribution, with a few found only from the Amazons. Comparative views of male genitalia and habitus images are in
Because of the heavy emphasis on male genitalic characters for grouping species, four species described only from females are not placed in any of the species groups defined in the above. These are: Zelus fasciatus Champion, 1899, Zelus plagiatus (Signoret, 1852), Zelus sphegeus Fabricius, 1803 and Zelus means Fabricius, 1803. Zelus fasciatus is similar to the females of some of the species in the Zelus panamensis species group and also occurs in an overlapping geographical region (southern Central America). Zelus plagiatus and Z. sphegeus show resemblance to the females of Z. versicolor, which is in the Zelus erythrocephalus species group. Zelus means, by possessing a rounded humeral angle and spinelike setae, aligns most closely with the Zelus vagans species group and the Zelus longipes species group. A future cladistic analysis, including morphological and molecular data, is needed to test the monophyly of these species groups and may also have the potential to place these female-based species.
Species removed from Zelus
Five species are removed from Zelus: Z. araneiformis, Zelus gradarius Bergroth, 1905, Z. modestus (Stål, 1862), Zelus subfasciatus Stål, 1860 and Zelus vittaticeps Stål, 1866. These species represent an undescribed genus "Hartzelus" and will be treated in a separate study. They will be listed as Harpactorini incertae sedis until their generic placement is formally clarified.
Zelus aithaleos , sp. n.
-
scientificName: Zelus aithaleos; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Huanuco; locality:Aerro Puerto, Tingo Maria; verbatimElevation:671 m; decimalLatitude:-9.3; decimalLongitude:-76.01666; georeferenceSources:Gazetteer; eventDate:1946-10-22; sex:Adult Male; catalogNumber:UCR_ENT 00047314; recordedBy:J. C. Pallister; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus aithaleos; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:BOLIVIA; stateProvince:La Paz; locality:Guanay; decimalLatitude:-15.4833; decimalLongitude:-67.8833; georeferenceSources:Gazetteer; eventDate:1993-10-01 to 1993-11-01; sex:Adult Female; catalogNumber:UCR_ENT 00009327; recordedBy:L. Pena; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus aithaleos; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:BOLIVIA; stateProvince:La Paz; locality:Guanay; decimalLatitude:-15.4833; decimalLongitude:-67.8833; georeferenceSources:Gazetteer; eventDate:1993-10-01 to 1993-11-01; sex:Adult Female; catalogNumber:UCR_ENT 00009328; recordedBy:L. Pena; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus aithaleos; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:BRAZIL; stateProvince:Goias; locality:Annapolis; decimalLatitude:-16.3333; decimalLongitude:-48.9667; georeferenceSources:Gazetteer; eventDate:1936-02-07; sex:Adult Female; catalogNumber:UCR_ENT 00071251; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:TAMU
-
scientificName: Zelus aithaleos; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PARAGUAY; stateProvince:Guaira; locality:Paso-Yobai; verbatimElevation:280 m; decimalLatitude:-25.72344; decimalLongitude:-55.9969; georeferenceSources:Google Earth; eventDate:1951-09-28; sex:Adult Female; catalogNumber:UCR_ENT 00071252; recordedBy:Foerster; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:TAMU
Description
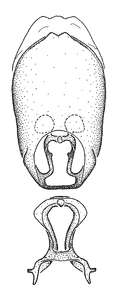
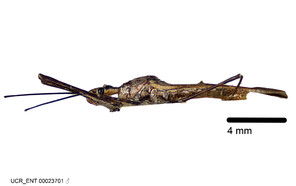
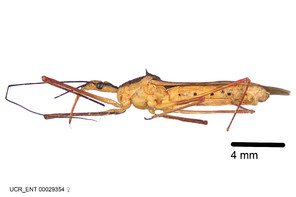
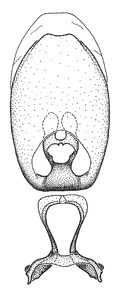
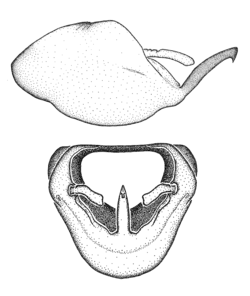
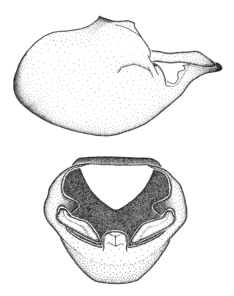
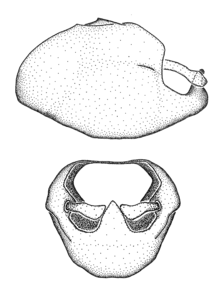
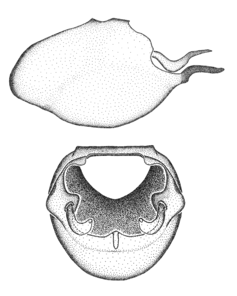
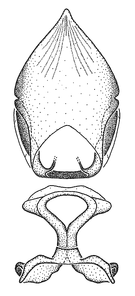
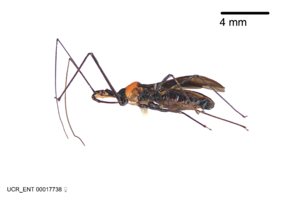
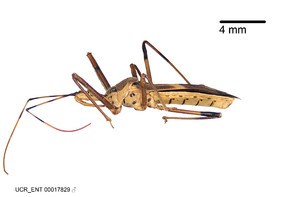
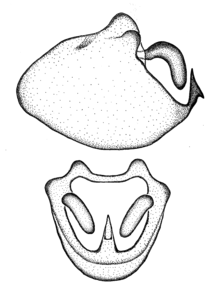
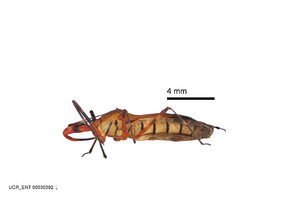
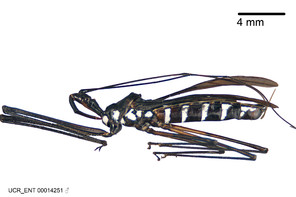
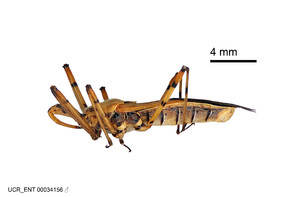
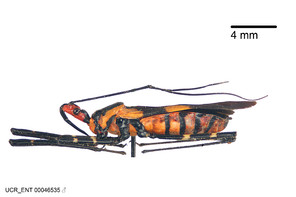
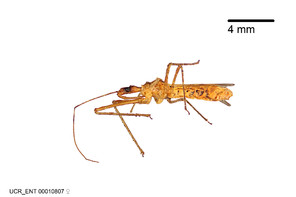
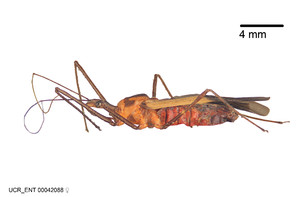
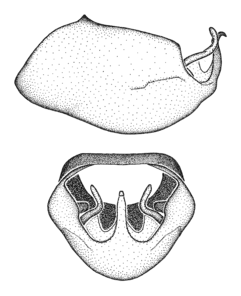
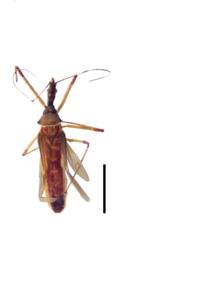
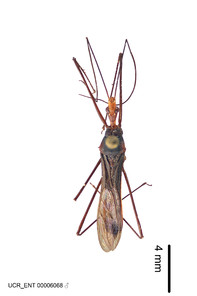
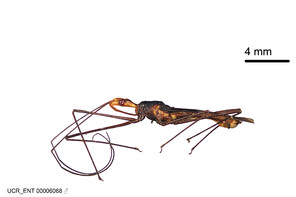
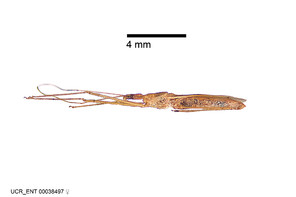
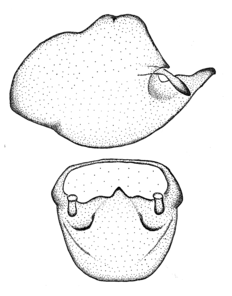
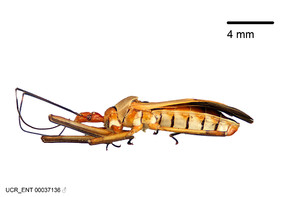
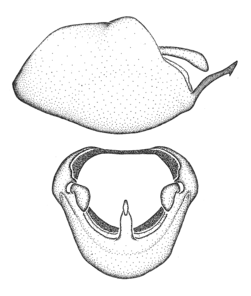
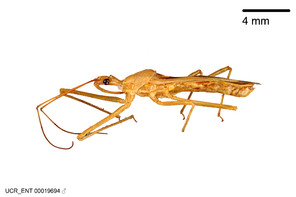
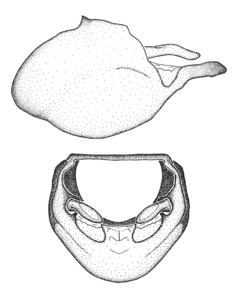
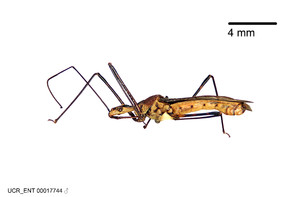
Zelus aithaleos Zhang & Hart, sp. n., habitus
b: Zelus aithaleos Zhang & Hart, sp. n., male, lateral view (UCR_ENT 00047314, Huanuco, Peru)
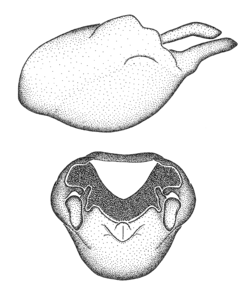
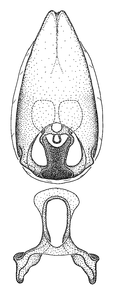
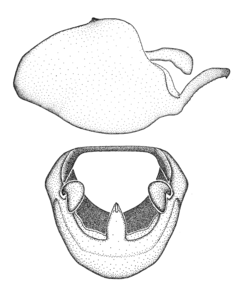
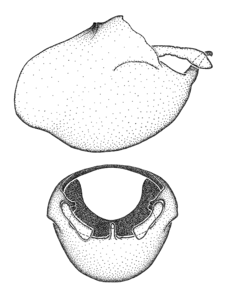
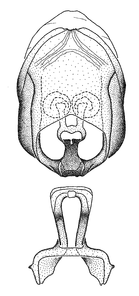
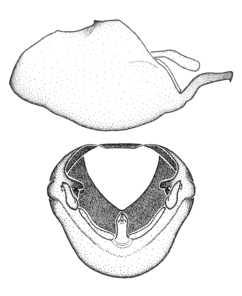
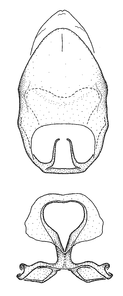
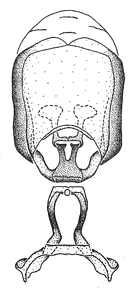
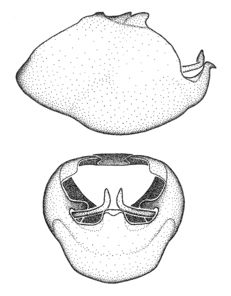
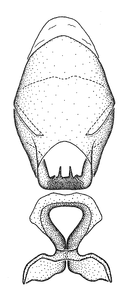
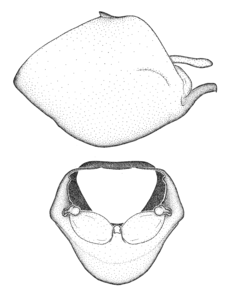
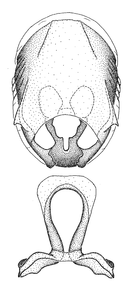
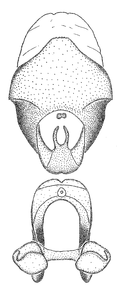
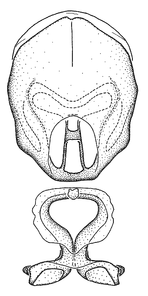
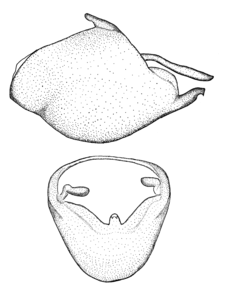
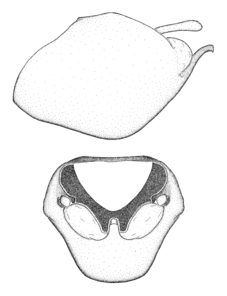
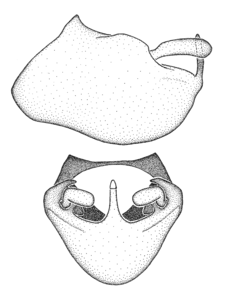
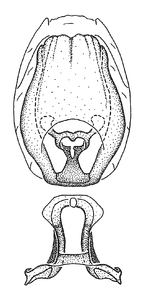
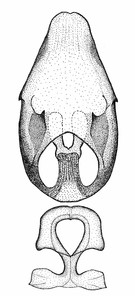
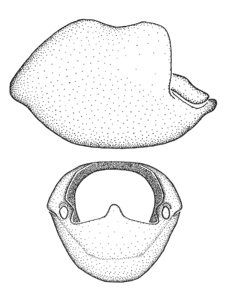
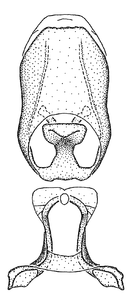
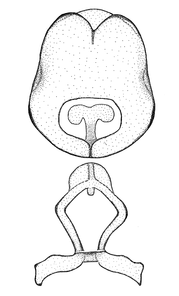
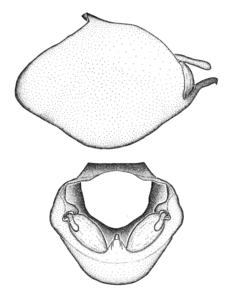
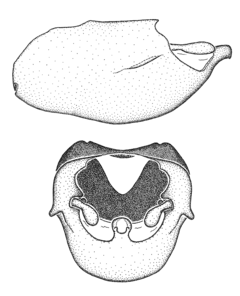
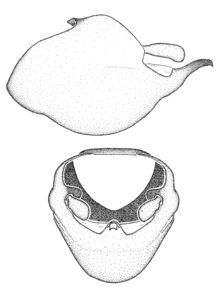
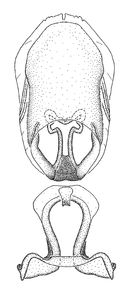
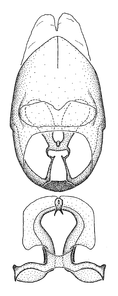
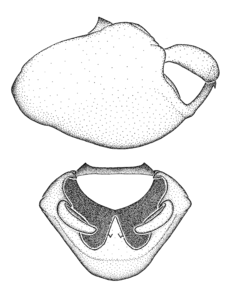
Zelus aithaleos Zhang & Hart, sp. n., male genitalic structures
b: Zelus aithaleos Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: Similar to male, except for the following. Larger than male, total length 13.87–17.61 mm (mean 16.27 mm,
Diagnosis
The nearly colorless cells of the membrane of the hemelytron contrast markedly with the dark veins, making Z. aithaleos an easily recognizable species in this genus. Also recognized by the following combination of characters: the postocular lobe short, 1.7x of the length of anteocular lobe in males and 1.2x in females; the anterior pronotal lobe short, abbreviated; the pronotum strongly convex; the humeral angle of pronotum rounded, unarmed; the cranium, the pronotum, the pleura and the scutellum with spinelike, short, stout setae (the last two characters also seen in the Zelus longipes species group and the Zelus vagans species group).
Males can also be recognized by the medial process laterally compressed, posteriorly directly and almost horizontal (also seen in the Zelus vagans species group). Within the Zelus vagans species group (
A unicolourous near-black dorsum, including the head, the pronotum and the corium, separates Z. aithaleos from both sexes of Z. gracilipes, Z. vagans, and Z. means (known from females only), all of which have some orange, yellow or reddish colors. The dark dorsal profile is shared with Z. championi (only the male is known) and Z. fuliginatus. A longitudinal lateral patch of whitish recumbent setae on the postocular lobe serves to separate this species from Z. fuliginatus. It is distinguished by a dark abdomen from Z. championi, which has a brightly red abdomen.
Etymology
From Greek aithales.
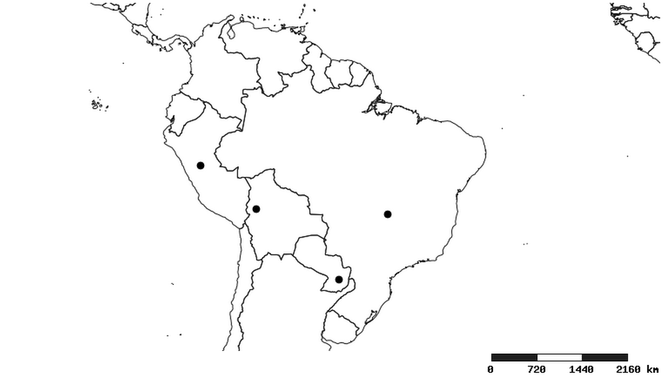
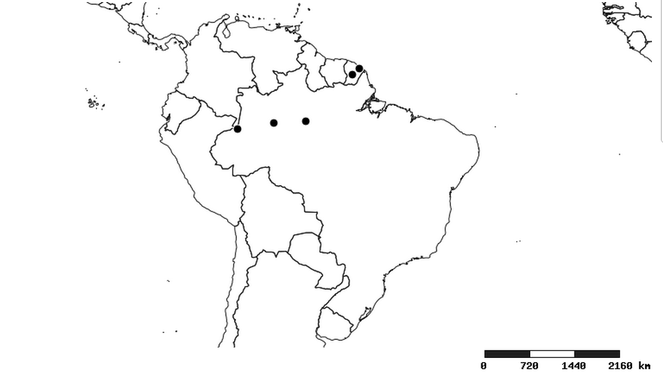
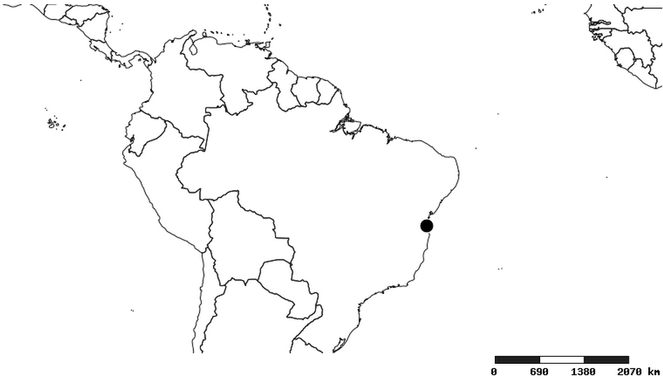
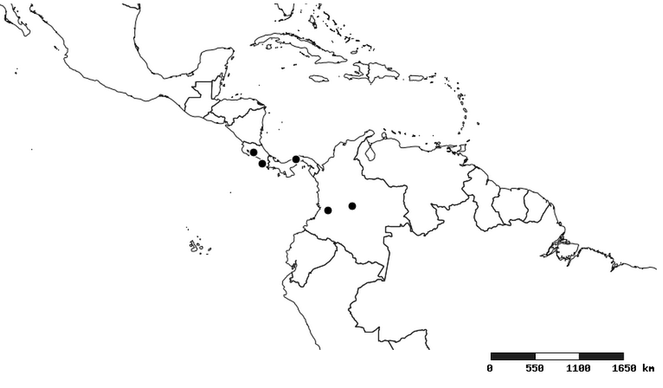
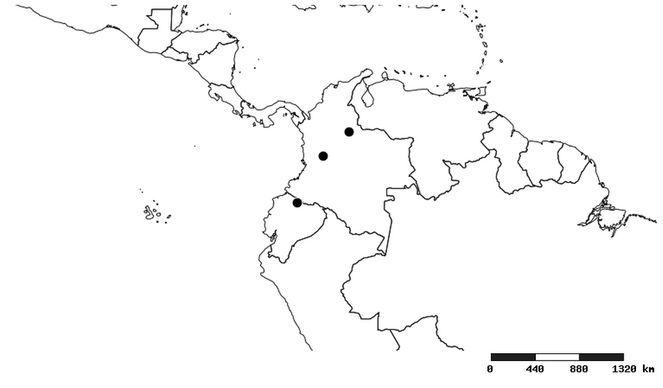
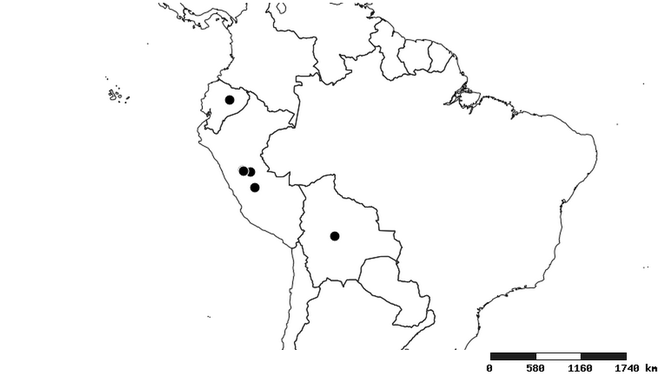
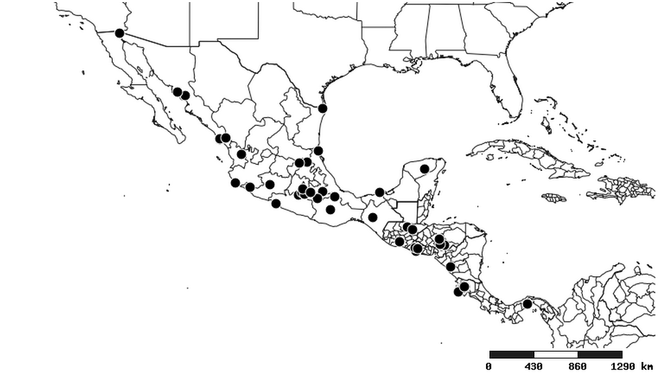
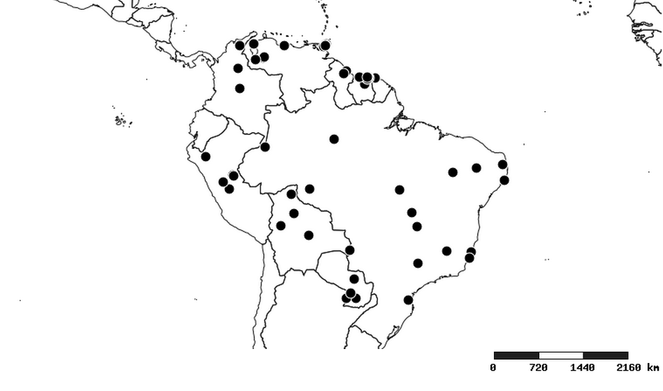
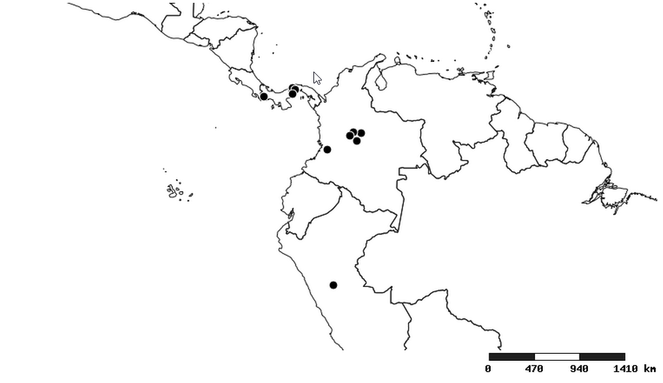
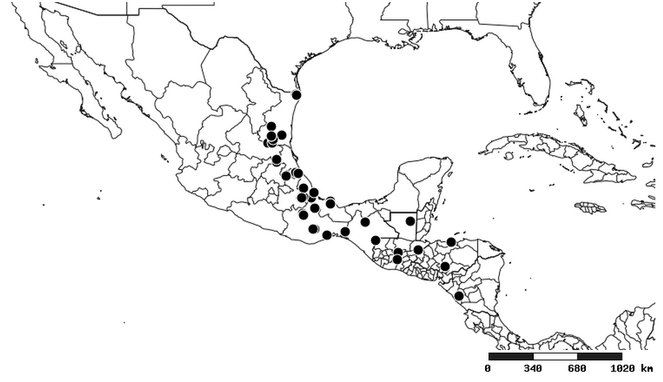
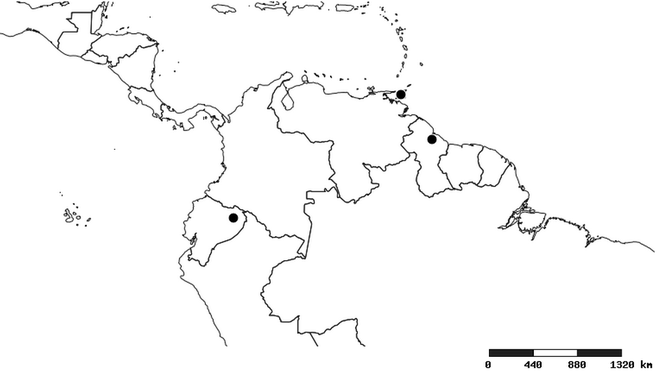
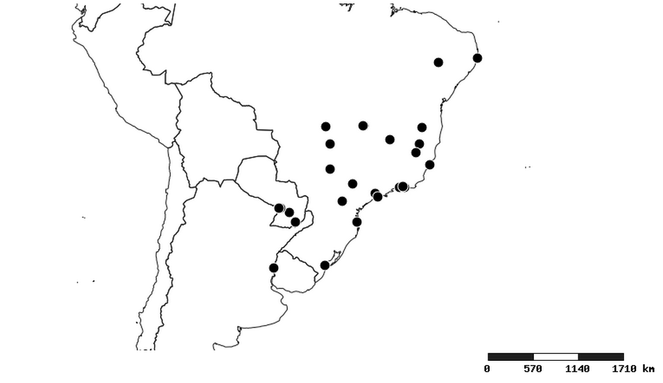
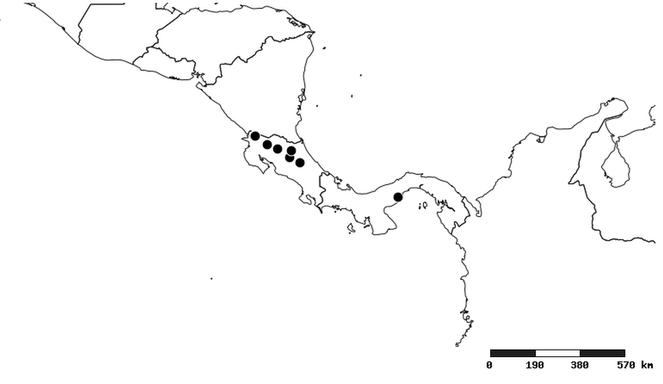
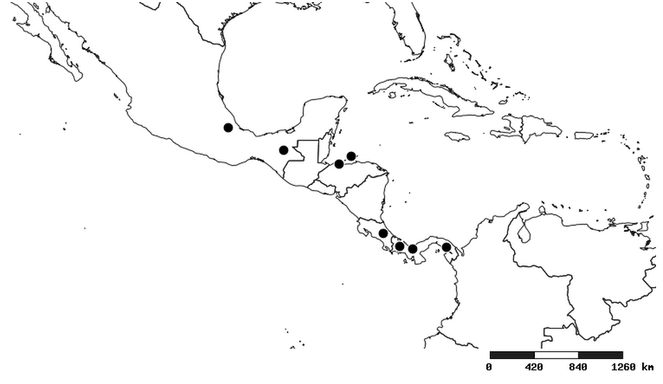
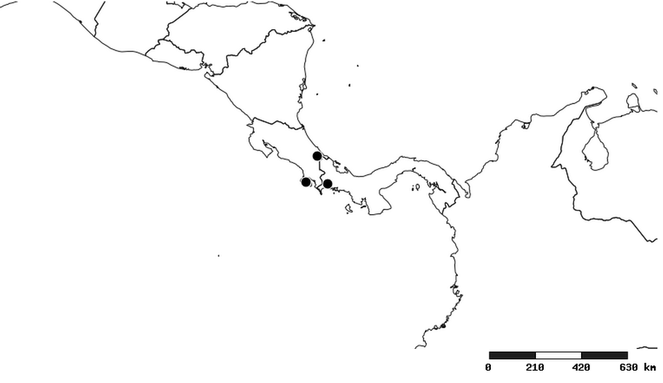
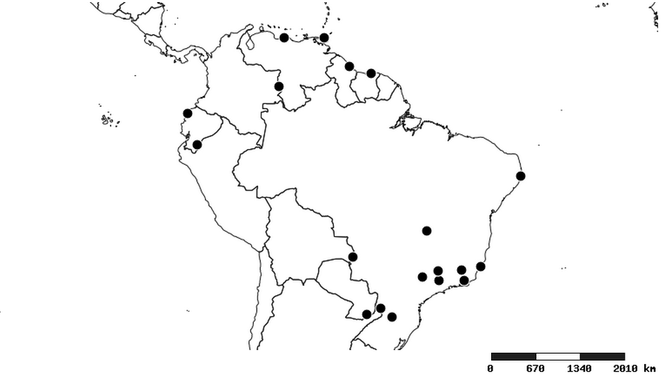
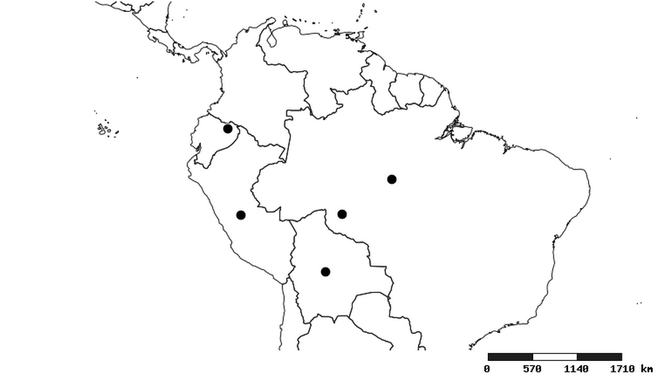
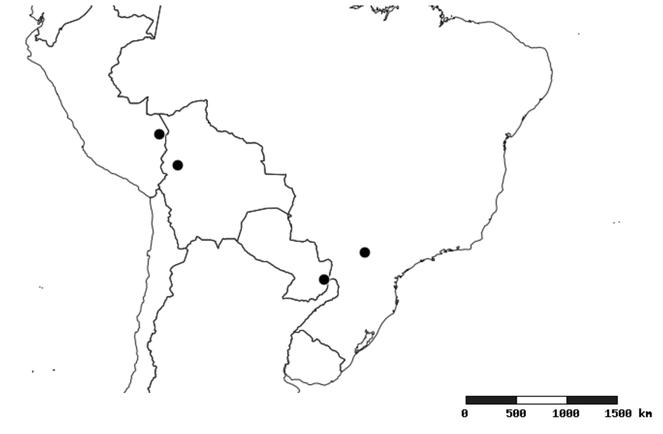
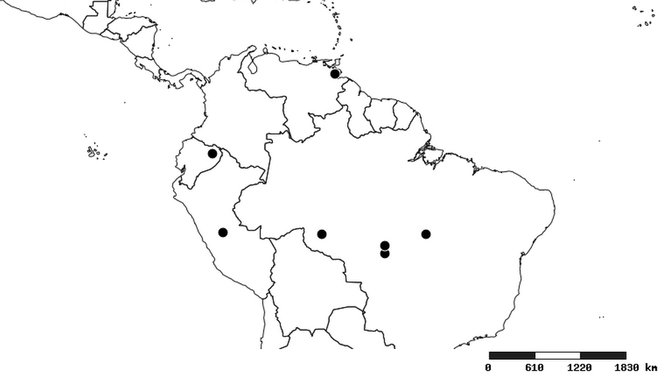
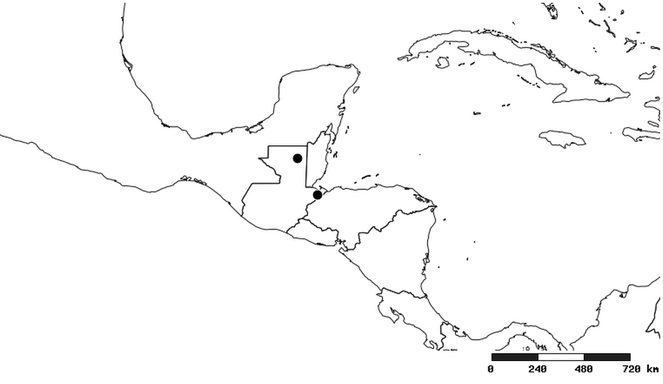
Distribution
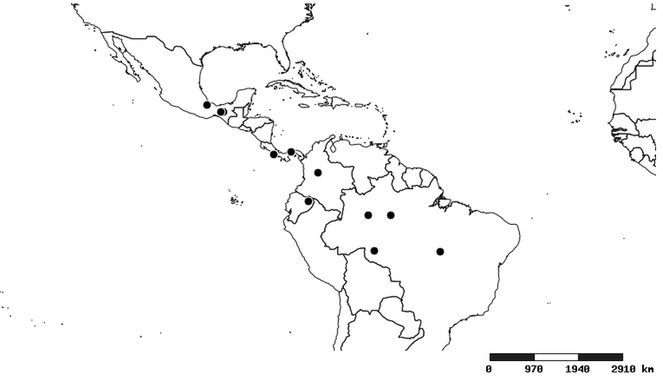
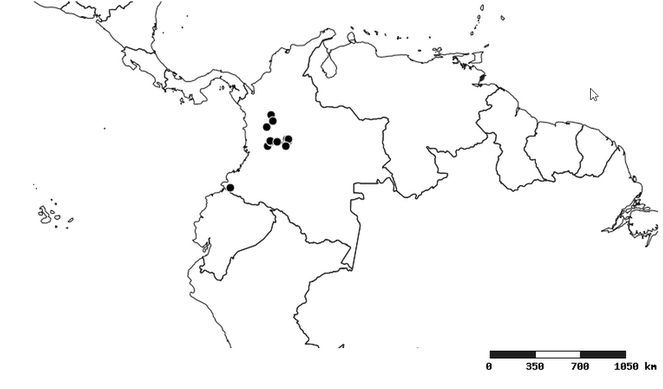
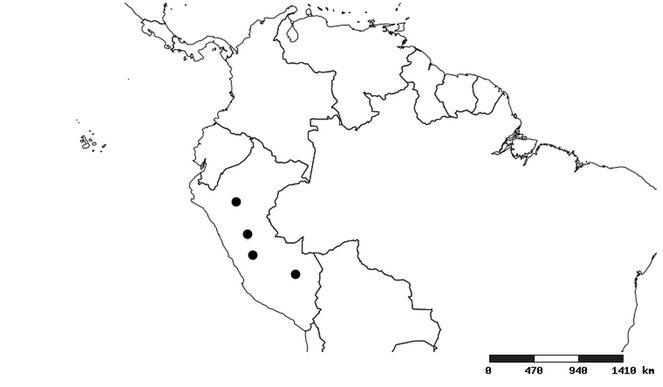
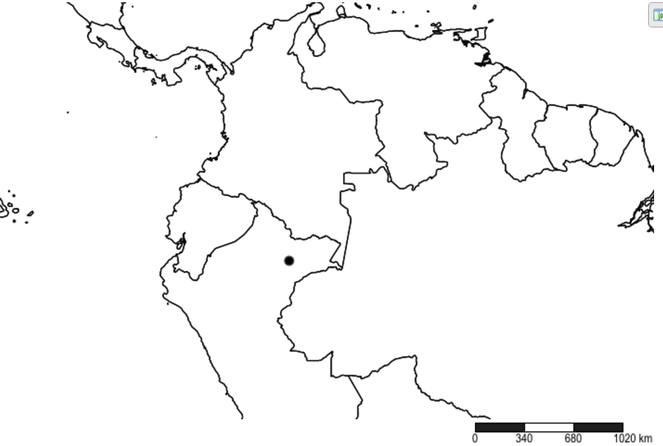
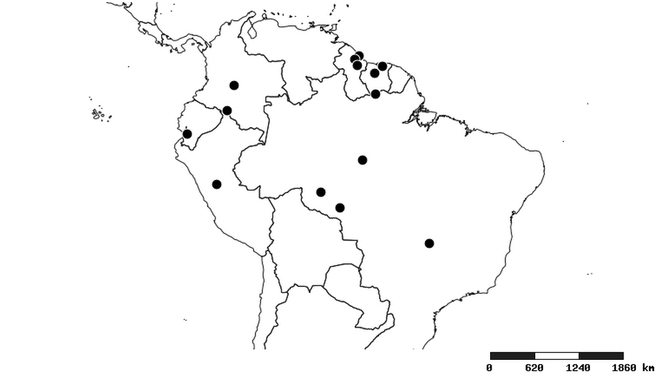
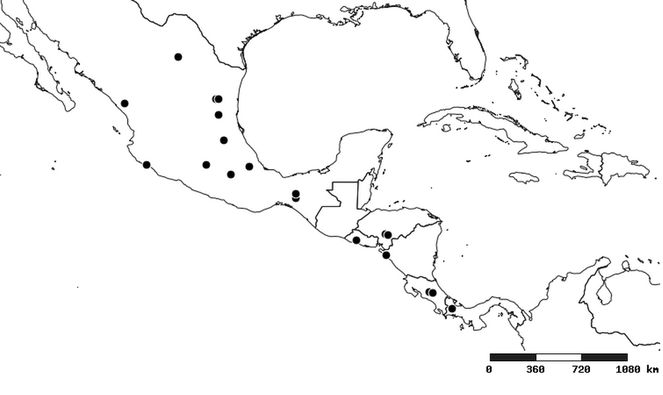
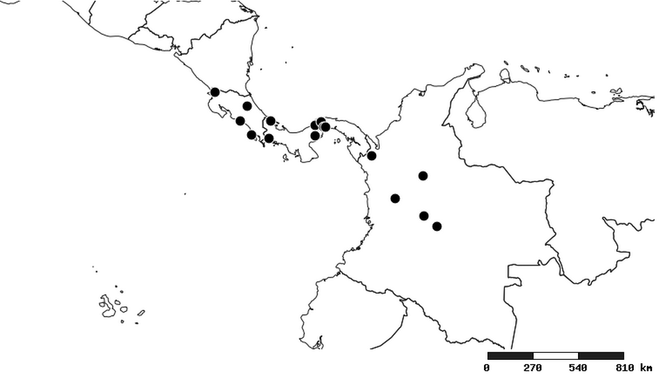
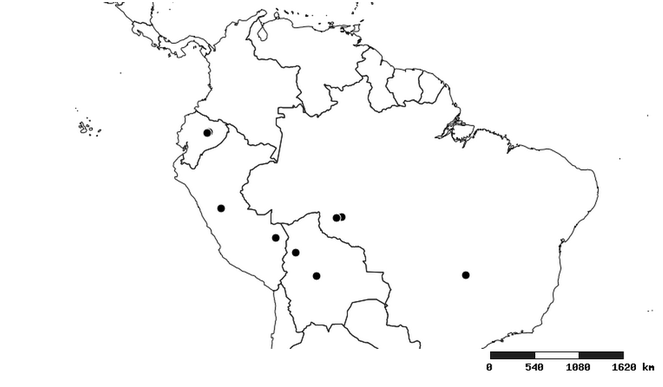
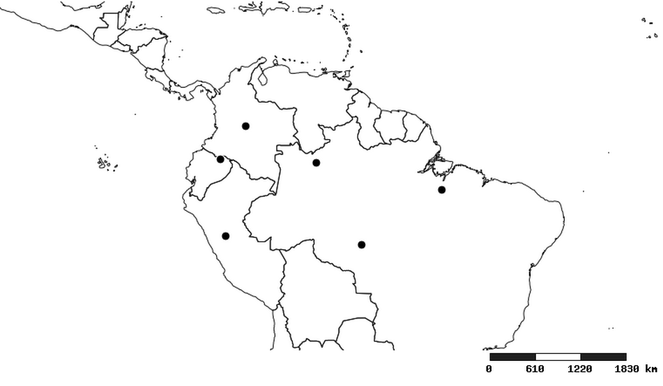
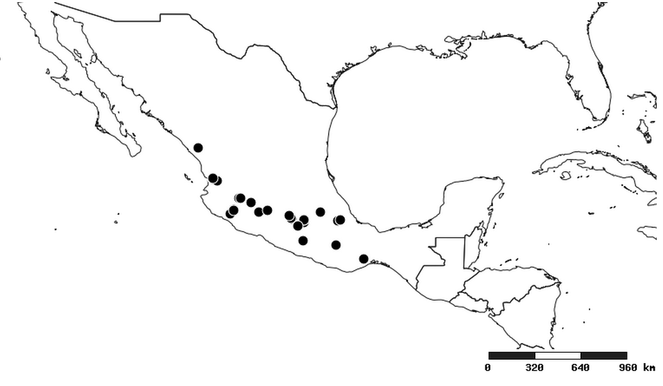
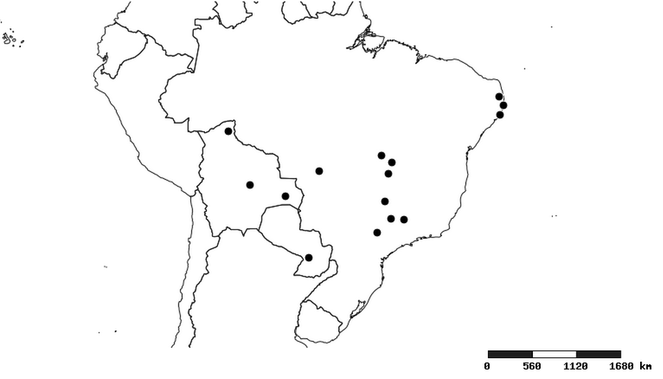
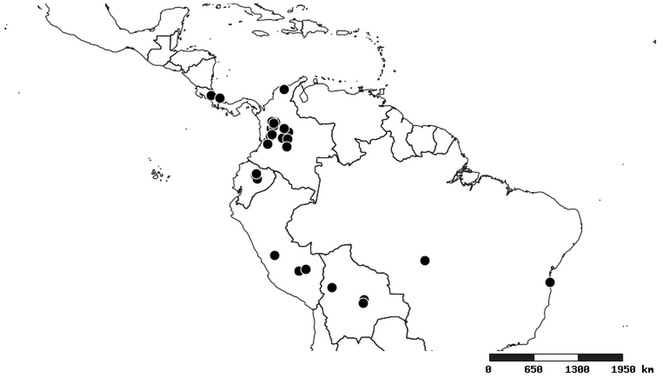
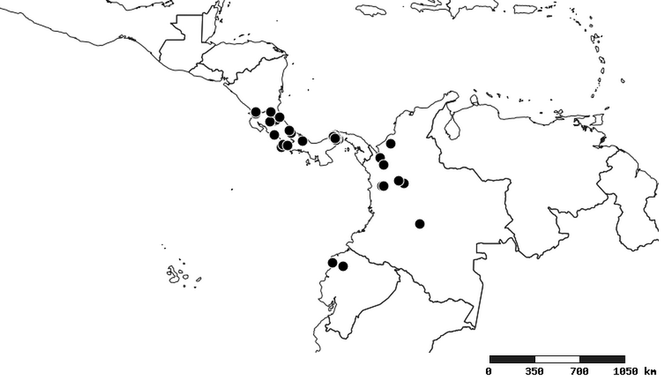
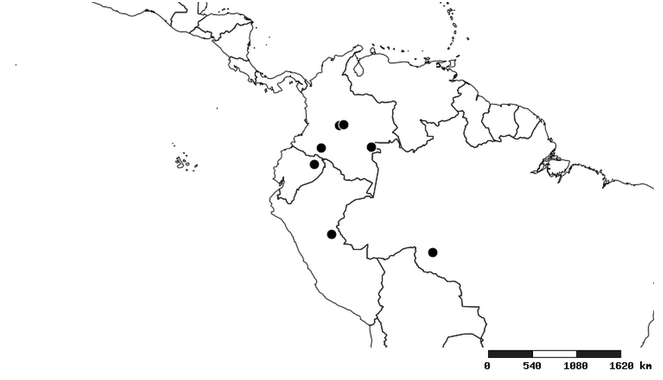
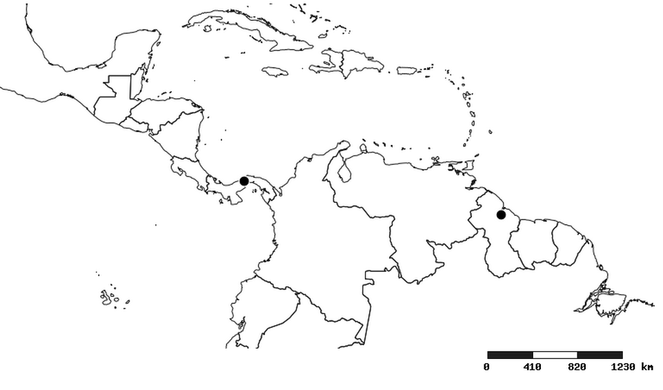
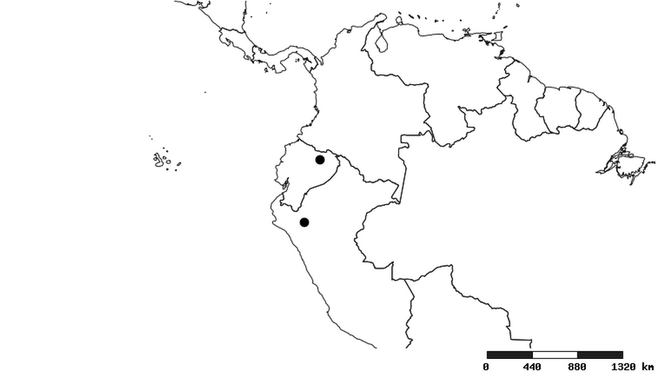
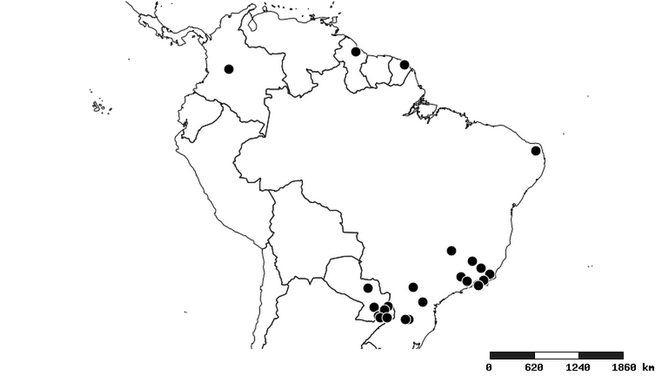
South America (
Zelus amblycephalus , sp. n.
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Golfito; decimalLatitude:8.6407; decimalLongitude:-83.1686; georeferenceSources:Google Earth; eventDate:1957-07-13; sex:Adult Male; catalogNumber:UCR_ENT 00022669; occurrenceRemarks:Holotype of Zelus amblycephalus Zhang and Hart, 2016; recordedBy:A. S. Menke; otherCatalogNumbers:LACM ENT 160232; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:LACM
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Amazonas; locality:Rio Janauaca, 40 km SW Manaus; decimalLatitude:-3.33333; decimalLongitude:-60.28333; georeferenceSources:Label; eventDate:1979-03-10; sex:Adult Male; catalogNumber:UCR_ENT 00009315; occurrenceRemarks:Paratype of Zelus amblycephalus Zhang and Hart, 2016; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Amazonas; locality:Teffe; decimalLatitude:-3.3667; decimalLongitude:-64.7; eventDate:1918-12-06; sex:Adult Male; catalogNumber:UCR_ENT 00009316; occurrenceRemarks:Paratype of Zelus amblycephalus Zhang and Hart, 2016; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Mato Grosso; locality:Barra do Tapirape; decimalLatitude:-10.46666; decimalLongitude:-50.51667; georeferenceSources:Gazetteer; eventDate:1962-12-30; sex:Adult Male; catalogNumber:UCR_ENT 00006070; occurrenceRemarks:Paratype of Zelus amblycephalus Zhang and Hart, 2016; recordedBy:B. Malkin; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km SW of Ariquemes, near Fzda. Rancho Grande; decimalLatitude:-10.32921; decimalLongitude:-63.46881; eventDate:1996-12-03 to 1996-12-15; sex:Adult Male; catalogNumber:UCR_ENT 00029368; occurrenceRemarks:Paratype of Zelus amblycephalus Zhang and Hart, 2016. Drake Collection; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Cundinamarca; locality:Villeta; verbatimElevation:799 m; decimalLatitude:5.01444; decimalLongitude:-74.47305; georeferenceSources:Label; eventDate:2003-05-10; sex:Adult Male; catalogNumber:UCR_ENT 00025328; occurrenceRemarks:Paratype of Zelus amblycephalus Zhang and Hart, 2016; recordedBy:C. Ardila, A. Montano, A. Pachon; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:UNAB
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Golfito; decimalLatitude:8.6407; decimalLongitude:-83.1686; georeferenceSources:Google Earth; eventDate:1957-07-13; sex:Adult Female; catalogNumber:UCR_ENT 00022670; occurrenceRemarks:Paratype of Zelus amblycephalus Zhang and Hart, 2016. Previously designated as 'allotype' of his manuscript name Zelus amblycephalus by Hart, a type status not used in the formal publication of this name (Zhang, Hart & Weirauch, 2016).; recordedBy:A. S. Menke; otherCatalogNumbers:LACM ENT 160233; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:LACM
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Orellana; locality:Reserva Etnica Waorani, 1 km S. Onkone Gare Camp, Transect Ent.; verbatimElevation:216 m; decimalLatitude:-0.65714; decimalLongitude:-76.453; georeferenceSources:Label; samplingProtocol:Fogging; eventDate:1994-10-09; sex:Adult Male; catalogNumber:UCR_ENT 00009473; occurrenceRemarks:Paratype of Zelus amblycephalus Zhang and Hart, 2016; recordedBy:T. L. Erwin et al.; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:MEXICO; stateProvince:Chiapas; locality:10 m N of Mexico 190 Tuztla Gutierrez; decimalLatitude:16.90574; decimalLongitude:-93.16486; georeferenceSources:Google Earth; eventDate:1956-08-24 to 1956-08-28; sex:Adult Male; catalogNumber:UCR_ENT 00010840; occurrenceRemarks:Paratype of Zelus amblycephalus Zhang and Hart, 2016; recordedBy:A. Lewis; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:LACM
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:MEXICO; stateProvince:Chiapas; locality:Reserva El Ocote; decimalLatitude:16.99502; decimalLongitude:-93.64056; georeferenceSources:Google Earth; eventDate:1993-12-02 to 1993-12-10; sex:Adult Female; catalogNumber:UCR_ENT 00034277; occurrenceRemarks:Paratype of Zelus amblycephalus Zhang and Hart, 2016; recordedBy:G. Ortega, E. Barrera, A. Casasola; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:IBUNAM
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:MEXICO; stateProvince:Oaxaca; locality:Temascal; decimalLatitude:18.23882; decimalLongitude:-96.40034; eventDate:1963-10-31; sex:Adult Male; catalogNumber:UCR_ENT 00009493; occurrenceRemarks:Paratype of Zelus amblycephalus Zhang and Hart, 2016. Drake Collection; recordedBy:D.H. Jansen; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:MEXICO; stateProvince:Oaxaca; locality:Temascal; decimalLatitude:18.23882; decimalLongitude:-96.40034; eventDate:1963-10-31; sex:Adult Male; catalogNumber:UCR_ENT 00009494; occurrenceRemarks:Paratype of Zelus amblycephalus Zhang and Hart, 2016. Drake Collection; recordedBy:D.H. Jansen; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:MEXICO; stateProvince:Oaxaca; locality:Temascal; decimalLatitude:18.23882; decimalLongitude:-96.40034; eventDate:1963-10-31; sex:Adult Male; catalogNumber:UCR_ENT 00009495; occurrenceRemarks:Paratype of Zelus amblycephalus Zhang and Hart, 2016. Drake Collection; recordedBy:D.H. Jansen; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:MEXICO; stateProvince:Oaxaca; locality:Temascal; decimalLatitude:18.23882; decimalLongitude:-96.40034; eventDate:1963-10-31; sex:Adult Male; catalogNumber:UCR_ENT 00009496; occurrenceRemarks:Paratype of Zelus amblycephalus Zhang and Hart, 2016. Drake Collection; recordedBy:D.H. Jansen; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:MEXICO; stateProvince:Oaxaca; locality:Temascal; decimalLatitude:18.23882; decimalLongitude:-96.40034; eventDate:1963-10-31; sex:Adult Male; catalogNumber:UCR_ENT 00009497; occurrenceRemarks:Paratype of Zelus amblycephalus Zhang and Hart, 2016. Drake Collection; recordedBy:D.H. Jansen; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Canal Zone; locality:Barro Colorado; decimalLatitude:9.16666; decimalLongitude:-79.83333; georeferenceSources:Google Earth; eventDate:1941-04-01; sex:Adult Male; catalogNumber:UCR_ENT 00009270; occurrenceRemarks:Paratype of Zelus amblycephalus Zhang and Hart, 2016. Additional label: Collected at Night.; recordedBy:J. Zetek; otherCatalogNumbers:41-7231; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:SURINAME; stateProvince:Unknown; locality:unknown; decimalLatitude:5.804157; decimalLongitude:-55.149886; eventDate:1965-12-12; sex:Adult sex unknown; catalogNumber:UCR_ENT 00023698; occurrenceRemarks:Paratype of Zelus amblycephalus Zhang and Hart, 2016; recordedBy:Geyskes; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:RMNH
-
scientificName: Zelus amblycephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:MEXICO; stateProvince:Chiapas; locality:Tuxtla Gutierrez; verbatimElevation:549 m; decimalLatitude:16.75469; decimalLongitude:-93.11485; eventDate:1955-07-06 to 1955-07-10; sex:Adult Female; catalogNumber:UCR_ENT 00017182; occurrenceRemarks:Paratype of Zelus amblycephalus Zhang and Hart, 2016; recordedBy:P. & C. Vaurie; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
Description
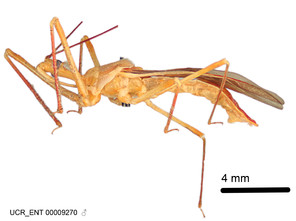
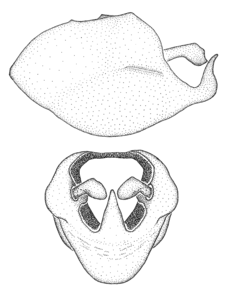
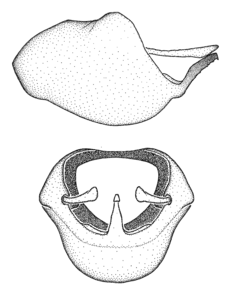
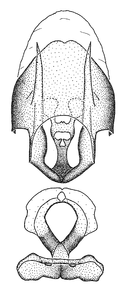
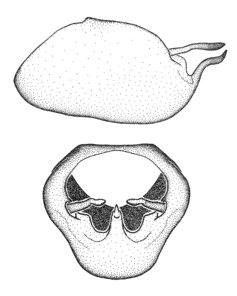
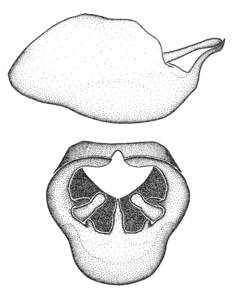
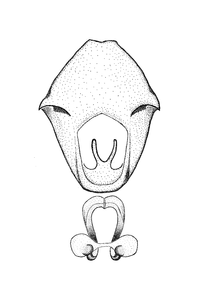
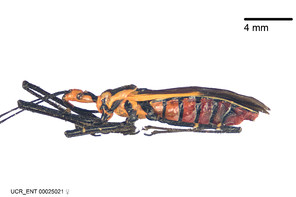
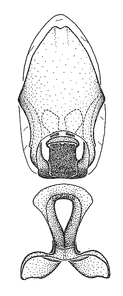
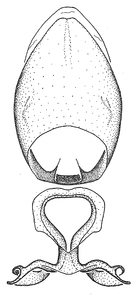
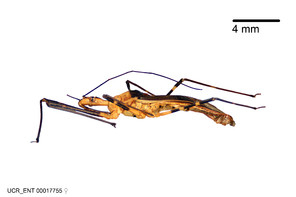
Zelus amblycephalus Zhang & Hart, sp. n., habitus
b: Zelus amblycephalus Zhang & Hart, sp. n., male, lateral (UCR_ENT 00009270, Canal Zone, Panama)
c: Zelus amblycephalus Zhang & Hart, sp. n., female, dorsal (UCR_ENT 00017182, Chiapas, Mexico)
d: Zelus amblycephalus Zhang & Hart, sp. n., female, lateral (UCR_ENT 00017182, Chiapas, Mexico)
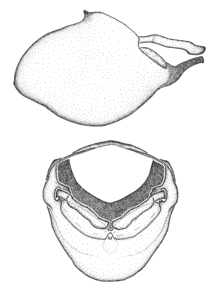
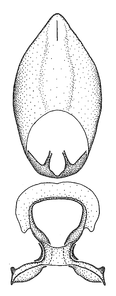
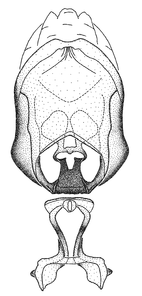
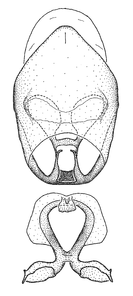
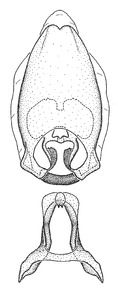
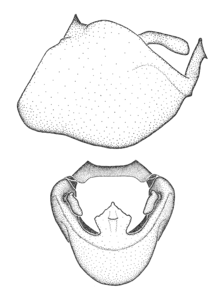
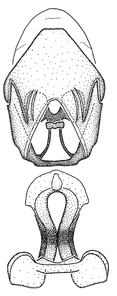
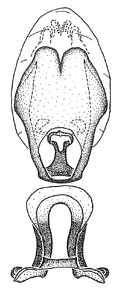
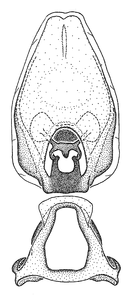
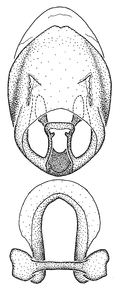
Zelus amblycephalus Zhang & Hart, sp. n., male genitalic structures
b: Zelus amblycephalus Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: (
Diagnosis
Can be recognized by the uniform pale coloration, the unpatterned legs (
Among species of the Zelus armillatus group (
Distribution
Southern Mexico to northern South America and part of Brazil (
Zelus ambulans
Nomenclature
Zelus ambulans Stål, 1862, p. 451, orig. descr.; Stål, 1872, p. 91, cat. (subgenus Diplodus); Lethierry and Severin, 1896, p. 151, cat.; Champion, 1898, p. 259–260, Tab. XV. fig. 23, 23a, junior syn. of Z. exsanguis; Maldonado, 1990, p. 327. cat. and junior syn. of Z. exsanguis. stat. rev. (current study).
Diplodus ambulans: Uhler, 1886, p. 24, checklist; Walker, 1873, cat.
-
scientificName: Zelus ambulans; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; stateProvince:unknown; eventDate:No date provided; sex:Adult Male; catalogNumber:UCR_ENT 00040998; occurrenceRemarks:Lectotype of Zelus ambulans Stål, 1862 (New Designation by Zhang, Hart & Weirauch, 2016). Verbatim label info: Mexico / Salle / ambulans Stal. / Lectotype Zelus ambulans Stal / designated by E. R. Hart / Typus / NHRS-GULI 000000318; recordedBy:Salle; otherCatalogNumbers:NHRS-GULI 000000318; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:NHRS
-
scientificName: Zelus ambulans; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; stateProvince:unknown; eventDate:No date provided; sex:Adult Male; occurrenceRemarks:Paralectotype of Zelus ambulans Stål, 1862 (New Designation by Zhang, Hart & Weirauch, 2016). Verbatim label info: Mexico / Salle / Allotypus / Zelus ambulans Stal; recordedBy:Salle; institutionCode:NHRS
-
scientificName: Zelus ambulans; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; stateProvince:unknown; eventDate:No date provided; sex:Adult Male; occurrenceRemarks:Paralectotype of Zelus ambulans Stål, 1862. (New Designation by Zhang, Hart & Weirauch, 2016). Verbatim label info: Mexico Coll. Signoret / det. Stal; recordedBy:Signoret; institutionCode:NHMW
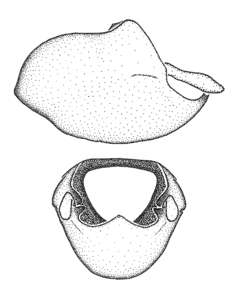
Description
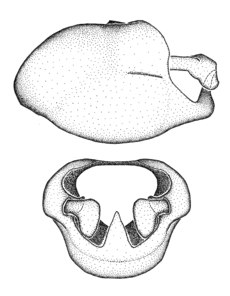
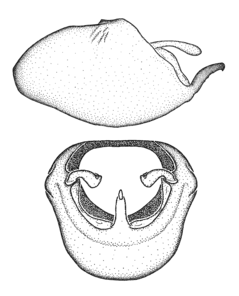
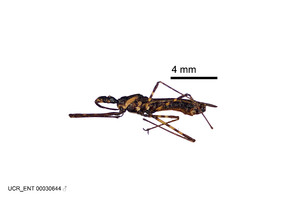
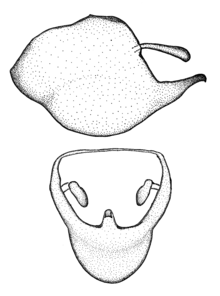
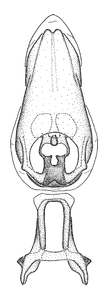
Zelus ambulans Stål, 1862, habitus
b: Zelus ambulans Stål, 1862, male, lateral (UCR_ENT 00009285, Veracruz, Mexico)
c: Zelus ambulans Stål, 1862, female, dorsal (UCR_ENT 00017884, Chiriqui, Panama)
Zelus ambulans Stål, 1862, male genitalic structures
b: Zelus ambulans Stål, 1862, phallus, dorsal view
Male: (
Female: (
Diagnosis
Among the species of Zelus luridus group, Z. ambulans has the humeral angle elevated to level of, and continuous with, disc of the posterior pronotal lobe, a condition that is also present in Z. exsanguis, but it can be separated from that species by the yellowish veins on corium, contrasting to the brown corium, whereas the entire corium is more or less uniformly colored in Z. exsanguis.
Among species of the Zelus luridus species group (
Distribution
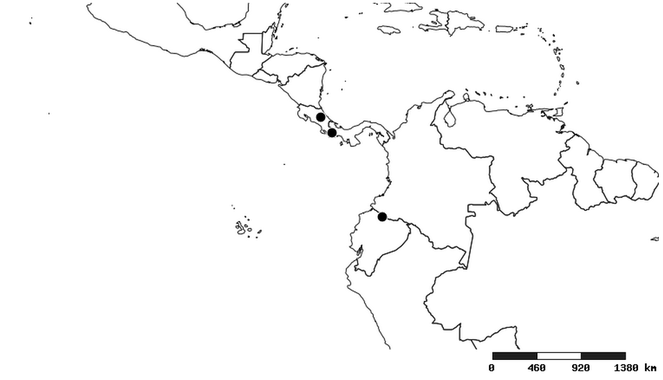
North and Central America (
Taxon discussion
Although this species shows very little morphological variations, color patterns within an area do vary considerably. The dark area at the posterior margin of the longitudinal medial sulcus of the anterior lobe, which serves to easily distinguish Z. ambulans from Z. exsanguis, is relatively constant. Other colors, specifically that of the posterior pronotal lobe and the femoral apices vary from quite light to very dark brown in any given locality. There is also an occasional specimen with somewhat darker hemelytron, but this does not show the wide range of variations of the aforementioned characters.
Most specimens examined have been collected from moderate to high altitudes.
Zelus annulosus
Nomenclature
Diplodus annulosus Stål, 1866, p. 299, orig. descr.; Walker, 1873, p. 126, cat.
Zelus annulosus: Stål, 1872, p. 92, cat. (subgenus Diplodus); Lethierry and Severin, 1896, p. 151, cat.; Fracker and Bruner, 1924, p. 170, note; Wygodzinsky, 1949a, p. 48, checklist; Maldonado, 1990, p. 326, cat.
-
scientificName: Zelus annulosus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:(Stål, 1866); country:unknown; stateProvince:unknown; locality:Amazon; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00040999; occurrenceRemarks:Verbatim label info: Amazon / Stevens. / annulosus Stal. / Typus / NHRS-GULI 000000319; recordedBy:Stevens; otherCatalogNumbers:NHRS-GULI 000000319; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:NHRS
Description
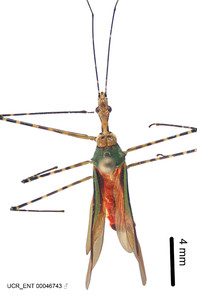
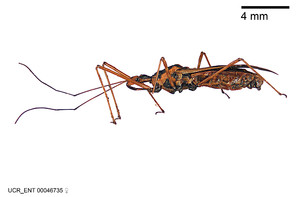
Zelus annulosus (Stål, 1866), habitus
b: Zelus annulosus (Stål, 1866), male, lateral (UCR_ENT 00046743, French Guiana)
Zelus annulosus (Stål, 1866), male genitalic structures
b: Zelus annulosus (Stål, 1866), phallus, dorsal view
Male: (
Female: Similar to male, except for the following. Larger than male, total length 21.19–22.72 mm (mean 21.91 mm,
Diagnosis
Recognized by the following combination of characters: the posterior pronotal and corium dark green; the legs with four to five alternative yellow and black bands; the head, pronotum, scutellum and corium with moderately dense, black, erect, spine-like setae; the rather long and slender legs, the profemur 1/2 of body length; the rather long postocular lobe, enlarged at posterior 3/4; and the quadrate cell on corium rather slender, length more than 2x width.
Males can also be recognized by the long paramere, reaching apex of medial process; the apex of paramere recurved; the medial process apically with two lateral sharp projections; the membranous sclerite between paramere and medial process, not distinctly protruding posteriorly; and the dorsal phallothecal sclerite with lateral expansion close to basal arm, sharp, dorsad.
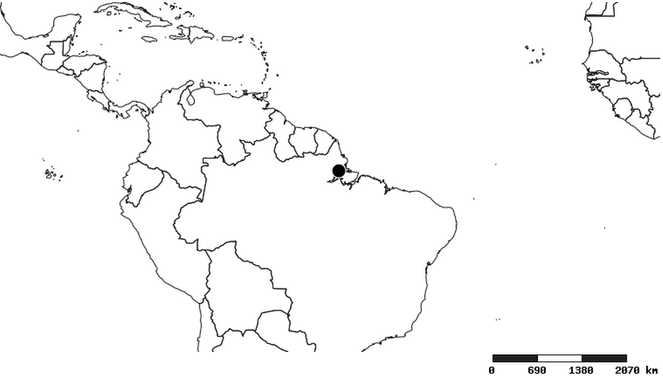
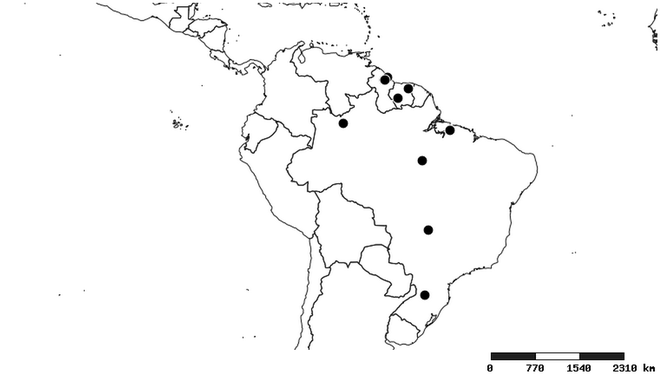
Distribution
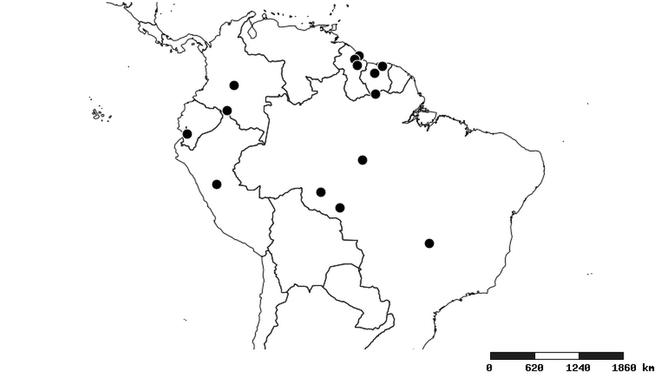
South America (
Zelus antiguensis , sp. n.
-
scientificName: Zelus antiguensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:GUATEMALA; stateProvince:Sacatepequez; locality:Antigua; verbatimElevation:1583 m; decimalLatitude:14.5611; decimalLongitude:-90.7344; georeferenceSources:Gazetteer; eventDate:no date provided; sex:Adult Male; catalogNumber:UCR_ENT 00007995; recordedBy:B. Lott; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus antiguensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:GUATEMALA; stateProvince:Sacatepequez; locality:Antigua; verbatimElevation:1583 m; decimalLatitude:14.5611; decimalLongitude:-90.7344; georeferenceSources:Gazetteer; eventDate:no date provided; sex:Adult Male; catalogNumber:UCR_ENT 00007955; occurrenceRemarks:Genitallia dissected; recordedBy:B. Lott; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus antiguensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:GUATEMALA; stateProvince:Sacatepequez; locality:Antigua; decimalLatitude:14.56667; decimalLongitude:-90.73333; georeferenceSources:Gazetteer; eventDate:1965-10-01; sex:Adult Female; catalogNumber:UCR_ENT 00009305; recordedBy:N. L. H. Krauss; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus antiguensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:GUATEMALA; stateProvince:Sacatepequez; locality:Antigua; verbatimElevation:1583 m; decimalLatitude:14.5611; decimalLongitude:-90.7344; georeferenceSources:Gazetteer; eventDate:1930-07-01; sex:Adult Female; catalogNumber:UCR_ENT 00015069; recordedBy:D. M. Bates; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus antiguensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:GUATEMALA; stateProvince:Sacatepequez; locality:Antigua; verbatimElevation:1583 m; decimalLatitude:14.5611; decimalLongitude:-90.7344; georeferenceSources:Gazetteer; eventDate:1951-09-12; sex:Adult Female; catalogNumber:UCR_ENT 00029478; recordedBy:R. H. Painter; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus antiguensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:MEXICO; stateProvince:Chiapas; locality:Tuxtla Gutierrez; verbatimElevation:549 m; decimalLatitude:16.75469; decimalLongitude:-93.11485; eventDate:1955-07-06 to 1955-07-10; sex:Adult Female; catalogNumber:UCR_ENT 00017184; recordedBy:P. & C. Vaurie; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus antiguensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:MEXICO; stateProvince:Jalisco; locality:Pine Forst 87 miles S of Manzamitla; decimalLatitude:19.17323; decimalLongitude:-103.66112; georeferenceSources:Google Earth; eventDate:1948-12-01; sex:Adult Female; catalogNumber:UCR_ENT 00006071; recordedBy:H. B. Leech; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus antiguensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:MEXICO; stateProvince:Jalisco; locality:Pine Forst 87 miles S of Manzamitla; decimalLatitude:19.17323; decimalLongitude:-103.66112; georeferenceSources:Google Earth; eventDate:1948-12-01; sex:Adult Female; catalogNumber:UCR_ENT 00019699; recordedBy:E. S. Ross; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus antiguensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:MEXICO; stateProvince:Jalisco; locality:6 mi W of Chapala; decimalLatitude:20.29709; decimalLongitude:-103.28149; georeferenceSources:Google Earth; eventDate:1963-06-30; sex:Adult Female; catalogNumber:UCR_ENT 00038423; recordedBy:J. Doyen; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:UCB
-
scientificName: Zelus antiguensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:MEXICO; stateProvince:Veracruz; locality:Jalapa; decimalLatitude:19.54381; decimalLongitude:-96.90993; georeferenceSources:Google Earth; eventDate:no date provided; sex:Adult Female; catalogNumber:UCR_ENT 00023699; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:RMNH
Description
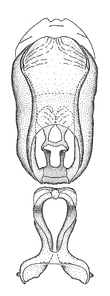
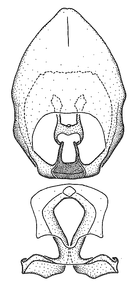
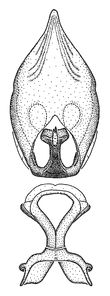
Zelus antiguensis Zhang & Hart, sp. n., habitus
b: Zelus antiguensis Zhang & Hart, sp. n., male, lateral (UCR_ENT 00007995, Sacatepequez, Guatemala)
c: Zelus antiguensis Zhang & Hart, sp. n., female, lateral (UCR_ENT 00029478, Sacatepequez, Guatemala)
d: Zelus antiguensis Zhang & Hart, sp. n., female, lateral (UCR_ENT 00029478, Sacatepequez, Guatemala)
Zelus antiguensis Zhang & Hart, sp. n., male genitalic structures
b: Zelus antiguensis Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: (
Diagnosis
As with several members of the Zelus luridus species group, the coloration is greenish-brown, rather uniform. The medial process is triangular, its base distinct from the rest of pygophore ventral rim and apex without modification. Can be distinguished from males of other species of the Zelus luridus species group (
Zelus antiguensis is similar in appearance to Z. luridus and might easily be confused with that species. The comparatively broader medial process and posterior protrusion of the base of the medial process are readily evident in Z. antiguensis (
Etymology
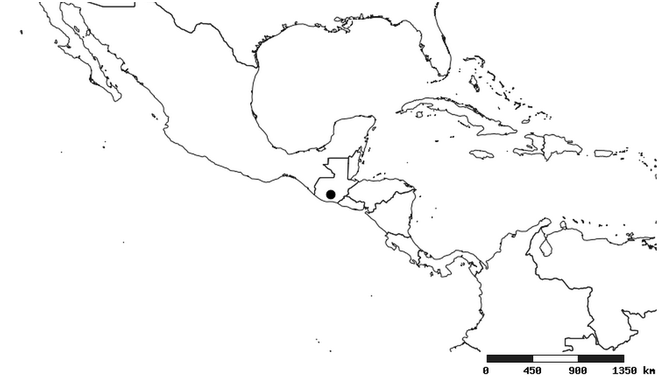
Named after the type locality, Antigua, in Guatemala.
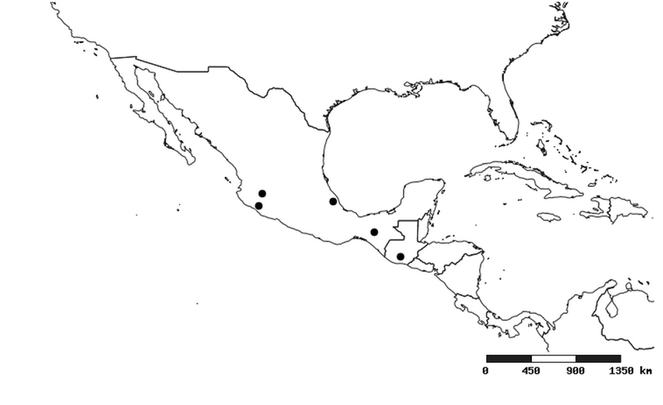
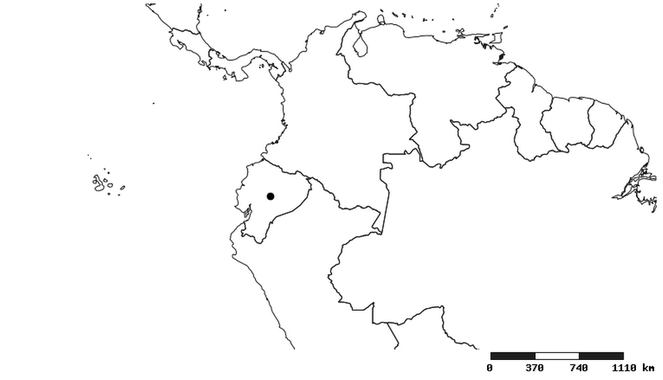
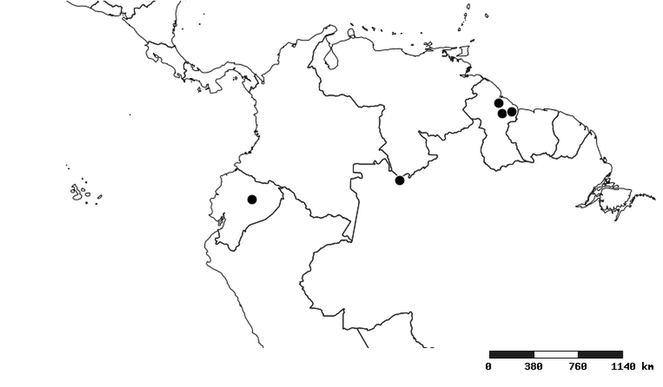
Distribution
Southern Mexico and Guatemala (
Zelus armillatus
Nomenclature
Reduvius armillatus Lepeletier and Serville, 1825, p. 278, orig. descr.
Diplodus armillatus: Amyot and Serville, 1843, p. 370, descr.; Stål, 1860, p. 75, list; Walker, 1873, p. 123, cat.
Euagoras armillatus: Herrich-Schaeffer, 1853, p. 91, list.
Zelus armillatus: Stål, 1872, p. 90, cat. (subgenus Diplodus); Lethierry and Severin, 1896, p. 151, cat.; Wygodzinsky, 1949a, p. 49, checklist; Mayr, 1866, p. 138-139, senior syn. of Z. brasiliensis, Z. aurantiacus, Z. guttifer and Z. conjungens; Berg, 1879, p. 151-152, list and nymphs (subgenus Diplodus); Costa Lima, 1940, 218, list (subgenus Diplocodus); Wygodzinsky, 1957, p. 268, note; Wygodzinsky, 1960; p. 307, list; Maldonado, 1990, p. 326, cat.; Van der Heyden, p. 85-90, new record (misidentification, should be Zelus atripes).
Reduvius brasiliensis Lepeletier and Serville, 1825, p. 278, orig. descr.
Diplodus brasiliensis: Amyot and Serville, 1843, p. 370, descr.; Stål, 1860, p. 75, note; Mayr, 1866, p. 138- 139, junior syn. of Z. armillatus; Walker, 1873, p. 123, cat.
Euagoras brasiliensis: Herrich-Schaeffer, 1853, p. 91, list.
Zelus brasiliensis: Stål, 1872, p. 90, cat. (subgenus Diplodus); Lethierry and Severin, 1896, p. 151, junior syn. of Z. armillatus.
Arilus aurantiacus Herrich-Schaeffer, 1848, p. 35-36 Tab. CCLXI. fig. 809, orig. descr. and fig.; Mayr, 1866, p. 138-139, junior syn. of Z. armillatus; Stål, 1872, p. 90, junior syn. of Z. armillatus.
Euagoras aurantiacus: Herrich-Schaeffer, 1853, p. 91, list (aurantius (sic)).
Ploeogaster aurantiacus: Herrich-Schaeffer, 1853, p. 168, list.
Arilus guttifer Herrich-Schaeffer, 1848, p. 36, Tab. CCLXI, fig. 810, orig. descr. and fig.; Mayr, 1866, p. 138-139, junior syn. of Z. armillatus; Stål, 1872, p. 90, junior syn. of Z. brasiliensis.
Euagoras guttifer: Herrich-Schaeffer, 1853, p. 92, list.
Ploeogaster guttifer: Herrich-Schaeffer, 1853, p. 168, list.
Diplodus guttifer: Stål , 1860, p. 74, descr.; Walker, 1873, p. 126, cat.
Zelus guttifer: Stål 1862, p. 453, note.
Arilus guttifer , Mayr, 1866, p. 138-139, junior syn. of Z. armillatus .
Description
Zelus armillatus (Lepeletier & Serville, 1825), habitus, males
b: Zelus armillatus (Lepeletier & Serville, 1825) male, lateral (UCR_ENT 00008021, Santa Catarina, Brazil)
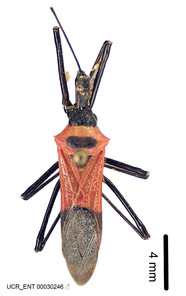
c: Zelus armillatus (Lepeletier & Serville, 1825) male, dorsal (UCR_ENT 00030246, Santa Catarina, Brazil)
d: Zelus armillatus (Lepeletier & Serville, 1825) male, dorsal (UCR_ENT 00019103, Santa Catarina, Brazil)
Zelus armillatus (Lepeletier & Serville, 1825), habitus, females
b: Zelus armillatus (Lepeletier & Serville, 1825), female, lateral (UCR_ENT 00019117, Huanuco, Peru)
c: Zelus armillatus (Lepeletier & Serville, 1825), female, dorsal (UCR_ENT 00019118, Huanuco, Peru)
d: Zelus armillatus (Lepeletier & Serville, 1825), female, dorsal (UCR_ENT 00030183, Junin, Peru)
e: Zelus armillatus (Lepeletier & Serville, 1825), female, dorsal (UCR_ENT 00019112, Huanuco, Peru)
f: Zelus armillatus (Lepeletier & Serville, 1825), female, dorsal (UCR_ENT 00019119, Huanuco, Peru)
Zelus armillatus (Lepeletier & Serville, 1825), habitus, females
b: Zelus armillatus (Lepeletier & Serville, 1825), female, dorsal (UCR_ENT 00029536, Junin, Peru)
c: Zelus armillatus (Lepeletier & Serville, 1825), female, dorsal (UCR_ENT 00017777, Huanuco, Peru)
d: Zelus armillatus (Lepeletier & Serville, 1825). Female specimens collected from Oct-Nov 1993 from Guanay, La Paz, Bolivia, showing a large range of color variations
Zelus armillatus (Lepeletier & Serville, 1825), male genitalic structures
b: Zelus armillatus (Lepeletier & Serville, 1825), phallus, dorsal
Male: (
Female: (
Diagnosis
The large and robust body, the dorsal coloration usually bright, yellow or red with black, the medial process short and relatively slender are characteristic to Z. armillatus. Male genitalic structures of Z. armillatus and Z. janus are nearly identical, but these two species do not overlap in range and are sufficiently different in non-genitalic morphological characters, which allow them to be easily separated.
The only species with which Z. armillatus is sympatric which may cause some identification problems is Z. conjungens. It may be distinguished from that species by the characters discussed under Z. conjungens.
Distribution
South America (
Taxon discussion
Zelus armillatus is a very common, widespread, variable species in South America. It is known to occur in nearly all areas of the continent from central Argentina and northward, at altitudes from sea level to several thousand feet, and dry temperate to moist tropical areas. The coloration and markings of Z. armillatus are highly variable throughout the range and appear to be as variable in any given area (e.g.,
Zelus auralanus , sp. n.
-
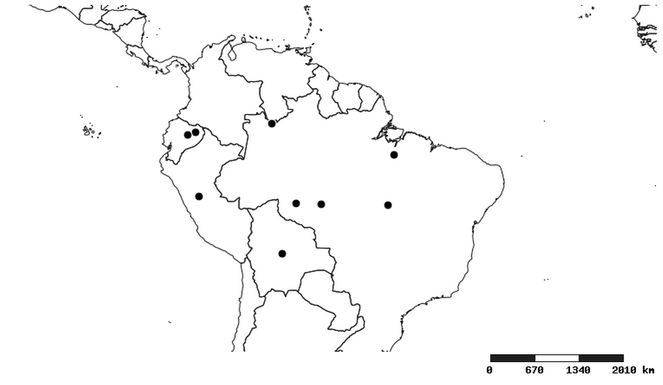
scientificName: Zelus auralanus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Amazonas; locality:Vista Alegre [in formerly-named 'Rio Branco' territory]; decimalLatitude:0.4578; decimalLongitude:-66.2489; georeferenceSources:Gazetteer; eventDate:1924-09-06; sex:Adult Male; catalogNumber:UCR_ENT 00069892; recordedBy:J. R. de la Torre-Bueno; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:KU
-
scientificName: Zelus auralanus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BOLIVIA; stateProvince:Cochabamba; locality:Sajta, Chapare; decimalLatitude:-17.00861; decimalLongitude:-64.78663; georeferenceSources:Google Earth; eventDate:1992-03-01; sex:Adult Female; catalogNumber:UCR_ENT 00009502; occurrenceRemarks:Drake Collection; recordedBy:L. E. Peńa; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus auralanus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Amazonas; locality:Vista Alegre [in formerly-named 'Rio Branco' territory]; decimalLatitude:0.4578; decimalLongitude:-66.2489; georeferenceSources:Gazetteer; eventDate:1924-09-06; sex:Adult Female; catalogNumber:UCR_ENT 00069893; occurrenceRemarks:Previously designated as 'allotype' of his manuscript name Zelus auralanus by Hart. This type status is not used in the formal publication of this name (Zhang et al.) and this specimen is instead designated as a paratype.; recordedBy:J. R. de la Torre-Bueno; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:KU
-
scientificName: Zelus auralanus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Amazonas; locality:Vista Alegre [in formerly-named 'Rio Branco' territory]; decimalLatitude:0.4578; decimalLongitude:-66.2489; georeferenceSources:Gazetteer; eventDate:1924-09-06; sex:Adult Male; catalogNumber:UCR_ENT 00069894; recordedBy:J. R. de la Torre-Bueno; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:KU
-
scientificName: Zelus auralanus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Mato Grosso; locality:Barra do Tapirape; decimalLatitude:-10.46666; decimalLongitude:-50.51667; georeferenceSources:Gazetteer; eventDate:1962-12-26; sex:Adult Female; catalogNumber:UCR_ENT 00006073; recordedBy:B. Malkin; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus auralanus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Mato Grosso; locality:Barra do Tapirape; decimalLatitude:-10.46666; decimalLongitude:-50.51667; georeferenceSources:Gazetteer; eventDate:1962-12-26; sex:Adult Male; catalogNumber:UCR_ENT 00019695; recordedBy:B. Malkin; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus auralanus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Mato Grosso; locality:Barra do Tapirape; decimalLatitude:-10.46666; decimalLongitude:-50.51667; georeferenceSources:Gazetteer; eventDate:1962-12-26; sex:Adult Female; catalogNumber:UCR_ENT 00019696; recordedBy:B. Malkin; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus auralanus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Mato Grosso; locality:Barra do Tapirape; decimalLatitude:-10.46666; decimalLongitude:-50.51667; georeferenceSources:Gazetteer; eventDate:1962-12-26; sex:Adult Female; catalogNumber:UCR_ENT 00019697; recordedBy:B. Malkin; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus auralanus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Mato Grosso; locality:Barra do Tapirape; decimalLatitude:-10.46666; decimalLongitude:-50.51667; georeferenceSources:Gazetteer; eventDate:1962-12-26; sex:Adult Female; catalogNumber:UCR_ENT 00019698; recordedBy:B. Malkin; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus auralanus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Mato Grosso; locality:Mato Gr.; decimalLatitude:-10.41666; decimalLongitude:-59.46667; georeferenceSources:Label; eventDate:1977-03-17 to 1977-03-22; sex:Adult Female; catalogNumber:UCR_ENT 00046999; recordedBy:D. Engleman; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus auralanus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Para; locality:Tucurui; decimalLatitude:-3.7; decimalLongitude:-49.7; georeferenceSources:Gazetteer; eventDate:1979-01-01; sex:Adult Female; catalogNumber:UCR_ENT 00047080; recordedBy:M. Alvarenga; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus auralanus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km S Ariquemes, Fazenda Rancho Grande; verbatimElevation:300 m; decimalLatitude:-10.3; decimalLongitude:-62.86666; eventDate:1991-11-11 to 1991-11-22; sex:Adult Female; catalogNumber:UCR_ENT 00009459; recordedBy:B. C. Ratcliffe; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus auralanus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Napo; locality:30 km E of Pto Napo; verbatimElevation:410 m; decimalLatitude:-1.04256; decimalLongitude:-77.60111; georeferenceSources:Label; eventDate:2005-03-04; sex:Adult Male; catalogNumber:UCR_ENT 00072667; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:UCR
-
scientificName: Zelus auralanus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Napo; locality:30 km E of Pto Napo; verbatimElevation:410 m; decimalLatitude:-1.04256; decimalLongitude:-77.60111; georeferenceSources:Label; eventDate:2005-03-06; sex:Adult Female; catalogNumber:UCR_ENT 00072668; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:UCR
-
scientificName: Zelus auralanus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Orellana; locality:Reserva Etnica Waorani, 1 km S. Onkone Gare Camp, Transect Ent.; verbatimElevation:216 m; decimalLatitude:-0.65714; decimalLongitude:-76.453; georeferenceSources:Label; samplingProtocol:Fogging; eventDate:1995-02-10; sex:Adult Male; catalogNumber:UCR_ENT 00009474; occurrenceRemarks:Lot#992 - Collection code moved to this field to prevent duplication; Drake Collection; recordedBy:T. L. Erwin et al.; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus auralanus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PERU; stateProvince:Huanuco; locality:Monzon valley, Tingo Maria; decimalLatitude:-9.27816; decimalLongitude:-76.05562; georeferenceSources:Google Earth; eventDate:1954-12-11; sex:Adult Male; catalogNumber:UCR_ENT 00006072; recordedBy:E. I. Schlinger & E. S. Ross; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus auralanus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Pastaza; locality:Cuisimi, on Rio Cuisimi, 150km SE of Puyo; verbatimElevation:350 m; decimalLatitude:-2.43129; decimalLongitude:-77.03292; eventDate:1971-06-01 to 1971-06-05; sex:Adult Female; catalogNumber:UCR_ENT 00047094; recordedBy:B. Malkin; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
Description
Zelus auralanus Zhang & Hart, sp. n., habitus
b: Zelus auralanus Zhang & Hart, sp. n., male, lateral (UCR_ENT 00009474, Orellana, Ecuador)
c: Zelus auralanus Zhang & Hart, sp. n., male, dorsal (UCR_ENT 00019695, Mato Grosso, Brazil)
d: Zelus auralanus Zhang & Hart, sp. n., male, lateral (UCR_ENT 00019695, Mato Grosso, Brazil)
e: Zelus auralanus Zhang & Hart, sp. n., female, dorsal (UCR_ENT 00047094, Pastaza, Ecuador)
f: Zelus auralanus Zhang & Hart, sp. n., female, lateral (UCR_ENT 00047094, Pastaza, Ecuador)
Zelus auralanus Zhang & Hart, sp. n., male genitalic structures
b: Zelus auralanus Zhang & Hart, sp. n., pygophore, posterior view
Male: (
Female: (
Diagnosis
Can be readily recognized by the uniformly brown dorsal coloration; the darkened tibial apex; the humeral angle elevated to level of disc; the dorsal setae on head and pronotum appearing somewhat golden, shining when viewed under magnification. Males can also be recognized by the gradually enlarged paramere; the triangular medial process, curved slightly posteriad in the middle, apex with a hooklike projection; and the dorsal phallothecal sclerite with short, dorsad projections sub-laterally.
Etymology
The species epithet indicates the somewhat reddish tone of the coloration.
Distribution
South America (
Zelus bahiaensis , sp. n.
-
scientificName: Zelus bahiaensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:BRAZIL; stateProvince:Bahia; locality:Agua Preta; decimalLatitude:-14.58333; decimalLongitude:-39.26666; samplingProtocol:Unknown; eventDate:No date provided; sex:Adult Male; catalogNumber:UCR_ENT 00071255; occurrenceRemarks:Name from type locality 'Bahia'. Wrongly spelled in Hart 1972, and changed to bahiaensis.; recordedBy:P. Silva; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:TAMU
Description
Zelus bahiaensis Zhang & Hart, sp. n., habitus
b: Zelus bahiaensis Zhang & Hart, sp. n., male, lateral (UCR_ENT 00071255, Bahia, Brazil)
Zelus bahiaensis Zhang & Hart, sp. n., male genitalic structures
b: Zelus bahiaensis Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: Unknown.
Diagnosis
Recognized by the following combination of characters: the anterior pronotal lobe dark brown and the posterior pronotal lobe orange; the 1A an Pcu not intersecting, short crossvein between them; the long and slender, cylindrical medial process; the medial process apically folded posteriad; and the rather long paramere.
Etymology
Named after the Brazilian state Bahia, where the holotype was collected.
Distribution
South America (
Zelus banksi , sp. n.
-
scientificName: Zelus banksi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PANAMA; stateProvince:Canal Zone; county:none; locality:Barro Colorado; decimalLatitude:9.16666; decimalLongitude:-79.83333; georeferenceSources:Google Earth; eventDate:1924-06-25; sex:Adult Male; catalogNumber:UCR_ENT 00057804; recordedBy:N. Banks; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus banksi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:COLOMBIA; stateProvince:Meta; county:none; locality:Rio Guayuriba; verbatimElevation:400 m; decimalLatitude:4.01978; decimalLongitude:-73.60807; georeferenceSources:Google Earth; eventDate:1947-09-06; sex:Adult Male; catalogNumber:UCR_ENT 00071253; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:TAMU
-
scientificName: Zelus banksi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:COLOMBIA; stateProvince:Valle del Cauca; county:none; locality:Palmira; decimalLatitude:3.5364; decimalLongitude:-76.3036; georeferenceSources:Gazetteer; eventDate:1939-08-25; sex:Adult Male; catalogNumber:UCR_ENT 00009559; occurrenceRemarks:Drake Collection; recordedBy:F. J. Otoya; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus banksi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:COSTA RICA; stateProvince:Alajuela; county:none; locality:Higuito, San Mateo; verbatimElevation:254 m; decimalLatitude:9.95; decimalLongitude:-84.55; georeferenceSources:Gazetteer; eventDate:no date provided; sex:Adult Male; catalogNumber:UCR_ENT 00029366; occurrenceRemarks:Genitalia dissected.; recordedBy:Pablo Schild; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus banksi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; county:none; locality:Rancho Quemado, Peninsula de Osa; verbatimElevation:200 m; decimalLatitude:8.67776; decimalLongitude:-83.56478; georeferenceSources:Label; eventDate:1991-11-01; sex:Adult Male; catalogNumber:UCR_ENT 00014431; recordedBy:F. Quesada; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus banksi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PANAMA; stateProvince:Canal Zone; county:none; locality:Barro Colorado Island; decimalLatitude:9.15562; decimalLongitude:-79.84895; georeferenceSources:Google Earth; eventDate:1924-06-26; sex:Adult Male; catalogNumber:UCR_ENT 00015110; recordedBy:N. Banks; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
Description
Zelus banksi Zhang & Hart, sp. n., habitus
b: Zelus banksi Zhang & Hart, sp. n., male, lateral (UCR_ENT 00014431, Puntarenas, Costa Rica)
Zelus banksi Zhang & Hart, sp. n., male genitalic structures
b: Zelus banksi Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: Unknown.
Diagnosis
Recognized by the following combination of characters: the posterior pronotal lobe usually orangish-brown; the rather long paramere, apex obliquely truncate; and the medial process nearly straight, curvature small. Among the males of the Zelus panamensis species group (
Etymology
Named after N. Banks, the collector of the type specimen.
Distribution
Southern Central America and northern South America (
Zelus bruneri
Nomenclature
Zelus bruneri De Zayas, 1960, p. 125–127, orig. descr. and fig; Alayo, 1967, p. 5, 36, 37, list, key and note; Hart, 1987, p. 296-297, note and key; Maldonado, 1990, p. 326, cat.
-
scientificName: Zelus bruneri; family:Reduviidae; genus:Zelus; scientificNameAuthorship:de Zayas, 1960; country:Cuba; locality:Piloto, Moa, Oriente; decimalLatitude:20.55; decimalLongitude:-75.783333; georeferenceSources:Gazetteer; eventDate:1954-06; sex:Adult male; occurrenceRemarks:Deposited m the collection of Prof. F. de Zayas in Havana, Cuba. Not available for examination in current study.
-
scientificName: Zelus bruneri; family:Reduviidae; genus:Zelus; scientificNameAuthorship:de Zayas, 1960; country:Cuba; locality:Piloto, Moa, Oriente; decimalLatitude:20.55; decimalLongitude:-75.783333; georeferenceSources:Gazetteer; eventDate:1954-06; sex:Adult male; occurrenceRemarks:Deposited m the collection of Prof. F. de Zayas in Havana, Cuba. Not available for examination in current study.
Distribution
Known only from Cuba.
Taxon discussion
Two male specimens are known from Cuba, which were not physically examined, but the original description and illustration provide a strong basis for placing this species in the Zelus puertoricensis species group. This is confirmed by the narrow, elongated body form; the flat and rectangular pronotum; the general genitalic shape indicated in the figure. The much smaller size and flat postocular lobe negates it being a male of Z. subimpressus. It is more likely to be the male of Z. zayasi.
Zelus casii , sp. n.
-
scientificName: Zelus casii; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:BRAZIL; stateProvince:Amapa; locality:Villa Amazonas; decimalLatitude:0.03333; decimalLongitude:-51.05; eventDate:1964-05-29; sex:Adult Male; catalogNumber:UCR_ENT 00048228; occurrenceRemarks:CAS Type No. 12716; recordedBy:C. E. & E. S. Ross; otherCatalogNumbers:CAS Type No. 12716; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
Description
Zelus casii Zhang & Hart, sp. n., habitus
b: Zelus casii Zhang & Hart, sp. n., male, lateral
Zelus casii Zhang & Hart, sp. n., male genitalic structures
b: Zelus casii Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: Unknown.
Diagnosis
Recognized by the uniform dark brown coloration; the extremely long postocular lobe; and the rather broad medial process, apex emarginate in the middle and bearing a pair of ventrally directed projections.
Etymology
Named after Casi.
Distribution
South America (
Taxon discussion
Several characters of Z. casii are highly unique among all species of Zelus. It has an extraordinarily long postocular lobe. The medial process is very broad, has lateral ridge-like elevations, and the apex is emarginate.
Zelus cervicalis
Nomenclature
Zelus cervicalis Stål, 1872, p. 90, orig. descr. (subgenus Zelus); Uhler, 1876, p. 61, list (reprint); Uhler, 1886, p. 24, checklist; Lethierry and Severin, 1896, p. 151, cat.; Champion, 1898, p. 255, cat.; Banks, 1910, p. 16, cat.; Torre-Bueno and Engelhardt, 1910, p. 150, note; Van Duzee, 1912, p. 324, senior syn. of Z. marginata (Provancher); Fracker, 1913, p. 239, 240, key and list (subgenus Zelus); Torre-Bueno, 1913, p. 60, list; Barber, 1914, p. 506, list; Van Duzee, 1916, p. 30, checklist (subgenus Zelus) ; Van Duzee, 1917, p. 260, cat. (subgenus Zelus); Dozier, 192,0 p. 357, list; Blatchley, 1926, p. 569, key and note (subgenus Zelus); Readio, 1927, p. 169, 170, key and descr.; Wygodzinsky, 1949a, p. 48, checklist; Elkins, 1951, p. 410, list; Sibley, 1951, p. 92, list; Kelton, 1968, p. 1071, note; Snow, 1906, p. 180, list; Van Duzee, 1909, p. 177, list; Osborne and Drake, 1915, p. 531, note; Brimley, 1938, p. 73, list; Elliott, 1938, p. 39, list; Tenhet and Howe, 1939, p. 24, note; Drew and Schaeffer, 1962, p. 106, list; Oliver, 1964, p. 316, note; Whitcomb and Bell , 1964, p. 22, List and note; Hart, 1986, p. 542-543, lectotype desig., redescription, note, fig. and key; Maldonado, 1990, p. 326, cat.
Evagoras marginata Provancher, 1887, p. 182–183, orig. descr.; Van Duzee, 1912, p. 324, junior syn. of Z. cervicalis; Kelton, 1968, p. 1071, note.
Zelus marginatus: Lethierry and Severin, 1896, p. 152, cat.; Banks, 1910, p. 16, cat.
Zelus pictipes Champion, 1898, p. 255, Tab. XV, fig. 14, orig. descr, and fig.; Fracker, 1913, p. 239, 240, key and list; Van Duzee, 1916, p. 30, checklist (subgenus Zelus) ; Van Duzee, 1917, p. 259, cat. (subgenus Zelus); Readio, 1927, p. 169, 170, key and descr.; Wygodzinsky, 1949a, p. 50, checklist; Snow, 1906, p. 180, list; Elkins, 1951, p. 410, list; Sibley, 1951, p. 92, list; Drew and Schaeffer, 1962, p. 106, list; Hart, 1986, p. 542, lectotype desig. and junior syn. of Z. cervicalis.
Description
Zelus cervicalis Stål, 1872, habitus
b: Zelus cervicalis Stål, 1872, male, dorsal (UCR_ENT 00034075, Puebla, Mexico)
c: Zelus cervicalis Stål, 1872, male, lateral (UCR_ENT 00034075, Puebla, Mexico)
d: Zelus cervicalis Stål, 1872, female, dorsal (UCR_ENT 00034044, Guerrero, Mexico)
e: Zelus cervicalis Stål, 1872, female, lateral (UCR_ENT 00034044, Guerrero, Mexico)
f: Zelus cervicalis Stål, 1872, female, dorsal (UCR_ENT 00032055, Georgia, USA)
Zelus cervicalis Stål, 1872, male genitalic structures
b: Zelus cervicalis Stål, 1872, Gulf Coast-US population, pygophore, lateral and posterior views
c: Zelus cervicalis Stål, 1872, Mexico-Central America population, phallus, dorsal view
d: Zelus cervicalis Stål, 1872, Gulf Coast-US population, phallus, dorsal view
Male: (
Female: (
Diagnosis
The rather slender body form makes this species easy to separate from other species that occur in the same geographic region. Males can also be recognized by the paramere apically greatly enlarged; the medial process apically curved ventrad, hooklike; the lateral margin of the dorsal phallothecal sclerite recurved. Zelus cervicalis is most similar to Z. renardii and the two share a number putatively synapomorphic characters of structures of male genitalia. The more slender body separates both sexes of Z. cervicalis from Z. renardii. Males of Z. cervicalis also have the apex of medial process not bent as strongly as that in Z. renardii.
Distribution
South Atlantic and Gulf Coast states of the United States, southeastern Arizona, most of Mexico, Central America and Northern Colombia (
Taxon discussion
Hart (1986) stated that, based on male genitalic characters and pilosity, Z. cervicalis and Z. renardii are closely related species, and we agree with that view. We also corroborate, using a larger specimen sample, the western and eastern parapatric distribution pattern for Z. renardii and Z. cervicalis found by Hart. Based mainly on the coloration of the legs, Hart (1986) delimited two populations of Z. cervicalis, i.e., a South Atlantic and Gulf Coast population and a Mexico-Central America population, the latter also extending to southeastern Arizona and northern Colombia. Most individuals of the South Atlantic and Gulf Coast population have unicolorous legs, or, at most, only a few brownish to reddish spots. Specimens of the Mexico-Central America population have heavily spotted or banded legs. This pattern is also recovered in the current study. However, contrary to Hart's claim that "occasional specimens from either population may occur that do not conform to the normal pattern for that population", we found that all specimens of the Mexico-Central America population have spotted or banded legs. This condition also appears in a small number of specimens in other populations (e.g., UCR_ENT 00016129, UCR_ENT 00039079, UCR_ENT 00042740, UCR_ENT 00042741, UCR_ENT 39522, UCR_ENT 00039519, UCR_ENT 00039531, UCR_ENT 00039525, UCR_ENT 00039561, UCR_ENT 00039560, UCR_ENT 00039559, UCR_ENT 00039557, and more specimens from Texas). We also observed that compared to populations in other US states, specimens from southern Texas tend to have spotted legs, but the density of spots is lower than that in the Mexico-Central America population. By examining previously unstudied Mexican specimens from southern Sonora and northern Sinaloa, we also support Hart's second theory that the Arizona specimens are in continuity with the remainder of the population. The male genitalia are also variable in a number of respects between the two populations (
The images of the lectotype of Z. cervicalis are available on the 'Types of Heteroptera' website of the Swedish Museum of Natural History.
Zelus chamaeleon
Nomenclature
Zelus chamaeleon Stål, 1872, p. 90–91, orig. descr. and cat. (subgenus Diplodus); Lethierry and Severin, 1896, p. 151, cat.; Champion, 1898, p. 260, note; Wygodzinsky, 1949a, p. 48–49, checklist; Maldonado, 1990, p. 326, cat.
-
scientificName: Zelus chamaeleon; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1872; country:COLOMBIA; stateProvince:Cundinamarca; locality:Bogota; decimalLongitude:-75.16833; georeferenceSources:Gazetteer; eventDate:Date not provided; sex:Adult Male; catalogNumber:UCR_ENT 00041001; occurrenceRemarks:Lectotype of Zelus chamaeleon Stål, 1872. (New designation by Zhang, Hart & Weirauch, 2016). Verbatim label info: Bogota. / Lindig / v. niger Stal / designated by E.R.Hart / Lectotype Zelus chamaeleon Stal / NHRS-GULI 000000322; recordedBy:Lindig; otherCatalogNumbers:NHRS-GULI 000000322; dateIdentified:2012; institutionCode:NHRS
-
scientificName: Zelus chamaeleon; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1872; country:COLOMBIA; stateProvince:Cundinamarca; locality:Bogota; decimalLongitude:-75.16833; georeferenceSources:Gazetteer; eventDate:Date not provided; sex:Adult Female; catalogNumber:None; occurrenceRemarks:Allolectotype of Zelus chamaeleon Stål, 1872. (New designation by Zhang, Hart & Weirauch, 2016). Labels: Bogota / Lindig / Typus / v. fasciativentris Stal.; recordedBy:Lindig; institutionCode:NHRS
-
scientificName: Zelus chamaeleon; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1872; country:COLOMBIA; stateProvince:Cundinamarca; locality:Bogota; decimalLongitude:-75.16833; georeferenceSources:Gazetteer; eventDate:Date not provided; sex:Adult sex unknown; catalogNumber:UCR_ENT 00041002; occurrenceRemarks:Paralectotype of Zelus chamaeleon Stål, 1872 (New designation by Zhang, Hart & Weirauch, 2016). Verbatim label info: Lindig / Bogota. / Paralectotype Zelus chamaeleon Stal / designated by E.R.Hart / NHRS-GULI 000000323; recordedBy:Lindig; otherCatalogNumbers:NHRS-GULI 000000323; dateIdentified:2012; institutionCode:NHRS
-
scientificName: Zelus chamaeleon; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1872; country:COLOMBIA; stateProvince:Cundinamarca; locality:Bogota; decimalLongitude:-75.16833; georeferenceSources:Gazetteer; eventDate:Date not provided; sex:Adult sex unknown; catalogNumber:UCR_ENT 00041003; occurrenceRemarks:Paralectotype of Zelus chamaeleon Stål, 1872 (New designation by Zhang, Hart & Weirauch, 2016). Verbatim label info: Lindig / Bogota. / v. mainticollis[?] Stal / Paralectotype Zelus chamaeleon Stal / designated by E.R.Hart / Typus / NHRS-GULI 000000327; recordedBy:Lindig; otherCatalogNumbers:NHRS-GULI 000000327; dateIdentified:2012; institutionCode:NHRS
-
scientificName: Zelus chamaeleon; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1872; country:COLOMBIA; stateProvince:Cundinamarca; locality:Bogota; decimalLongitude:-75.16833; georeferenceSources:Gazetteer; eventDate:Date not provided; sex:Adult sex unknown; catalogNumber:UCR_ENT 00041013; occurrenceRemarks:Paralectotype of Zelus chamaeleon Stål, 1872 (New designation by Zhang, Hart & Weirauch, 2016). Verbatim label info: Bogota / Lindig / Paratypus / Lectotype Zelus chamaeleon Stal / designated by E.R.Hart / NHRS-GULI 0000003958; recordedBy:Lindig; otherCatalogNumbers:NHRS-GULI 0000003958; dateIdentified:2012; institutionCode:NHRS
Description
Zelus chamaeleon Stål, 1872, habitus
b: Zelus chamaeleon Stål, 1872, male, lateral view (UCR_ENT 00022985, Cundinamarca, Colombia)
c: Zelus chamaeleon Stål, 1872, female, dorsal view (UCR_ENT 00025243, Cundinamarca, Colombia)
d: Zelus chamaeleon Stål, 1872, female, lateral view (UCR_ENT 00025243, Cundinamarca, Colombia)
Zelus chamaeleon Stål, 1872, male genitalic structures
b: Zelus chamaeleon Stål, 1872, phallus, dorsal view
Male: (
Female: (
Diagnosis
Can be recognized by the following combination of characters: the long, erect, fine setae on head, anterior pronotal lobe, pleura and sternites; the stout and short head and the nearly hemispherical postocular lobe; the short paramere, not exceeding medial process; the medial process short and triangular, apex as hooklike process, extending ventrally as transverse ridge; and the apical surface of dorsal phallothecal sclerite medially with keel-like elevation.
Distribution
South America (
Zelus championi , sp. n.
-
scientificName: Zelus championi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PANAMA; stateProvince:Chiriqui; locality:Bugaba; verbatimElevation:457 m; decimalLatitude:8.4833; decimalLongitude:-82.6167; georeferenceSources:Gazetteer; eventDate:No date provided; sex:Adult Male; catalogNumber:UCR_ENT 00048759; occurrenceRemarks:Verbatim label info: B.C.A.Rhyn.II. Zelus inconstans Ch. / Bugaba, 800-1,500 ft. Champion. / Holotype Zelus championi Hart / [genitalia vial]; recordedBy:G.C. Champion; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:BMNH
-
scientificName: Zelus championi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:ECUADOR; stateProvince:Napo; locality:10 km W Cosanga; verbatimElevation:2114 m; decimalLatitude:0.59094; decimalLongitude:-77.88086; eventDate:2005-03-10; sex:Adult Male; catalogNumber:UCR_ENT 00004770; occurrenceRemarks:Drake Collection; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:UCR
-
scientificName: Zelus championi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:Costa Rica; stateProvince:Cartago; locality:Monumento Nacional Guayabo, Turrialba; verbatimElevation:1100 m; decimalLatitude:9.97159; decimalLongitude:-83.69072; georeferenceSources:Gazetteer; eventDate:1903-01-04; sex:Adult Male; catalogNumber:UCR_ENT 00014406; occurrenceRemarks:Additional information on label: L N 217200_570300; recordedBy:G. Fonseca; otherCatalogNumbers:INBIO CRI002 040338; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBio
Description
Zelus championi, Zhang & Hart, sp. n., habitus
b: Zelus championi, Zhang & Hart, male, lateral view (UCR_ENT 00004770)
Zelus championi, Zhang & Hart, sp. n., pygophore, lateral and posterior views
b: Zelus championi, Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: Unknown.
Diagnosis
The strongly contrasting black dorsal surface and red abdomen is distinctive of this species. The features of the genitalia are rather similar to those of other species in the Zelus vagans species group (
Etymology
This species epithet is a patronym, in honor of entomologist George C. Champion (1851-1927), who authored several volumes on Rhyncophora (Heteroptera) in the Biologia Centrali Americana series.
Distribution
Central and South America (
Ecology
No natural history or ecological knowledge is known, but we hypothesize that the strikingly contrasting black and red coloration is at the same time cryptic and aposematic, and may also be mimetic. Based on observations of other species, we know that low vegetation is a common habitat of members of this genus. In a dense forest, predators from above may confuse the black dorsum of Z. championi with dark forest background, while the strong contrast formed by black and red colors is highly visible to predators (e.g., lizards) at the same level or approaching from below. Like many assassin bugs, species of Zelus may inflict a painful bite when attacked. Besides, harpactorines, including Zelus spp., emit a foul odor when handled. We do not know if vertebrate predators are deterred by this odor, but it is strong enough to be detected by a human even meters away. Hence Z. championi may be well defended against predators and the contrasting coloration serves as a signal for unpalatability. Of course, many other species of Zelus are dull colored, but expected to have the same kind of physical or chemical defense. There may be other ecological factors that determine the coloration of Z. championi. We suspect that mimicry is one. Many other unpalatable insects show similar contrasting bright red and black color patterns. Zelus championi may participate in Müllerian mimicry with those species.
Taxon discussion
The type specimen of this species was originally described as the male of Z. inconstans, a species very similar in general form to Z. championi. On the basis of pubescence, pronotal armature and whitish exudation, Champion himself questioned the conspecificity of this male with the three females of the original type series. As more material was available for the present work, his doubts have been substantiated, the male of Z. inconstans identified and this particular specimen found to be a male of a new species. The two species belong to different species groups, as verified by pubescence and genitalic characters.
Zelus cognatus
Nomenclature
Diplodus cognatus Costa, 1864, p. 81, orig. descr.; Uhler, 1886, p. 24, checklist; Walker, 1873, p. 125, cat.
Zelus cognatus: Stål, 1872, p. 91, cat. (subgenus Diplodus); Lethierry and Severin, 1896, p. 151, cat.; Champion, 1898, p. 259–260, junior syn. of Z. exsanguis Stål. stat. rev. (current study).
Description
The following is a translation of the original description:
"Closely related to the preceding species [Z. ambulans]; differing in that the spines of the humeral angle of the pronotum are conspicuously directed obliquely upward; dorsal surface of the head black, longitudinal line and transverse sulcus yellowish; first and second antennal segments testaceous, apex black. -- length 15 mm."
Taxon discussion
This species was originally described from a single specimen from Mexico. The original description did not indicate its sex. Champion’s synonymy was apparently based on the description and not upon examination of the specimen. Attempts to locate the holotype were unsuccessful. From the above original description it is impossible to determine whether this species may be synonymous with Z. exsanguis or is a separate species. It is reasonably certain, however, that it belongs to the Zelus luridus species group, as the comparison with Z. ambulans precludes any similarity to other reduviid genera or even groups in this genus within Mexico.
Zelus conjungens
Nomenclature
Diplodus conjungens Stål, 1860, p. 75, orig. descr.; Mayr, 1866, p. 138–139, junior syn. of Z. armillatus; Walker, 1873, p. 125, cat.
Zelus conjungens: Stål, 1872, p. 90, cat. (subgenus Diplodus); Lethierry and Severin, 1896, p. 151, junior syn. of Z. armillatus; Wygodzinsky, 1949a, p. 49, checklist. stat. rev. (current study).
Zelus atripes Champion, 1898, p. 259, Tab. XV. fig. 22, orig. descr. and fig.; Wygodzinsky, 1949a, p. 48, checklist; Maldonado, 1990, p. 326, cat. syn. nov. (current study).
-
scientificName: Zelus conjungens; family:Reduviidae; genus:Zelus; scientificNameAuthorship:(Stål, 1860); country:BRAZIL; stateProvince:Rio de Janeiro; locality:unknown; verbatimElevation:NHRS-GULI 000000320; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00041000; occurrenceRemarks:Lectotype of Zelus conjungens (Stål, 1860). (New Designation by Zhang, Hart & Weirauch, 2016). Verbatim label info: Rio Jan / Stal / Lectotype Zelus conjungens (Stal) / designated by E.R.Hart / NHRS-GULI 000000320; recordedBy:Stal; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:NHRS
-
scientificName: Zelus conjungens; family:Reduviidae; genus:Zelus; scientificNameAuthorship:(Stål, 1860); country:BRAZIL; stateProvince:Rio de Janeiro; locality:unknown; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00041000; occurrenceRemarks:Paralectotype of Zelus conjungens (Stål, 1860). (New Designation by Zhang, Hart & Weirauch, 2016). Verbatim label info: Rio Jan / Stal / Lectotype Zelus conjungens (Stal) / designated by E.R.Hart / NHRS-GULI 000000320; recordedBy:Stal; institutionCode:NHRS
-
scientificName: Zelus conjungens; family:Reduviidae; genus:Zelus; scientificNameAuthorship:(Stål, 1860); country:PANAMA; sex:Adult Female; occurrenceRemarks:Holotype of Zelus atripes Champion, 1898 (junior synonym of Zelus conjungens). Bears the following labels: Type / Panama (Bouchard) / Zelus atripes Ch. / B.C.A. Sp. figured; recordedBy:Boucard; institutionCode:BMNH
Description
Zelus conjungens (Stål, 1860), habitus
b: Zelus conjungens (Stål, 1860), male, lateral (UCR_ENT 00047657, Santa Catarina, Brazil)
c: Zelus conjungens (Stål, 1860), male, dorsal (UCR_ENT 00047100, Meta, Colombia)
d: Zelus conjungens (Stål, 1860), female, dorsal (UCR_ENT 00030295, Santa Catarina, Brazil)
e: Zelus conjungens (Stål, 1860), female, lateral (UCR_ENT 00047609, Santa Catarina, Brazil)
f: Zelus conjungens (Stål, 1860), female, dorsal (UCR_ENT 00017733, Sau Paulo, Brazil)
Zelus conjungens (Stål, 1860), male genitalic structures
b: Zelus conjungens (Stål, 1860), phallus, dorsal view
Male: (
Female: (
Diagnosis
Among species of the Zelus armillatus species group, the medial process in Z. conjungens is the broadest, being more than 2x the diameter of paramere and more than 1.5x the diameter of ocellus. Other characters helpful for identification may include the lateral processes on apex of medial process minute, inconspicuous. Most similar to Z. armillatus, but can be separated by characters aforementioned, and also the lateral expansion on dorsal phallothecal sclerite close to basal arm not as sharp process. In females the mesofemur is swollen nearly throughout, much thicker than profemur, which will serve as a basis for separation from the females of Z. armillatus.
Distribution
Southern Central America, northern South America and Southern Brazil (
Taxon discussion
Mayr (1866) synonymized Z. conjungens with Z. armillatus, the former as the junior synonym. We here resurrect this species on the basis of the characters described in the diagnosis. Two disjunct populations are recognized for this species, one in Southern Brazil and another in Central America and Northern South America. The latter represents a species formerly described by Champion, Z. atripes, which is here considered conspecific with Z. conjungens, as in both species the females show a swollen femur and the medial processes of males are broad. However, male genitalia of the two populations are somewhat different and may warrant a further closer examination. In any area in which the species occurs there is apparently a great range of color and pattern variations, but there does seem to be a general trend toward the lighter colorations in Colombia, Costa Rica and Panama.
Zelus cordazulus , sp. n.
-
scientificName: Zelus cordazulus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Huanuco; locality:Divisoria, Cordillera Azul; verbatimElevation:1300 m; decimalLatitude:-9.215; decimalLongitude:-75.846; georeferenceSources:Google Earth; eventDate:1947-05-01; sex:Adult Male; catalogNumber:UCR_ENT 00046973; recordedBy:Wygodzinsky; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus cordazulus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Huanuco; locality:Divisoria, Cordillera Azul; verbatimElevation:1300 m; decimalLatitude:-9.215; decimalLongitude:-75.846; georeferenceSources:Google Earth; eventDate:1947-05-01; sex:Adult Male; catalogNumber:UCR_ENT 00009529; recordedBy:Ranile; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus cordazulus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Junin; locality:Chanchamayo; decimalLatitude:-11.125; decimalLongitude:-75.357; georeferenceSources:Google Earth; eventDate:no date provided; sex:Adult Male; catalogNumber:UCR_ENT 00023701; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:RMNH
-
scientificName: Zelus cordazulus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Huanuco; locality:Divisoria, Cordillera Azul; verbatimElevation:1300 m; decimalLatitude:-9.215; decimalLongitude:-75.846; georeferenceSources:Google Earth; eventDate:1947-05-01; sex:Adult Female; catalogNumber:UCR_ENT 00026168; recordedBy:Ranile; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus cordazulus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Cusco; locality:S. Amer, Santa Isabel, Valley of River Ccosnipata; decimalLatitude:-13; decimalLongitude:-71.3; georeferenceSources:Gazetteer; eventDate:1952-01-02; sex:Adult Female; catalogNumber:UCR_ENT 00029354; recordedBy:F. Woytkowski; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus cordazulus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:San Martin; locality:15 kms SE of Moyobamba; verbatimElevation:890 m; decimalLatitude:-6.09796; decimalLongitude:-76.8605; georeferenceSources:Google Earth; eventDate:1947-08-10; sex:Adult Female; catalogNumber:UCR_ENT 00029355; recordedBy:F. Woytkowski; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus cordazulus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Huanuco; locality:Divisoria (Cordillera Azul); verbatimElevation:1500 m; decimalLatitude:-9.219; decimalLongitude:-75.829; georeferenceSources:Google Earth; eventDate:1905-04-25; sex:Adult Female; catalogNumber:UCR_ENT 00071256; recordedBy:Weyrauch; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:TAMU
Description
Zelus cordazulus Zhang & Hart, sp. n., habitus
b: Zelus cordazulus Zhang & Hart, sp. n., male, lateral view (UCR_ENT 00023701, Junin, Peru)
c: Zelus cordazulus Zhang & Hart, sp. n., female, dorsal view (UCR_ENT 00029354, Cusco, Peru)
d: Zelus cordazulus Zhang & Hart, sp. n., female, lateral view (UCR_ENT 00029354, Cusco, Peru)
Zelus cordazulus Zhang & Hart, sp. n., male genitalic structures
b: Zelus cordazulus Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: (
Diagnosis
The nearly uniform brown dorsal coloration; the dorsum with short, erect, somewhat spine-like setae; and the posterior margin of the pronotum smoothly convex. The paramere short, broad; the apical part of medial process laterally compressed and ridged on the anterior surface; the posterolateral rim of pygophore with lightly sclerotized expansion below paramere; and the basal plate arm separate, not fused. In females the dorsum is nearly uniformly brown, the lateral and ventral surfaces yellowish, the legs apically reddish-brown, not conspicuously banded and the abdominal segment with single dark spot.
Etymology
Named after the type locality "Cordillera" in Peru.
Distribution
South America (
Zelus couturieri
Nomenclature
Iquitozelus couturieri Bérenger, 2003, p. 23, orig. descr. and fig.
Zelus couturieri (Bérenger, 2003), comb. nov. (current study).
-
scientificName: Zelus couturieri; family:Reduviidae; genus:Zelus; scientificNameAuthorship:(Berenger, 2003); country:Peru; stateProvince:Loreto; locality:Iquitos; eventDate:1992-07-20; sex:Adult Female; occurrenceRemarks:Host plant: Psidium guajava; recordedBy:Couturier, G; institutionCode:Muséum national d'Histoire naturelle
-
scientificName: Zelus couturieri; family:Reduviidae; genus:Zelus; scientificNameAuthorship:(Berenger, 2003); country:Peru; stateProvince:Loreto; locality:Iquitos; eventDate:1992-07-20; sex:Adult Female; occurrenceRemarks:Host plant: Psidium guajava; recordedBy:Couturier, G; institutionCode:Museo de Entomologia, Universidad Agraria La Molina, Lima
-
scientificName: Zelus couturieri; family:Reduviidae; genus:Zelus; scientificNameAuthorship:(Berenger, 2003); country:Peru; stateProvince:Loreto; locality:Iquitos; eventDate:1992-07-20; sex:Adult Female; occurrenceRemarks:Host plant: Psidium guajava; recordedBy:Couturier, G; institutionCode:USNM
-
scientificName: Zelus couturieri; family:Reduviidae; genus:Zelus; scientificNameAuthorship:(Berenger, 2003); country:Peru; stateProvince:Loreto; locality:Iquitos; eventDate:1992-07-20; sex:Adult Female; occurrenceRemarks:Host plant: Psidium guajava; recordedBy:Couturier, G; institutionCode:Jean-Michel Berenger Collection
Description
Diagnosis
The connexivum segment VI with foliaceous expansion is unique among all females of Zelus spp.
Distribution
South America (
Taxon discussion
Zelus errans
Nomenclature
Zelus errans Fabricius, 1803, p. 282, orig. descr.; Stål, 1868, p.108, descr., note and senior syn. of Z. cursitans; Stål, 1872, p.88, cat.; Walker, 1873, p. 135, cat.; Wygodzinsky, 1949a, p. 49, checklist; Maldonado, 1990, p. 326, cat.
Zelus cursitans Fabricius, 1803, p. 284, orig. descr.; Blanchard, 1840, p. 101, descr.; Stål, 1868,p. 108, junior syn. of Z. errans.
-
scientificName: Zelus errans; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Fabricius, 1803; country:unknown; stateProvince:unknown; locality:Habitat in America meridionali; eventDate:No date provided; sex:Adult Male; occurrenceRemarks:Lectotype of Zelus errans Fabricius, 1803 (New designation by Zhang, Hart & Weirauch, 2016). Labels: Z. errans in Am. mer. Schmidt / Type; recordedBy:Dom. Smidt; institutionCode:ZMUC
-
scientificName: Zelus errans; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Fabricius, 1803; country:unknown; stateProvince:unknown; locality:Habitat in America meridionali; eventDate:No date provided; sex:Adult Male; occurrenceRemarks:Paralectotype of Zelus errans Fabricius, 1803 (New designation by Zhang, Hart & Weirauch, 2016). Labels: Z. errans in Am. mer. Schmidt / Type; recordedBy:Dom. Smidt; institutionCode:ZMUC
-
scientificName: Zelus errans; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Fabricius, 1803; occurrenceRemarks:Paralectotype of Zelus errans Fabricius, 1803 (New designation by Zhang, Hart & Weirauch, 2016). Label: errans; institutionCode:ZMUC
Description
Zelus errans Fabricius, 1803, habitus
b: Zelus errans Fabricius, 1803, male, lateral view
c: Zelus errans Fabricius, 1803, female, dorsal view (UCR_ENT 00017742, El Beni, Bolivia)
d: Zelus errans Fabricius, 1803, female, lateral view (UCR_ENT 00017742, El Beni, Bolivia)
e: Zelus errans Fabricius, 1803, female, dorsal view (UCR_ENT 00017743, Parana, Brazil)
Zelus errans Fabricius 1803, male genitalic structures
b: Zelus errans Fabricius 1803, phallus, dorsal view
Male: (
Female: (
Diagnosis
May be confused with Z. vespiformis and Z. gracilipes, species that have similar appearances. Distinguished from Z. vespiformis by the more elongated body; the longer medial process (
Distribution
South America (
Taxon discussion
Zelus errans is quite closely related to Z. vespiformis, and they are possibly sister species. These two species appear to be allopatric, with their boundaries roughly lying across central Colombia and southern Venezuela.
Zelus erythrocephalus
Nomenclature
Zelus erythrocephalus Fabricius, 1803, p. 283, orig. descr.; Blanchard, 1840, p. 101, cat. (erytrocephalus sic.); Stål, 1872, p. 92, cat. (subgenus Diplodus); Bergroth, 1893, p. 63, note; Lethierry and Severin, 1896, p. 152, cat.; Champion, 1898, p. 257, note; Brindley, 1931, p. 137, 151, list and note; Wygodzinsky, 1949a, p. 49, checklist; Zimsen, 1964, p. 338, list; Maldonado, 1990, p. 327, cat.
Euagoras erythrocephalus: Burmeister, 1835, p. 227, list.
Diplodus erythrocephalus: Stål, 1868, p. 283, descr.; Walker, 1873, p. 125, cat.; Van Duzee, 1901, p. 351, note.
-
scientificName: Zelus erythrocephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Fabricius, 1803; country:unknown; stateProvince:unknown; locality:Habitat in America meridionali; eventDate:No date provided; sex:Adult Male; catalogNumber:UCR_ENT 00075109; occurrenceRemarks:Lectotype of Zelus erythrocephalus Fabricius, 1803 (New Designation by Zhang, Hart & Weirauch, 2016). Bears labels: Type /Z. erythrocephalus in Am. mer. Schmidt; recordedBy:Dom. Smidt; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:ZMUC
Description
Zelus erythrocephalus Fabricius, 1803, habitus
b: Zelus erythrocephalus Fabricius, 1803, male, lateral view (UCR_ENT 00023652, Suriname)
c: Zelus erythrocephalus Fabricius, 1803, female, dorsal view (UCR_ENT 00023659, Suriname)
d: Zelus erythrocephalus Fabricius, 1803, female, lateral view (UCR_ENT 00023659, Suriname)
Zelus erythrocephalus Fabricius, 1803, male genitalic structures
b: Zelus erythrocephalus Fabricius, 1803, phallus, dorsal view
Male: (
Female: (
Diagnosis
Recognized by the following combination of characters: the dorsal coloration nearly uniformly dark brown, the head reddish-brown, and the membrane with indistinct iridescence. Most similar to Z. paracephalus and Z. russulumus; can be distinguished from both by the rather slender medial process. Females of Z. erythrocephalus, Z. paracephalus and Z. russulumus are difficult to separate.
Distribution
South America (
Taxon discussion
Zelus erythrocephalus and two other species in the same species group, Z. paracephalus and Z. russulumus superficially resemble Z. panamensis, a species in a different group. All have a orange, reddish head and a uniformly dark dorsum. These can be separated from Z. panamensis based on male genitalia and iridescence on membrane.
Zelus exsanguis
Nomenclature
Zelus exsanguis Stål, 1862, p. 452, orig. descr.; Stål, 1872, p. 91, cat. (subgenus Diplodus); Walker, 1873, p. 124, cat.; Uhler, 1894, p. 283, list; Lethierry and Severin, 1896, p. 152, cat.; Champion, 1898, p. 259–260, senior syn. of Z. luridus Stål, Z. ambulans Stål and Z. cognatus Costa; Banks, 1910, p. 16, cat.; Fracker, 1913, p- 239, 240, 241, key, list and note (subgenus Diplodus); Van Duzee, 1916, p, 30, checklist (subgenus Diplodus); Van Duzee, 1917, p. 260, cat. (subgenus Diplodus); Wygodzinsky, 1949a, p. 49, checklist; Hart, 1986, p. 539, redescription, lectotype desig., note, fig. and key; Maldonado, 1990, p. 327, cat.
Diplodus exsanguis: Uhler, 1886, p. 24, checklist.
-
scientificName: Zelus exsanguis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; stateProvince:unknown; locality:unknown; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00075071; occurrenceRemarks:Lectotype of Zelus exsanguis Stål, 1862, designated by Hart (1986). Verbatim label info: Mexico coll. Signoret / exsanguis det. Stal / B.C.A. Rhyn.II. Zelus exsanguis St. / Lectotype Zelus exsanguis Stal / designated by E. R. Hart / Lectotypus Zelus exsanguis STAL, 1862 etik. Hecher 1996 REDV. 470/1; recordedBy:Signoret; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:NHMW
Description
Zelus exsanguis Stål, 1862, habitus
b: Zelus exsanguis Stål, 1862, male, lateral view (UCR_ENT 00037113, Chiriqui, Panama)
c: Zelus exsanguis Stål, 1862, female, dorsal view (UCR_ENT 00037115, Veracruz, Mexico)
d: Zelus exsanguis Stål, 1862, female, lateral view (UCR_ENT 00037115, Veracruz, Mexico)
Zelus exsanguis Stål, 1862, male genitalic structures
b: Zelus exsanguis Stål 1862, northern population, pygophore, lateral and posterior views
c: Zelus exsanguis Stål 1862, southern population, phallus, dorsal view
d: Zelus exsanguis Stål 1862, northern population, phallus, dorsal view
Male: (
Female: (
Diagnosis
As with some species of the Zelus luridus species group, Z. exsanguis has a rather uniform greenish-brown coloration. Can be distinguished from most other species of the same species group by the humeral angle elevated to same level of and nearly continuous with disc. This is also seen in Z. ambulans, but the two species can be easily separated on the basis of coloration (
Distribution
Mexico to Panama (
Taxon discussion
Zelus fasciatus
Nomenclature
Zelus fasciatus Champion, 1899, p. 257, Tab. XV. fig. 18, orig. descr. and fig.; Kuhlgatz, 1902, p. 266, note; Wygodzinsky, 1949a, p. 49, checklist; Maldonado, 1990, p. 327, cat.
-
scientificName: Zelus fasciatus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Champion, 1899; country:PANAMA; stateProvince:Chiriqui; locality:Bugaba; verbatimElevation:457 m; decimalLatitude:8.4833; decimalLongitude:-82.6167; georeferenceSources:Gazetteer; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00048758; occurrenceRemarks:Verbatim label info: Type / B.C.A.Rhyn.II. Zelus fasciatus Ch. / Sp. figured. / Bugaba, 800-1,500 ft. Champion. / Holotype Sel. E.R. Hart letter II.iii.76; recordedBy:G.C. Champion; institutionCode:BMNH
Description
Zelus fasciatus Champion, 1899, habitus
b: Zelus fasciatus Champion, 1899, female, lateral view (UCR_ENT 00017227, Colon, Panama)
Male: unknown.
Female: (
Diagnosis
The rather unique dorsal color pattern easily distinguishes this species from all other species in the genus.
Distribution
Southern Central America and northern South America (
Zelus filicauda
Nomenclature
Zelus filicauda Bergroth, 1893, p. 63, orig. descr.; Lethierry and Severin, 1896, p. 152, cat.; Wygodzinsky, 1949a, p. 49, checklist; Maldonado, 1990, p. 327, cat.
-
scientificName: Zelus filicauda; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Bergroth, 1893; country:ECUADOR; stateProvince:Loja; locality:Loja; decimalLatitude:-4.003057; decimalLongitude:-79.207349; georeferenceSources:Google Earth; sex:Adult Male; recordedBy:G. Fallou; otherCatalogNumbers:259-95; identifiedBy:ER Hart; dateIdentified:1972; institutionCode:Muséum national d'histoire naturelle
-
scientificName: Zelus filicauda; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Bergroth, 1893; country:ECUADOR; stateProvince:Tungurahua; locality:13 mi W. Mera, Napo-Pastaza; decimalLatitude:-1.4628; decimalLongitude:-78.28538; georeferenceSources:Google Earth; eventDate:1955-02-12; sex:Adult Male; catalogNumber:UCR_ENT 00019043; recordedBy:E. I. Schlinger & E. S. Ross; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:CAS
Description
Zelus filicauda Bergroth, 1893, habitus
b: Zelus filicauda Bergroth, 1893, male, lateral view (UCR_ENT 00019043, Tungurahua, Ecuador)
Zelus filicauda Bergroth, 1893, male genitalic structures
b: Zelus filicauda Bergroth, 1893, phallus, dorsal view
Male: (
Female: Unknown.
Diagnosis
Recognized by the entire body dark brown, the posterior pronotal lobe slightly lighter and somewhat reddish, the legs without bands; the humeral angle projected into spinous process. Distinguished among species of the Zelus panamensis species group by the curved medial process (
Distribution
South America (
Zelus fuliginatus , sp. n.
-
scientificName: Zelus fuliginatus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:COLOMBIA; stateProvince:Quindio; locality:Salento; verbatimElevation:1895 m; decimalLatitude:4.6375; decimalLongitude:-75.57028; georeferenceSources:Gazetteer; eventDate:1939-07-14; sex:Adult Male; catalogNumber:UCR_ENT 00007997; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus fuliginatus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:COLOMBIA; stateProvince:Santander; locality:Rio Suarez; decimalLatitude:6.76667; decimalLongitude:-73.26667; georeferenceSources:GeoLocate Software; eventDate:1946-01-16; sex:Adult Female; catalogNumber:UCR_ENT 00009462; occurrenceRemarks:Previously designated as 'allotype' of his manuscript name Zelus fuliginatus by Hart, a type status not used in the formal publication of this name (Zhang et al.); recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus fuliginatus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:Ecuador; stateProvince:Napo; locality:W bank of Rio Quijos; verbatimElevation:1750 m; decimalLatitude:0.43333; decimalLongitude:-77.88333; georeferenceSources:Label; eventDate:2006-03-03; sex:Adult Male; catalogNumber:AMNH_PBI 00218883; recordedBy:J.S. Miller & E. Tapia; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:UCR
Description
Zelus fuliginatus Zhang & Hart, sp. n., habitus
b: Zelus fuliginatus Zhang & Hart, sp. n., male, lateral view (UCR_ENT 00007997, Colombia)
c: Zelus fuliginatus Zhang & Hart, sp. n., female, dorsal view (UCR_ENT 00009462, Santander, Colombia)
d: Zelus fuliginatus Zhang & Hart, sp. n., female, lateral view (UCR_ENT 00009462, Santander, Colombia)
b: Zelus fuliginatus Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: (
Diagnosis
Recognized by the strongly contrasting black dorsum and yellow abdomen, the rather short postocular lobe, and the Sc not reaching apex of cubital cell. Other diagnostic characters shared with members of the Zelus vagans species group and the Zelus longipes species group include the unarmed rounded humeral angle and the spine-like setae on pronotum. Males can also be separated from other species of the Zelus vagans species group by the medial process apically tapered, somewhat pointed (
Etymology
The species epithet means 'soot' or painted black, referring to the black dorsal coloration of this species.
Distribution
Northern South America (
Zelus gilboventris , sp. n.
-
scientificName: Zelus gilboventris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:BOLIVIA; stateProvince:Cochabamba; locality:Villa Tunari, Chapare; verbatimElevation:500 m; decimalLatitude:-16.91666; decimalLongitude:-65.36667; eventDate:1958-01-09; sex:Adult Male; catalogNumber:UCR_ENT 00016769; recordedBy:Wygodzinsky and Monros; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus gilboventris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:ECUADOR; stateProvince:Napo; locality:Jatun Sacha Biological Station, 20 km E of Puerto Napo; verbatimElevation:450 m; decimalLatitude:-1.0644; decimalLongitude:-77.613; georeferenceSources:Google Earth; eventDate:1989-01-14; sex:Adult Male; catalogNumber:UCR_ENT 00038447; recordedBy:E.A. Bergey and K.R. Hobson; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:UCB
-
scientificName: Zelus gilboventris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Huanuco; county:Leoncio Prado; locality:Monzon Valley, Tingo Maria; verbatimElevation:648 m; decimalLatitude:-9.29528; decimalLongitude:-75.99754; georeferenceSources:Google Earth; eventDate:1954-10-15; sex:Adult Female; catalogNumber:UCR_ENT 00006074; recordedBy:E. I. Schlinger & E. S. Ross; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus gilboventris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Huanuco; county:Leoncio Prado; locality:Tingo Maria; verbatimElevation:671 m; decimalLatitude:-9.3; decimalLongitude:-75.9833; eventDate:1946-11-02; sex:Adult Male; catalogNumber:UCR_ENT 00016997; recordedBy:J. C. Pallister; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus gilboventris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Huanuco; locality:Upper Rio Huallaga; decimalLatitude:-9.2975; decimalLongitude:-76.005; georeferenceSources:Google Earth; eventDate:1928-01-02; sex:Adult Female; catalogNumber:UCR_ENT 00016998; recordedBy:H. Bassler; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus gilboventris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Huanuco; county:Leoncio Prado; locality:Tingo Maria; verbatimElevation:671 m; decimalLatitude:-9.3; decimalLongitude:-75.9833; eventDate:1905-04-29; sex:Adult Female; catalogNumber:UCR_ENT 00016999; recordedBy:J. C. Pallister; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus gilboventris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Huanuco; locality:Monzon valley, Tingo Maria; decimalLatitude:-9.27816; decimalLongitude:-76.05562; georeferenceSources:Google Earth; eventDate:1954-09-23; sex:Adult Female; catalogNumber:UCR_ENT 00019700; recordedBy:E. I. Schlinger & E. S. Ross; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus gilboventris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Huanuco; locality:Monzon valley, Tingo Maria; decimalLatitude:-9.27816; decimalLongitude:-76.05562; georeferenceSources:Google Earth; eventDate:1954-11-02; sex:Adult Female; catalogNumber:UCR_ENT 00019701; recordedBy:E. I. Schlinger & E. S. Ross; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus gilboventris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Huanuco; locality:Monzon valley, Tingo Maria; decimalLatitude:-9.27816; decimalLongitude:-76.05562; georeferenceSources:Google Earth; eventDate:1954-11-21; sex:Adult Female; catalogNumber:UCR_ENT 00019702; recordedBy:E. I. Schlinger & E. S. Ross; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus gilboventris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Huanuco; locality:Tingo Maria (Town of); verbatimElevation:671 m; decimalLatitude:-9.3; decimalLongitude:-75.9833; georeferenceSources:GeoLocate Software; eventDate:1960-08-10; sex:Adult Male; catalogNumber:UCR_ENT 00029356; occurrenceRemarks:Genitalia dissected. Paratype designated by Hart, unpublished; recordedBy:D. A. Young; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus gilboventris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Huanuco; county:Leoncio Prado; locality:Tingo Maria; verbatimElevation:671 m; decimalLatitude:-9.3; decimalLongitude:-75.9833; eventDate:1940-11-23; sex:Adult Female; catalogNumber:UCR_ENT 00057801; occurrenceRemarks:Previously designated as 'allotype', a concept not used in the formal publication of this name (Zhang et al.); recordedBy:J. C. Pallister; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus gilboventris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Junin; locality:Satipo; decimalLatitude:-11.2667; decimalLongitude:-74.6833; georeferenceSources:Gazetteer; eventDate:1940-07-01; sex:Adult Male; catalogNumber:UCR_ENT 00029357; occurrenceRemarks:Drake collection; recordedBy:P. Papraychi; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus gilboventris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:unknown; locality:Pachitea; decimalLatitude:-9.41667; decimalLongitude:-75.16667; georeferenceSources:Gazetteer; eventDate:no date provided; sex:Adult Male; catalogNumber:UCR_ENT 00023700; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:RMNH
-
scientificName: Zelus gilboventris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:unknown; locality:Pachitea; decimalLatitude:-9.41667; decimalLongitude:-75.16667; georeferenceSources:Gazetteer; eventDate:no date provided; sex:Adult Male; catalogNumber:UCR_ENT 00023702; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:RMNH
Description
Zelus gilboventris Zhang & Hart, sp. n., habitus
b: Zelus gilboventris Zhang & Hart, sp. n., male, lateral view (UCR_ENT 00016997, Huanuco, Peru)
c: Zelus gilboventris Zhang & Hart, sp. n., female, dorsal view (UCR_ENT 00019700, Huanuco, Peru)
d: Zelus gilboventris Zhang & Hart, sp. n., female, lateral view (UCR_ENT 00019700, Huanuco, Peru)
Zelus gilboventris Zhang & Hart, sp. n., male genitalic structures
b: Zelus gilboventris Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: (
Diagnosis
The posterior pronotal lobe usually orangish-brown; the medial process rather long, much longer than paramere; and the anterior side of medial process keeled medially at apex. In females the dorsal surface is nearly uniformly brown, the lateral and ventral surfaces yellowish, and the quadrate cell and proximal margin of postcubital cell yellowish.
Distribution
South America (
Zelus gracilipes , sp. n.
-
scientificName: Zelus gracilipes; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:BOLIVIA; stateProvince:La Paz; locality:Tumupasa; verbatimElevation:457 m; decimalLatitude:-14.1487; decimalLongitude:-67.8876; georeferenceSources:Google Earth; eventDate:Dec 1921-22; sex:Adult Male; catalogNumber:UCR_ENT 00007998; occurrenceRemarks:Genitalia dissected.; recordedBy:Mulford Bio. Expl; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus gracilipes; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:BOLIVIA; stateProvince:Cochabamba; locality:Chapare; decimalLatitude:-16.711; decimalLongitude:-65.663; georeferenceSources:Google Earth; eventDate:no date provided; sex:Adult Female; catalogNumber:UCR_ENT 00009463; recordedBy:Zischka; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus gracilipes; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:BOLIVIA; stateProvince:Cochabamba; locality:Chapare; decimalLatitude:-16.711; decimalLongitude:-65.663; georeferenceSources:Google Earth; eventDate:no date provided; sex:Adult Female; catalogNumber:UCR_ENT 00071194; recordedBy:Zischka; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:TAMU
-
scientificName: Zelus gracilipes; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:BOLIVIA; stateProvince:none; locality:Rio Negro; eventDate:Jan; sex:Adult Male; catalogNumber:UCR_ENT 00009296; occurrenceRemarks:Additional Labels Mulford BioExpl 1921-1922; recordedBy:W. M. Mann; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus gracilipes; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:BRAZIL; stateProvince:Goias; locality:Campinas; decimalLatitude:-16.66785; decimalLongitude:-49.29149; georeferenceSources:Google Earth; eventDate:1905-04-17; sex:Adult Female; catalogNumber:UCR_ENT 00009297; recordedBy:T. Borgmeier; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus gracilipes; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km S Ariquemes, Fazenda Rancho Grande; verbatimElevation:300 m; decimalLatitude:-10.3; decimalLongitude:-62.86666; eventDate:1996-07-03 to 1996-07-15; sex:Adult Female; catalogNumber:UCR_ENT 00009450; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus gracilipes; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km S Ariquemes, Fazenda Rancho Grande; verbatimElevation:300 m; decimalLatitude:-10.3; decimalLongitude:-62.86666; eventDate:1997-11-04 to 1997-11-16; sex:Adult Male; catalogNumber:UCR_ENT 00009499; occurrenceRemarks:Drake Collection; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus gracilipes; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km SW of Ariquemes, near Fzda. Rancho Grande; decimalLatitude:-10.32921; decimalLongitude:-63.46881; eventDate:1997-11-04 to 1997-11-16; sex:Adult Male; catalogNumber:UCR_ENT 00026166; occurrenceRemarks:Drake Collection; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus gracilipes; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km SW of Ariquemes, near Fzda. Rancho Grande; decimalLatitude:-10.32921; decimalLongitude:-63.46881; eventDate:1997-11-04 to 1997-11-16; sex:Adult Male; catalogNumber:UCR_ENT 00026167; occurrenceRemarks:Drake Collection; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus gracilipes; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:ECUADOR; stateProvince:Napo; locality:Puerto Misahuali; verbatimElevation:541 m; decimalLatitude:-1.0345; decimalLongitude:-77.66366; georeferenceSources:Label; eventDate:1998-09-06 to 1998-09-19; sex:Adult Male; catalogNumber:UCR_ENT 00009449; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus gracilipes; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:ECUADOR; stateProvince:Napo; locality:Puerto Misahuali; verbatimElevation:541 m; decimalLatitude:-1.0345; decimalLongitude:-77.66366; georeferenceSources:Label; eventDate:1998-09-06 to 1998-09-19; sex:Adult Male; catalogNumber:UCR_ENT 00009492; occurrenceRemarks:Drake Collection; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus gracilipes; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:ECUADOR; stateProvince:Napo; locality:Alinahui, 20 km E of Puerto Napo; verbatimElevation:450 m; decimalLatitude:-1; decimalLongitude:-77.41666; eventDate:1992-12-01; sex:Adult Male; catalogNumber:UCR_ENT 00047971; recordedBy:E. S. Ross; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus gracilipes; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Huanuco; locality:Monzon valley, Tingo Maria; decimalLatitude:-9.27816; decimalLongitude:-76.05562; georeferenceSources:Google Earth; eventDate:1954-09-23; sex:Adult Female; catalogNumber:UCR_ENT 00006075; recordedBy:E. I. Schlinger & E. S. Ross; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus gracilipes; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:PERU; stateProvince:Madre de Dios; locality:Los Amigos Biol.Sta. trail 14; verbatimElevation:231 m; decimalLatitude:-12.57141; decimalLongitude:-70.09538; georeferenceSources:GPS; samplingProtocol:General Collecting; eventDate:2010-12-20; sex:Adult Female; catalogNumber:UCR_ENT 00044265; occurrenceRemarks:Primary DNA voucher RCW_2474; recordedBy:C. Weirauch; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:UCR
Description
Zelus gracilipes Zhang & Hart, sp. n., habitus
b: Zelus gracilipes Zhang & Hart, sp. n., male, lateral view (UCR_ENT 00009499, Rondonia, Brazil)
c: Zelus gracilipes Zhang & Hart, sp. n., female, dorsal view (UCR_ENT 00006075, Huanuco, Peru)
d: Zelus gracilipes Zhang & Hart, sp. n., female, lateral view (UCR_ENT 00006075, Huanuco, Peru)
b: Zelus gracilipes Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: (
Diagnosis
Recognized by the posterior pronotal lobe orangish-brown; the legs uniformly blackish-brown, without bands; the humeral angle rounded; and the head and pronotum with spine-like setae (last two characters shared with species of the Zelus vagans species group and the Zelus longipes species group). Males can also be recognized by the medial process posteriorly directed and apical portion laterally compressed (diagnostic of Zelus vagans species group). The medial process is comparatively shorter than those in other species of the Zelus vagans species group (
Etymology
From Latin gracilis, meaning slender.
Distribution
South America (
Zelus grandoculus , sp. n.
-
scientificName: Zelus grandoculus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang & Hart, 2016; country:GUATEMALA; stateProvince:Sacatepequez; locality:Antigua; verbatimElevation:1583 m; decimalLatitude:14.5611; decimalLongitude:-90.7344; georeferenceSources:Gazetteer; eventDate:no date provided; sex:Adult Male; catalogNumber:UCR_ENT 00007999; occurrenceRemarks:Genitalia dissected; recordedBy:B. Lott; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
Description
Zelus grandoculus Zhang & Hart, sp. n., habitus
b: Zelus grandoculus Zhang & Hart, sp. n., male, lateral view (UCR_ENT-00007999, Sacatepequez, Guatemala)
Zelus grandoculus Zhang & Hart, sp. n., male genitalic structures
b: Zelus grandoculus Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: Unknown.
Diagnosis
This is the only species in the genus with the margins of the eye exceeding outlines of the head both dorsally and ventrally. Compared to other species of the Zelus luridus species group (
Etymology
The species epithet combines grandis, meaning large, with oculus, meaning eye, to indicate the prominently large-sized compound eye.
Distribution
Known only from type locality in Guatemala (
Zelus grassans
Nomenclature
Zelus grassans Stål, 1862, p, 450, orig. descr.; Stål, 1872, p. 91, cat.; Lethierry and Severin, 1896, p. 152, cat.; Champion, 1898, p. 256–257, Tab. XV, fig. 16, 17, note and fig.; Kuhlgatz, 1902, p. 266, note; Fracker, 1913, p. 239, 240, key and list (subgenus Diplodus); Wygodzinsky, 1949a, p. 49, checklist; Hart, 1986, p. 546-547, redescription, note, fig. and key; Maldonado, 1990, p. 327, cat.
Diplodus grassans: Walker, 1872, p. 124, cat.; Uhler, 1886, p. 24, checklist.
-
scientificName: Zelus grassans; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; sex:Adult Female; catalogNumber:UCR_ENT 00075073; occurrenceRemarks:Verbatim label info: Mexico coll. Signoret / grassans det. Stal / B.C.A. Rhyn.II. Zelus grassans St. / Zelus grassans Stal / Holotype / Lectotypus Zelus grassans STAL, 1862 etik. Hecher 1996 REDV. 471/1; recordedBy:Signoret; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:NHMW
Description
b: Zelus grassans Stål, 1862, male, lateral view (UCR_ENT 00025682, Morelos, Mexico)
c: Zelus grassans Stål, 1862, male, dorsal view (UCR_ENT 00034177, Guanacaste, Costa Rica)
d: Zelus grassans Stål, 1862, female, dorsal view (UCR_ENT 00030373, Tamaulipas, Mexico)
Zelus grassans Stål, 1862, male genitalic structures
b: Zelus grassans Stål, 1862, phallus, dorsal view
Male: (
Female: (
Diagnosis
Can be distinguished by the following combination of characters: Humeral angle without or with minute processes; head and legs predominantly reddish; abdominal segments usually banded; pronotum with erect, nearly spine-like setae. Males can also be recognized by the paramere greatly curved at middle and distinctly tapered apically; the medial process curved and directed posteriad; and the dorsal phallothecal sclerite constricted and the apex truncate, without emargination. The only species within the range of Z. grassans with which the females may be confused is Z. ruficeps. The pronotal armature readily separate them, that of Z. ruficeps consisting of broad dentate lateral processes while those of Z. grassans are as given above.
Distribution
From Mexico to Panama (
Taxon discussion
There is a great amount of size and color variations in Z. grassans. The dorsal coloration varies from almost entirely yellowish with only a narrow transverse dark band near the posterior margins of the pronotal lobes, to almost entirely dark brown. The legs show a similar range of coloration, from almost entirely light to entirely dark. The contrasting black and orange or red colors and the banded abdomen in many specimens of Z. grassans suggest that they may be mimics of Dysdercus, whose members have similarly strongly contrasting red and black colors. They have been observed to co-occur on the same plant (Zhang, unpublished), indicating that this may be a case of aggressive mimicry.
Zelus illotus
Nomenclature
Zelus illotus Berg, 1879, p. 153–154, orig. descr. (subgenus Diplodus); Lethierry and Severin, 1896, p. 152, cat.; Wygodzinsky, 1949a, p. 49, checklist; Wygodzinsky, 1949b, p. 336, note; Wygodzinsky, 1957, p. 264, 268, list and junior syn. of Z. obscuridorsis; Hart, 1987, p. 297, redescription, note, fig, key, lectotype desig. and stat. rev.; Maldonado, 1990, p. 330, cat.
Zelus carvalhoi Wygodzinsky, 1947, p. 428–431, orig. descr. and fig.; Zikan and Wygodzinsky, 1948, p. 17, list; Wygodzinsky, 1949a, p. 48, checklist; Wygodzinsky, 1949b, p. 336, note; wygodzinsky, 1957, p. 264, 268, list and junior syn. of Z. obscuridorsis; Hart, 1987, p. 297, junior syn. of Z. illotus Berg.
-
scientificName: Zelus illotus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Berg, 1879; country:ARGENTINA; stateProvince:Corrientes; locality:Corrientes; decimalLatitude:-27.484102; decimalLongitude:-58.809555; georeferenceSources:Google map; eventDate:no date provided; sex:Adult Male; occurrenceRemarks:Designated as lectotype by Hart (1987). Bears the following labels: Typus / Corrientes / 1554 / Lectotype, designated by E.R. Hart; recordedBy:Unknown; identifiedBy:E.R. Hart 1972; institutionCode:Unversidad Nacional de La Plata
-
scientificName: Zelus illotus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Berg, 1879; country:ARGENTINA; stateProvince:Corrientes; locality:Corrientes; decimalLatitude:-27.484102; decimalLongitude:-58.809555; georeferenceSources:Google map; eventDate:no date provided; sex:Adult Female; occurrenceRemarks:Designated as allolectotype by Hart (1987). Bears the following labels: Typus / Corrientes / Zelus illotus Bert / 168(9) / 1554 / Allolectotype, designated by E.R. Hart; recordedBy:Unknown; identifiedBy:E.R. Hart 1972; institutionCode:Unversidad Nacional de La Plata
-
scientificName: Zelus illotus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Berg, 1879; country:BRAZIL; stateProvince:Matto Grosso; locality:Chavantina, Rio des Mortes; decimalLatitude:-14.66667; decimalLongitude:-52.35; georeferenceSources:Gazetteer; eventDate:1947-06; sex:Adult Male; occurrenceRemarks:Holotype of Zelus carvalhoi Wygodzinsky, 1947, junior synonym of Zelus illotus Berg, 1879 (Hart, 1987); recordedBy:J.C.M Carvalho; identifiedBy:E.R. Hart 1972; institutionCode:Museu Nacionaldo Rio de Janeiro
-
scientificName: Zelus illotus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Berg, 1879; country:BRAZIL; stateProvince:Matto Grosso; locality:Chavantina, Rio des Mortes; decimalLatitude:-14.66667; decimalLongitude:-52.35; georeferenceSources:Gazetteer; eventDate:1947-06; sex:Adult Male; occurrenceRemarks:Paratype of Zelus carvalhoi Wygodzinsky, 1947, junior synonym of Zelus illotus Berg, 1879 (Hart, 1987); recordedBy:J.C.M Carvalho; identifiedBy:E.R. Hart 1972; institutionCode:Instituto de Ecologia e Experimentacao Agricola, Rio de Janeiro
-
scientificName: Zelus illotus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Berg, 1879; country:BRAZIL; stateProvince:Matto Grosso; locality:Chavantina; decimalLatitude:-14.66667; decimalLongitude:-52.35; georeferenceSources:Gazetteer; eventDate:1946-10; sex:Adult Male; occurrenceRemarks:Paratype of Zelus carvalhoi Wygodzinsky, 1947, junior synonym of Zelus illotus Berg, 1879 (Hart, 1987). Present location of specimen not known.; recordedBy:H. Sick; institutionCode:None
Description
Zelus illotus Berg, 1879, habitus
b: Zelus illotus Berg, 1879, male, lateral view (UCR_ENT 00013468, Suriname)
c: Zelus illotus Berg, 1879, female, dorsal view (UCR_ENT 00010836, Goias, Brazil)
Zelus illotus Berg, 1879, male genitalic structures
b: Zelus illotus Berg, 1879, phallus, dorsal view
Male: (
Female: (
Diagnosis
Among species of the Zelus nugax species group (
Distribution
South America and adjacent islands of the Caribbean (
Zelus impar
Nomenclature
Zelus impar Kuhlgatz, 1902, p. 264–266, Tab. IV, fig. 6, 6a, 6b, orig. descr. and fig.; Wygodzinsky, 1949a, p. 49, checklist; Hart, 1987, p. 297, redescription, note, fig., key and neotype desig.; Maldonado, 1990, p. 327, cat.
-
scientificName: Zelus impar; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Kuhlgatz & Melichar, 1902; country:COLOMBIA; stateProvince:Magdalena; locality:Santa Marta Mountains, Mount San Lorenzo; verbatimElevation:1524 m; decimalLatitude:11.12343; decimalLongitude:-74.0372; georeferenceSources:Gazetteer; eventDate:1926-02-02; sex:Adult Male; catalogNumber:UCR_ENT 00071190; occurrenceRemarks:Neotype designated by Hart (1987). Original type was destroyed; recordedBy:F. W. Walker; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:TAMU
Description
Zelus impar Kuhlgatz & Melichar, 1902, habitus
b: Zelus impar Kuhlgatz & Melichar, 1902, male, lateral view (UCR_ENT 00042091, Colombia)
c: Zelus impar Kuhlgatz & Melichar, 1902, male, dorsal view (UCR_ENT 00017740, Magdalena, Colombia)
d: Zelus impar Kuhlgatz & Melichar, 1902, male, lateral view (UCR_ENT 00017740, Magdalena, Colombia)
Zelus impar Kuhlgatz & Melichar, 1902, male genitalic structures
b: Zelus impar Kuhlgatz & Melichar, 1902, phallus, dorsal view
Male: (
Female: Unknown.
Diagnosis
Recognized by the slender, curved, laterally compressed, and apically tapered medial process (shared with members of the Zelus nugax species group;
Distribution
Panama and Northern South America and adjacent islands of the Caribbean (
Taxon discussion
Hart (1987) designated a neotype for Z. impar because the original type material of that species was destroyed during World War II. This neotype specimen was at that time deposited in the private collection of J. C. Elkins, Houston,Texas. This specimen was eventually transferred to and deposited at TAMU, instead of AMNH as the author had indicated.
Zelus inconstans
Nomenclature
Zelus inconstans Champion, 1898, p. 254–255, Tab. XV., fig. 13, orig. descr. and fig.; Wygodzinsky, 1947, p. 43, note; Wygodzinsky, 1949a, p. 49, checklist; Maldonado, 1990, p. 328, cat.
-
scientificName: Zelus inconstans; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Champion, 1899; country:PANAMA; stateProvince:Chiriqui; locality:Bugaba; decimalLatitude:8.4833; decimalLongitude:-82.6167; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00048756; occurrenceRemarks:Lectotype of Zelus inconstans Champion, 1898 (New Designation by Zhang, Hart & Weirauch, 2016). Verbatim label info: Type / B.C.A.Rhyn.II. Zelus inconstans Ch. / Sp. figured. / Bugaba, Panama. Champion. / Lectotype Zelus inconstans Champion des. by E.R. Hart/Lectotype Zelus inconstans Champion, 1898 designated and published by Zhang, Hart & Weirauch (2016); recordedBy:G.C. Champion; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:BMNH
-
scientificName: Zelus inconstans; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Champion, 1899; country:PANAMA; stateProvince:Chiriqui; locality:Bugaba; decimalLatitude:8.4833; decimalLongitude:-82.6167; eventDate:No date provided; sex:Adult Female; occurrenceRemarks:Paralectotype of Zelus inconstans Champion, 1898 (New Designation by Zhang, Hart & Weirauch, 2016). Verbatim label info: Type / B.C.A.Rhyn.II. Zelus inconstans Ch. / Bugaba, Panama. Champion. / Paralectotype Zelus inconstans Champion des. by E.R. Hart/Lectotype Zelus inconstans Champion, 1898 designated and published by Zhang, Hart & Weirauch (2016); recordedBy:G.C. Champion; identifiedBy:Hart; dateIdentified:1972; institutionCode:BMNH
-
scientificName: Zelus inconstans; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Champion, 1899; country:PANAMA; stateProvince:Chiriqui; locality:Bugaba; decimalLatitude:8.4833; decimalLongitude:-82.6167; eventDate:No date provided; sex:Adult Female; occurrenceRemarks:Paralectotype of Zelus inconstans Champion, 1898 (New Designation by Zhang, Hart & Weirauch, 2016). Verbatim label info: Type / B.C.A.Rhyn.II. Zelus inconstans Ch. / Bugaba, Panama. Champion. / Paralectotype Zelus inconstans Champion des. by E.R. Hart / Lectotype Zelus inconstans Champion, 1898 designated and published by Zhang, Hart & Weirauch (2016); recordedBy:G.C. Champion; identifiedBy:Hart; dateIdentified:1972; institutionCode:BMNH
Description
Zelus inconstans Champion, 1899, habitus
b: Zelus inconstans Champion, 1899, male, lateral view (UCR_ENT 00017735, Cundinamarca, Colombia)
c: Zelus inconstans Champion, 1899, female, dorsal view (UCR_ENT 00017738, Canal Zone, Panama)
d: Zelus inconstans Champion, 1899, female, lateral view (UCR_ENT 00017738, Canal Zone, Panama)
Zelus inconstans Champion, 1899, male genitalic structures
b: Zelus inconstans Champion, 1899, phallus, dorsal view
Male: (
Female: (
Diagnosis
The humeral angle rounded and the pronotum lacking conspicuous spine-like setae are diagnostic of this species. Most other species with unarmed humeral angle also have spine-like setae on pronotum and are in the Zelus longipes species group. Males can also be recognized by the paramere apically greatly projected dorsad (
Distribution
Southern Central America and northern South America (
Taxon discussion
Coloration variations are mainly seen on the pronotum. The two males from Panama show similar coloration, the central 1/3 of the pronotum being lighter than the margins. The Colombian male, however, has a nearly unicolorous pronotum. The females from Panama have pronotal coloration ranging from reddish-brown to brown, the lighter color apparently being more common. The single female from Colombia has the lighter pronotum while that of the Peruvian specimen is dark brown. The lectotype and two paralectotypes exhibit the lighter coloration. The banding patterns of the legs appear to be somewhat variable in Panama, the only area from which several specimens are available for comparison.
Zelus janus
Nomenclature
Zelus janus Stål, 1862, p. 452, orig. descr.; Stål, 1872, p. 90, cat. (subgenus Diplodus); Lethierry and Severin, 1896, p. 152, cat.; Champion, 1898, (in part), p. 257–258, Tab. XV. fig. 19, note, fig. and senior syn. of Z. litigiosus; Fracker, 1913, p. 239, 240, key and list (subgenus Diplodus); Fracker and Bruner, 1924, p. 170–171, note; Wygodzinsky, 1949a, p. 49, checklist; Hart, 1986, p. 543, redescription, lectotype desig., note, key and fig.; Maldonado, 1990, p. 328, cat.
Diplodus janus: Uhler, 1886, p. 24, checklist; Walker, 1873, p. 124, cat.
-
scientificName: Zelus janus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; sex:Adult Male; catalogNumber:UCR_ENT 00041005; occurrenceRemarks:Lectotype designated by Hart (1986). Verbatim label info: Mexico / Salle / janus Stal / Lectotype Zelus janus Stal / designated by E.R.Hart / Typus / NHRS-GULI 000000331; recordedBy:Salle; otherCatalogNumbers:NHRS-GULI 000000331; institutionCode:NHRS
-
scientificName: Zelus janus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; sex:Adult Female; occurrenceRemarks:Allolectotype designated by Hart (1986).; recordedBy:Salle; institutionCode:NHRS
-
scientificName: Zelus janus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; sex:Adult Male; occurrenceRemarks:Paralectotype designated by Hart (1986).; recordedBy:Salle; institutionCode:NHRS
-
scientificName: Zelus janus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; sex:Adult Female; occurrenceRemarks:Paralectotype designated by Hart (1986).; recordedBy:Salle; institutionCode:NHRS
-
scientificName: Zelus janus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; sex:Adult Male; occurrenceRemarks:Paralectotype designated by Hart (1986). Bears labels: Mexico / CoIl. Signoret / Janus, det. Stal.; recordedBy:Signoret; institutionCode:NHMW
-
scientificName: Zelus janus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; individualCount:3; sex:Adult Female; occurrenceRemarks:Paralectotypes designated by Hart (1986). Bear labels: Mexico / CoIl. Signoret / Janus, det. Stal.; recordedBy:Signoret; institutionCode:NHMW
Description
Zelus janus Stål, 1862, habitus
b: Zelus janus Stål, 1862, male, lateral view (UCR_ENT 00017829, Tamaulipas, Mexico)
c: Zelus janus Stål, 1862, female, dorsal view (UCR_ENT 00040424, Tamaulipas, Mexico)
d: Zelus janus Stål, 1862, female, lateral view (UCR_ENT 00040424, Tamaulipas, Mexico)
e: Zelus janus Stål, 1862, male, dorsal view (UCR_ENT 00034238, Oaxaca, Mexico)
Male: (
Female: (
Diagnosis
Among closely related species in the Zelus armillatus species group with overlapping distribution ranges, Z. janus is the only species with the humeral angle elevated to about same level as the disc of the posterior pronotal lobe. This species is much larger than and the coloration different from two other species sharing this feature, Z. exsanguis and Z. ambulans, both from a different species group. Other characters useful for diagnosis include the dorsal surface usually mostly brown and the lateral and ventral surfaces yellowish-brown; the abdominal segment each with single dark spot anteriorly; and in males the medial process narrower, relatively short.
Distribution
Southern Texas to Central America (
Taxon discussion
It is rather difficult to distinguish Z. janus from Z. armillatus on the basis of the male genitalia alone, the only two species in the genus where such distinction cannot be made. As the humeral angle of the posterior pronotal lobe are raised to the level of and are nearly continuous with the disc, however, it is quite easy to separate specimens of these species. There is further divergence in coloration and pattern between the two which may be seen in the descriptions of these species. A sympatric and closely related species, Z. litigiosus, also has the disc elevated above the humeral angle and is easily distinguished. Zelus janus has a somewhat more uniform brown dorsal coloration, whereas the color pattern in Z. litigiosus is more variable.
Zelus kartabensis
Nomenclature
Zelus kartabensis Haviland, 1931: 137, 148, 152 (checklist, fig. and orig. descr. [subgenus Diplodus]); Wygodzinsky, 1949a: 49 (checklist); Maldonado, 1990, p. 328, cat.
Zelus pallidinervus Haviland, 1931: 137, 148, 153 (checklist, fig and orig. descr. [subgenus Diplodus]); Wygodzinsky, 1949a: 50 (checklist); Maldonado, 1990, p. 330, cat. syn. nov. (current study).
-
scientificName: Zelus kartabensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Haviland, 1931; country:GUYANA; stateProvince:Cuyuni-Mazaruni Region; locality:Kartabo, British Guiana; decimalLatitude:6.384; decimalLongitude:-58.695; eventDate:1922-06; sex:Adult Male; catalogNumber:UCR_ENT 00048761; occurrenceRemarks:Lectotype of Zelus kartabensis Haviland, 1931 (New Designation by Zhang, Hart & Weirauch, 2016). Verbatim label info: Type / [blue label, no content] / Kartabo, Brit. Guiana June 1922 e coll.M.D. Haviland d.d.Collegium Newnhamense / Pres. by Mrs Brindley. B.M.1928-172. / Zelus kartabensis Haviland / Lectotype Zelus kartabensis Haviland des. by E.R. Hart; recordedBy:M.D. Haviland; institutionCode:BMNH
-
scientificName: Zelus kartabensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Haviland, 1931; country:GUYANA; stateProvince:Cuyuni-Mazaruni Region; locality:Kartabo, British Guiana; decimalLatitude:6.384; decimalLongitude:-58.695; eventDate:1922-07; sex:Adult Male; catalogNumber:UCR_ENT 00048761; occurrenceRemarks:Paralectotype of Zelus kartabensis Haviland, 1931 (New Designation by Zhang, Hart & Weirauch, 2016). Verbatim label info: Kartabo, Brit. Guiana July 1922 e coll.M.D. Haviland d.d.Collegium Newnhamense / Pres. by Mrs Brindley. B.M.1928-172. / Zelus Kartabensis Haviland / Paralectotype Zelus kartabensis Haviland des. by E.R. Hart; recordedBy:M.D. Haviland; institutionCode:BMNH
-
scientificName: Zelus kartabensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Haviland, 1931; country:GUYANA; stateProvince:Cuyuni-Mazaruni Region; locality:Kartabo, British Guiana; decimalLatitude:6.384; decimalLongitude:-58.695; eventDate:1922-08; sex:Adult Female; occurrenceRemarks:Lectotype of Zelus pallidinervus Haviland, 1931 (New Designation by Zhang, Hart & Weirauch, 2016), junior synonym of Zelus kartabensis Haviland, 1931. Bears labels: Type / Kartabo, Brit. Guiana, August 1922, e. coll. M. D. Haviland, d. d. collegium Newnhamense / Pres. by Mrs. Brindley, B. M. 1928-127 / Zelus pallidinervis Haviland; recordedBy:M.D. Haviland; institutionCode:BMNH
-
scientificName: Zelus kartabensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Haviland, 1931; country:GUYANA; stateProvince:Cuyuni-Mazaruni Region; locality:Kartabo, British Guiana; decimalLatitude:6.384; decimalLongitude:-58.695; eventDate:1922-08; sex:Adult Female; occurrenceRemarks:Paralectotype of Zelus pallidinervus Haviland, 1931 (New Designation by Zhang, Hart & Weirauch, 2016), junior synonym of Zelus kartabensis Haviland, 1931. Bears labels: Kartabo, Brit. Guiana, September 1922, e. coll. M. D. Haviland, d. d. collegium Newnhamense / Pres. by Mrs. Brindley, B. M. 1928-127 / Zelus pallidinervis Haviland; recordedBy:M.D. Haviland; institutionCode:BMNH
Description
Zelus kartabensis Haviland, 1931, habitus
b: Zelus kartabensis Haviland, 1931, male, lateral view (UCR_ENT 00047969, Para, Brazil)
c: Zelus kartabensis Haviland, 1931, female, dorsal view (UCR_ENT 00017859, Para, Brazil)
d: Zelus kartabensis Haviland, 1931, female, lateral view (UCR_ENT 00017859, Para, Brazil)
Zelus kartabensis Haviland, 1931, male genitalic structures
b: Zelus kartabensis Haviland, 1931, phallus, dorsal view
Male: (
Female: (
Diagnosis
The dorsal surface of posterior pronotal lobe uniformly dark brown. The paramere gradually enlarged, somewhat club-shaped; the medial process with ridge-like medial elevation through apical 1/2.
Zelus kartabensis is most similar to Z. kartaboides, and the differences between the two species are presented in the diagnosis of the latter species.
Distribution
South America (
Zelus kartaboides , sp. n.
-
scientificName: Zelus kartaboides; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Meta; locality:Rio Guayuriba; verbatimElevation:400 m; decimalLatitude:4.01978; decimalLongitude:-73.60807; georeferenceSources:Google Earth; eventDate:1947-09-06; sex:Adult Male; catalogNumber:UCR_ENT 00071182; occurrenceRemarks:Holotype of Zelus kartaboides Zhang & Hart, 2016; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:TAMU
-
scientificName: Zelus kartaboides; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Amazonas; locality:Tapurucuara Rio Negro; decimalLatitude:-0.4; decimalLongitude:-65.0333; georeferenceSources:Gazetteer; eventDate:1963-02-22; sex:Adult Male; catalogNumber:UCR_ENT 00042153; occurrenceRemarks:Paratype of Zelus kartaboides Zhang & Hart, 2016; recordedBy:C. Lindemann; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:ZSM
-
scientificName: Zelus kartaboides; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Mato Grosso; locality:Mato Gr.; decimalLatitude:-10.41666; decimalLongitude:-59.46667; georeferenceSources:Label; eventDate:1977-03-17; sex:Adult Male; catalogNumber:UCR_ENT 00017890; occurrenceRemarks:Paratype of Zelus kartaboides Zhang & Hart, 2016; recordedBy:D. Engleman; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus kartaboides; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Mato Grosso; locality:Mato Gr.; decimalLatitude:-10.41666; decimalLongitude:-59.46667; georeferenceSources:Label; eventDate:1977-03-17 to 1977-03-22; sex:Adult Male; catalogNumber:UCR_ENT 00046978; occurrenceRemarks:Paratype of Zelus kartaboides Zhang & Hart, 2016; recordedBy:D. Engleman; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus kartaboides; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Mato Grosso; locality:Mato Gr.; decimalLatitude:-10.41666; decimalLongitude:-59.46667; georeferenceSources:Label; eventDate:1977-03-17 to 1977-03-22; sex:Adult Male; catalogNumber:UCR_ENT 00046982; occurrenceRemarks:Paratype of Zelus kartaboides Zhang & Hart, 2016; recordedBy:D. Engleman; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus kartaboides; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Para; locality:Tucurui; decimalLatitude:-3.7; decimalLongitude:-49.7; georeferenceSources:Gazetteer; eventDate:1979-01-01; sex:Adult Male; catalogNumber:UCR_ENT 00047047; occurrenceRemarks:Paratype of Zelus kartaboides Zhang & Hart, 2016; recordedBy:M. Alvarenga; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus kartaboides; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Meta; locality:Rio Guayeriba, a triburary of Rio Meta; eventDate:1948-05-17; sex:Adult Male; catalogNumber:UCR_ENT 00017862; occurrenceRemarks:Paratype of Zelus kartaboides Zhang & Hart, 2016; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus kartaboides; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Meta; locality:Cano Grande; verbatimElevation:490 m; eventDate:1948-01-20; sex:Adult Male; catalogNumber:UCR_ENT 00071183; occurrenceRemarks:Paratype of Zelus kartaboides Zhang & Hart, 2016; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:TAMU
-
scientificName: Zelus kartaboides; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:unknown; locality:Buena Vista; verbatimElevation:1300 m; eventDate:1944-02-06; sex:Adult Male; catalogNumber:UCR_ENT 00071184; occurrenceRemarks:Paratype of Zelus kartaboides Zhang & Hart, 2016; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:TAMU
-
scientificName: Zelus kartaboides; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Sucumbios; locality:Dureno; verbatimElevation:150 m; decimalLatitude:0.0444; decimalLongitude:-76.6972; georeferenceSources:Gazetteer; eventDate:1977-23-09 to 1977-09-30; sex:Adult Male; catalogNumber:UCR_ENT 00009521; occurrenceRemarks:Paratype of Zelus kartaboides Zhang & Hart, 2016. Drake Collection; recordedBy:L. Pena; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus kartaboides; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PERU; stateProvince:Huanuco; locality:Monzon valley, Tingo Maria; decimalLatitude:-9.27816; decimalLongitude:-76.05562; georeferenceSources:Google Earth; eventDate:1954-10-15; sex:Adult Male; catalogNumber:UCR_ENT 00019083; occurrenceRemarks:Paratype of Zelus kartaboides Zhang & Hart, 2016; recordedBy:E. I. Schlinger & E. S. Ross; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
Description
Zelus kartaboides Zhang & Hart, sp. n., habitus
b: Zelus kartaboides Zhang & Hart, sp. n., male, lateral view (UCR_ENT 00071182, Meta, Colombia)
Zelus kartaboides Zhang & Hart, sp. n., male, genitalic structures
b: Zelus kartaboides Zhang & Hart, sp. n., phallus
Male: (
Female: Unknown.
Diagnosis
The posterior margin of the pronotum and the scutellum yellowish, strongly contrasting to the remaining dark brown dorsal surface, makes this species easily recognizable among all species of Zelus, including the very similarly looking Z. kartabensis. It can also be recognized by the medial process with ridge-like medial elevation through apical 1/2 (also in Z. kartabensis and Z. chamaeleon). It is separated from Z. kartabensis by the paramere uniquely shaped like a banana and its diameter uniform throughout. Some specimens of Z. armillatus, Z. conjungens, Z. longipes and Z. ruficeps also exhibit a predominantly dark brown pronotum with posterior and/or dorsolateral margins yellow or orange, but they are much more larger and robust than Z. kartaboides and the male genitalic structures are very different.
Etymology
The specific epithet indicates that this species is rather similar to Z. kartabensis.
Distribution
South America (
Zelus korystos
Nomenclature
Zelus korystos Hart, 1986, p. 303-304, figs. 25–27, orig. descri., note, fig. and key; Maldonado, 1990, p. 328, cat.
-
scientificName: Zelus korystos; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:TRINIDAD AND TOBAGO; stateProvince:Caroni; locality:Montserrado; verbatimElevation:101 m; decimalLatitude:10.41666; decimalLongitude:-61.35; eventDate:1929-06-01; sex:Adult Male; catalogNumber:UCR_ENT 00008000; occurrenceRemarks:Genitalia dissected; recordedBy:Aug Busck; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus korystos; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:GUYANA; stateProvince:Cuyuni-Mazaruni Region; locality:Bartica , or 'Kartabo-Bartica'; verbatimElevation:1 m; decimalLatitude:6.4; decimalLongitude:-58.6166; georeferenceSources:GeoLocate Software; eventDate:1922-04-08; sex:Adult Male; catalogNumber:UCR_ENT 00017230; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
Description
Zelus korystos Hart, 1986, habitus
b: Zelus korystos Hart, 1986, male, lateral view (UCR_ENT 00017230, Cuyuni-Mazaruni, Guyana)
Zelus korystos Hart, 1986, male genitalic structures
b: Zelus korystos Hart, 1986, phallus, dorsal view
Male: (
Female: Unknown.
Diagnosis
Recognized by the nearly uniformly dark brown dorsum; the abdomen light-colored, pale yellowish-brown; the posterolateral rim with lightly sclerotized expansion between paramere and medial process; the medial process curved at middle; the anterior surface of the medial process carinate; the apex of the medial process hooklike, the curvature of paramere small; the dorsal phallothecal sclerite with strong carination at apical part, the lateral expansion close to basal arm. Most similar to Z. filicauda, but the medial process is shorter and not as strongly curved and the paramere curvature is weaker.
Distribution
South America and adjacent islands of the Caribbean (
Zelus laticornis
Nomenclature
Euagoras laticornis Herrich-Schaeffer, 1853, p. 123, Tab. CCCIX. Fig. C, orig. descr. and fig.
Zelus laticornis: Stål, 1872, p. 92, cat.; Lethierry and Severin, 1896, p. 152, cat.; Wygodzinsky, l949a, p. 49, checklist; Maldonado, 1990, p. 328, cat.
Darbanus laticornis: Walker, 1873, p. 127, cat.
Zelus formosus Haviland, 1931, p. 137, 151–152, list and orig. descr. (subgenus Diplodus); Wygodzinsky, 1949a, p. 49, checklist; Maldonado, 1990, p. 327, cat. syn. nov. (current study).
Zelus tristis Haviland, 1931, p. 137, 154, list and orig. descr. (subgenus Diplodus); Wygodzinsky, 1949a, p. 50, checklist; Maldonado, 1990, p. 331, cat. syn. nov. (current study).
-
scientificName: Zelus laticornis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:(Herrich-Schaeffer, 1853); country:GUYANA; stateProvince:Cuyuni-Mazaruni Region; locality:Kartabo, British Guiana; decimalLatitude:6.384; decimalLongitude:-58.695; eventDate:1922-08-01; sex:Adult Female; catalogNumber:UCR_ENT 00048764; occurrenceRemarks:Holotype of Zelus formosus Haviland, 1931, junior syonym of Zelus laticornis (Herrich-Schaeffer, 1853) Verbatim label info: Type / [blue label, no content] / Kartabo, Brit. Guiana August 1922 e coll.M.D. Haviland d.d.Collegium Newnhamense / Pres. by Mrs Brindley. B.M.1928-172. / Zelus formosus Haviland / Holotype / Zelus laticornis (Herrich-Schaeffer) det. E.R.Hart 1972; recordedBy:M.D. Haviland; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:BMNH
Description
Zelus laticornis (Herrich-Schaeffer, 1853), habitus
b: Zelus laticornis (Herrich-Schaeffer, 1853), male, lateral view (UCR_ENT 00013482, Suriname)
c: Zelus laticornis (Herrich-Schaeffer, 1853), male, dorsal view (UCR_ENT 00029339, Concepción, Paraguay)
d: Zelus laticornisI (Herrich-Schaeffer, 1853), male dorsal (UCR_ENT 00030401, Zamora-Chinchipe, Ecuador)
e: Zelus laticornis (Herrich-Schaeffer, 1853), female, dorsal view (UCR_ENT 00030392, La Paz, Bolivia)
f: Zelus laticornis (Herrich-Schaeffer, 1853), female, lateral view (UCR_ENT 00030392, La Paz, Bolivia)
Zelus laticornis (Herrich-Schaeffer, 1853), male genitalic structures
b: Zelus laticornis (Herrich-Schaeffer, 1853), phallus, dorsal view
Male: (
Female: (
Diagnosis
The strongly convex pronotum distinguishes this species from most other species of the genus. The males can be distinguished by the relatively small size (mean 10.82 mm); the dorsum of the posterior pronotal lobe usually with lighter colored, pale brown, with medial stripe; the broad, pentagonal, apically angulate medial process; the short, blade-like process on dorsal phallothecal sclerite; and the ridge mesad to the blade-like process. In females the head, pronotum and corium are usually orangish-brown to reddish.
Distribution
Southern Central America (Panama) and South America (
Taxon discussion
The type material of Z. laticornis (under the name Euagoras laticornis) was destroyed during World War II. The female holotype of Zelus formosus Haviland, 1931 is deposited in the Natural History Museum, London.
Zelus leucogrammus
Nomenclature
Reduvius leucogrammus Perty, 1834, p. 174, pl. 34, fig. 14, orig. descr. and fig.
Zelus leucogrammus: Stål, 1872, p. 90, cat. (subgenus Diplodus); Berg, 1879, p. 152–153, cat., descr. and nymph (subgenus Diplodus); Lethierry and Severin, 1896, p. 152, cat.; Costa Lima, 1940, p. 7, 218, 224 illus., biol. notes (subgenus Diplocodus); Wygodzinsky, 1949a, p. 49, checklist; Wygodzinsky, 1957, p. 264, list (Z. leucogrammus (sic.)); Wygodzinsky, 1960, p. 307, locality; Maldonado, 1990, p. 328, cat.
Description
Zelus leucogrammus (Perty, 1833), habitus
b: Zelus leucogrammus (Perty, 1833), male, lateral view (UCR_ENT 00047623, Minas Gerais, Brazil)
c: Zelus leucogrammus (Perty, 1833), male, dorsal view (UCR_ENT 00017803, Itapua, Paraguay)
d: Zelus leucogrammus (Perty, 1833), male, lateral view (UCR_ENT 00017803, Itapua, Paraguay)
e: Zelus leucogrammus (Perty, 1833), female, dorsal view (UCR_ENT 00047638, Minas Gerais, Brazil)
f: Zelus leucogrammus (Perty, 1833), female, lateral view (UCR_ENT 00047638, Minas Gerais, Brazil)
Zelus leucogrammus (Perty, 1833), male genitalic structures
b: Zelus leucogrammus (Perty, 1833), phallus, dorsal view
Male: (
Female: (
Diagnosis
The black dorsal and red ventral coloration is distinctive of this species. Other diagnostic characters include the legs uniformly black and the posterior pronotal lobe with medial depression.
Distribution
South America (
Taxon discussion
Zelus leucogrammus is one of the most distinctive species among Zelus. It can be easily recognized the red and black coloration, the medial depression on the posterior pronotal lobe. Variations in coloration are minimal and is usually seen in the size the dark area on the posterior pronotal lobe.
According to Dr. Heinz Wundt at ZSM (pers. comm.), the type material for this species was destroyed during World War II. The original description lists this species from the Amazon River. As this is such a distinctive species, it is not felt that neotype is needed.
Zelus lewisi , sp. n.
-
scientificName: Zelus lewisi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Alajuela; locality:Sector San Ramon de Dos Rios; decimalLatitude:10.16667; decimalLongitude:-84.08333; georeferenceSources:Google Earth; eventDate:1995-06-26; sex:Adult Male; catalogNumber:UCR_ENT 00014251; occurrenceRemarks:Verbatim coordinates info LN L N 318100 381900. Site number 5367; recordedBy:F. Quesada; otherCatalogNumbers:INBIO CRI002211269; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus lewisi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Alajuela; locality:San Cristobal; decimalLatitude:10.49557; decimalLongitude:-84.55206; georeferenceSources:Gazetteer; eventDate:1997-10-01 to 1997-11-01; sex:Adult Male; catalogNumber:UCR_ENT 00014250; recordedBy:F. Quesada; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus lewisi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Guanacaste; locality:Parque Nacional Guanacaste, Finca Aguirrez, Lado N. Volcan Orosi; decimalLatitude:10.99992; decimalLongitude:-85.46858; georeferenceSources:Google Earth; eventDate:1994-03-01; sex:Adult Male; catalogNumber:UCR_ENT 00014261; recordedBy:C. Moraga; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus lewisi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Guanacaste; locality:Parque Nacional Guanacaste, Finca Aguirrez, Lado N. Volcan Orosi; decimalLatitude:10.99992; decimalLongitude:-85.46858; georeferenceSources:Google Earth; eventDate:1994-03-01; sex:Adult Female; catalogNumber:UCR_ENT 00014262; recordedBy:C. Moraga; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus lewisi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Guanacaste; locality:Parque Nacional Guanacaste, Finca Aguirrez, Lado N. Volcan Orosi; decimalLatitude:10.99992; decimalLongitude:-85.46858; georeferenceSources:Google Earth; eventDate:1994-03-01; sex:Adult Female; catalogNumber:UCR_ENT 00014263; recordedBy:C. Moraga; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus lewisi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Alajuela; locality:Parque Nacional Volcan Tenorio. Estacion El Pilon; decimalLatitude:10.6607; decimalLongitude:-84.96272; georeferenceSources:Google Earth; eventDate:2005-10-20; sex:Adult Female; catalogNumber:UCR_ENT 00014264; recordedBy:A. Azofeifa; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus lewisi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Cartago; locality:Monumento Nacional Guayabo, Turrialba; decimalLatitude:9.97159; decimalLongitude:-83.69072; georeferenceSources:Google Earth; eventDate:1994-06-21; sex:Adult Female; catalogNumber:UCR_ENT 00014265; recordedBy:J. F. Corrales; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus lewisi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Guanacaste; locality:Est. Pitilla, 9 km S. Santa Cecilia, P.N. Guanacaste, A.C. Guanacaste; decimalLatitude:10.99261; decimalLongitude:-85.42948; georeferenceSources:Label; eventDate:1994-08-01; sex:Adult Female; catalogNumber:UCR_ENT 00014409; recordedBy:C. Moraga; otherCatalogNumbers:INBIO CR1OO2 029708; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus lewisi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Panama; locality:Cerro Campana; decimalLatitude:8.66666; decimalLongitude:-79.93333; georeferenceSources:Label; eventDate:1977-07-01; sex:Adult Male; catalogNumber:UCR_ENT 00017828; recordedBy:H. A. Hespenheide; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus lewisi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Heredia; locality:La Selva, 3 km S Puerto Viejo; decimalLatitude:10.43333; decimalLongitude:-84.01666; georeferenceSources:Label; eventDate:1973-10-15; sex:Adult Male; catalogNumber:UCR_ENT 00038446; recordedBy:P. A. Opler; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:UCB
Description
Zelus lewisi Zhang & Hart, sp. n., habitus
b: Zelus lewisi Zhang & Hart, sp. n., male, lateral view (UCR_ENT 00014251, Alajuela, Costa Rica)
c: Zelus lewisi Zhang & Hart, sp. n., female, dorsal view (UCR_ENT 00014263, Guanacaste, Costa Rica)
d: Zelus lewisi Zhang & Hart, sp. n., female, lateral view (UCR_ENT 00014263, Guanacaste, Costa Rica)
e: Zelus lewisi Zhang & Hart, sp. n., female, dorsal view (UCR_ENT 00014409, Guanacaste, Costa Rica)
f: Zelus lewisi Zhang & Hart, sp. n., female, lateral view (UCR_ENT 00014409, Guanacaste, Costa Rica)
Male: (
Female: (
Diagnosis
Recognized by the large and slender body and the posterior pronotal lobe bearing a pair of tubercles. Males can be easily recognized by the black coloration with white markings on scutellum and abdomen and females yellowish or reddish with black spots and markings. Among males of the Zelus armillatus group (
Etymology
The specific epithet is a patronym, named after Dr. James Lewis, in honor of his contribution to the curation of Heteroptera of Costa Rica at INBio. Without his and his fellow scientists' work the discovery of this species would not have been possible.
Distribution
Central America (
Zelus litigiosus
Nomenclature
Zelus litigiosus Stål, 1862, p. 453, orig. descr.; Stål, 1872, p. 90, cat. (subgenus Diplodus); Lethierry and Severin, 1896, p. 152, cat.; Champion, 1898, p. 257, Tab. XV. fig. 20, 20a, fig. and junior syn. of Z. janus; Maldonado, 1990, p. 328, cat.
Diplodus litigiosus: Walker, 1873, p. 124, cat; Uhler, 1886, p. 24, checklist.
-
scientificName: Zelus litigiosus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; sex:Adult Female; catalogNumber:UCR_ENT 00041006; occurrenceRemarks:Lectotype of Zelus litigiosus Stål, 1862 (New Designation by Zhang, Hart & Weirauch, 2016) Verbatim label info: Mexico / Salle / litigiosus Stal / Lectotype Zelus litigiosus Stal / designated by E.R.Hart / Typus / NHRS-GULI 000000332; recordedBy:salle; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:NHRS
-
scientificName: Zelus litigiosus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; sex:Adult Female; occurrenceRemarks:Paralectotype of Zelus litigiosus Stål, 1862 (New Designation by Zhang, Hart & Weirauch, 2016) Verbatim label info: Mexico / Salle / litigiosus Stal / Paralectotype Zelus litigiosus Stal / designated by E.R.Hart; recordedBy:salle; identifiedBy:G. Zhang; institutionCode:NHRS
Description
Zelus litigiosus Stål, 1872, habitus
b: Zelus litigiosus Stål, 1872, male, lateral view (UCR_ENT 00034145, Mexico, Mexico)
c: Zelus litigiosus Stål, 1872, male, dorsal view (UCR_ENT 00034156, Jalisco, Mexico)
d: Zelus litigiosus Stål, 1872, male, lateral view (UCR_ENT 00034156, Jalisco, Mexico)
e: Zelus litigiosus Stål, 1872, female, dorsal view (UCR_ENT 00019131, Colima, Mexico)
f: Zelus litigiosus Stål, 1872, female, lateral view (UCR_ENT 00019131, Colima, Mexico)
Zelus litigiosus Stål, 1872, male genitalic structures
b: Zelus litigiosus Stål, 1872, phallus
Male: (
Female: (
Diagnosis
Among species in the Zelus armillatus species group occurring in overlapping geographical regions, Zelus litigiosus can be easily distinguished from Z. janus by the elevated disc of the posterior pronotal lobe. It can be separated from Z. sulcicollis by the flat or slightly convex disc of the posterior pronotal lobe, and that being depressed in Z. sulcicollis.
Distribution
Southwestern Mexico (
Zelus longipes
Nomenclature
Cimex longipes Linnaeus, 1767, p. 724, orig. descr.; Gmelin, 1788, p. 2197, list (Reduvius); Turton, 1806, p. 690, descr.
Reduvius longipes: Fabricius, 1775, p. 730, descr.; Fabricius, 1781, p. 378, descr.; Fabricius, 1787, p. 309, list; Fabricius, 1794, p. 196, descr.
Zelus longipes: Fabricius, 1803, p. 283, descr.; Stål, 1872, p. 88-89, cat. (subgenus Zelus); Blanchard, 1840, p. 101, descr.; Stål, 1862, p. 449-450, descr.; Uhler, 1878, p. 427, list; Uhler, 1886, p, 24, checklist; Lethierry and Severin, 1896, p. 152, cat.; Champion, 1898, p. 253, note; Kirkaldy, 1900a, p. 263, note; Fracker, 1913, p. 239, 240, key and list (subgenus Zelus); Cotton, 1917, p. 170-173, note; Barber, 1923, p. 27-28, note and syn.; Wygodzinsky, 1949a, p. 49, checklist; Wolcott, 1950 (1948), p. 212, list and note; Elkins, 1951, p. 410, list; Guagliumi, 1953, p. 16, note; Barber, 1954, p. 13-14, list; Elkins, 1954, p. 44, 45, note and fig.; Simmonds, 1956, p. 232, note; Alayo, 1967, p. 5, 36-37, list and note; Hart, 1986, p. 543-546, redescription, note, fig. and key; Hart, 1987, p. 304, note and key; Maldonado, 1990, p. 328, cat.
Euagoras longipes: Walker, 1873, p. 117-118, cat.
Reduvius rubidus Lepeletier and Serville, 1825, p. 278, orig. descr,; Guerin-Meneville, 1857, p. 411-412, descr. and list (subgenus Evagoras).
Evagoras rubidus: Amyot and Serville, 1843, p. 368-369, descr. and senior syn. of Evagoras speciosus Burmeister. Stål, 1862, p. 449, junior syn. (in part) of Z. longipes. Walker, 1873, p. 117, junior syn. of Euagoras longipes.
Euagoras rubidus: Walker, 1873, p. 118, cat.
Zelus rubidus: Stål, 1872, p. 89, cat. and descr. (subgenus Zelus); Uhler, 1886, p. 24, checklist; Lethierry and Severin, 1896, p. 153, cat.; Champion, 1898, p. 252-253, cat. and note; Fracker, 1913, p. 238, 240, key and list (subgenus Zelus); Ballou, 1913, p. 65, note; Jones, 1914, p. 462, note; Osborne and Drake, 1915, p. 531, list; Cotton, 1917, p. 173, note; Ritchie, 1917, p. 94, note; Gibson, 1919, p. 276, list; Dash, 1920, p. 31, note; Barber, 1923, p. 27, junior syn. of Z. longipes; Bruner, 1926, p. 78, descr.; Gowdey, 1927, p. 16-17, note; Martorell, 1939, p. 189, list; Wygodzinsky, 1949a, p. 49, checklist and junior syn. of Z. longipes; Alayo, 1967, p. 36-37, note.
Reduvius phalangium Fabricius, 1794, p. 1966, orig. descr.; Zirnsen, 1964, p. 338, list; Hart, 1986, p. 543, junior syn. of Z. longipes.
Zelus phalangium: Fabricius, 1803, p. 283, descr.; Stål, 1872, p. 92, cat.; Lethierry and Severin, 1896, p. 153, cat.; Fracker, 1913, p. 240, descr. and list; Wygodzinsky, 1949a, p. 50, checklist.
Diplodus phalangium: Uhler, 1886, p. 24, checklist.
Zelus bilobus Say, 1832, p. 12, orig. descr.; LeConte, 1859, p. 306, descr.; Stål, 1862, p. 449, list (as variety of Z. longipes); Stål, 1872, p. 88, cat. (subgenus Zelus); Uhler,1876, p. 61, list; Uhler, 1886, p. 24, checklist; Lethierry and Severin, 1896, p. 151, cat.; Champion, 1898, p. 253, note; Van Duzee, 1909, p. 176, list; Torre-Bueno and Engelhardt, 1910, p. 150, list; Fracker, 1913, p. 239, 240, key and list (subgenus Zelus); Barber, 1914, p. 505, list; Van Duzee, 1916, p. 30, checklist (s .g. Zelus); Dozier, 1917, p. 542, note; Van Duzee, 1917, p. 259, cat. (subgenus Zelus); Dozier, 1920, p. 357, note; Blatchley, 1926, p. 568, 569, key and descr. (subgenus Zelus); Readio, 1927, p. 169-170, key, descr. and note; Miller, 1929, p. 462, note; Creighton 1936a p. 94, note; Creighton, 1936b, p. 382, note; Elliott , 1938, p. 39, key and list; Wygodzinsky, 1949a, p. 48, checklist; Elkins, 1951, p. 410, list; Sibley, 1951., p. 92, list ; Oliver, 1964, p. 316, note; Whitcomb and Bell , 1964, p. 22, list; Davis, 1969, p. 81, fig. and note (sic. Zellus bilobatus); Hart, 1986, p. 543, junior syn. of Z. longipes.
Euagoras speciosus Burmeister, 1835, p. 227, orig. descr.; Herrich-Schaeffer, 1848, p. 45, Tab. CCLXIV. fig. 817, descr. and fig; Hart, 1986, p. 543, junior syn. of Z. longipes.
Evagoras speciosus: Amyot and Serville, 1843, p. 368, junior syn. of Evagoras rubidus Le P. and Serv.
Zelus speciosus: Stål, 1862, p. 449, syn. (as variety of Z. longipes) Stål, 1872, p. 89, cat. (subgenus Zelus); Berg, 1879, p. 151, note; Uhler, 1886, p. 24, checklist; Lethierry and Severin, 1896, p. 153, cat.;, Kirkaldy, 1909, p. 32, list and syn.; Wygodzinsky, 1949a, p. 50, checklist.
Zelus speciosus var. stolli Lethierry and Severin, 1896, p. 152, nomen nudum; Champion, 1898, p. 253, syn. (=Zelus rubidus). Euagoras tricolor Herrich-Schaeffer, 1848, p. 45-46, Tab. CCLXIV, fig. 818, orig. descr. and fig.; Stål, 1862, p. 450, syn. (as variety of Zelus longipes); Stål, 1872, p. 89, syn. (as variety of Zelus speciosus); Champion, 1898, p. 253, junior syn. of Zelus rubidus; Fracker and Bruner, 1924, p. 170, list; Bruner, 1926, p. 79, descr; Wygodzinsky, 1949a, p. 50, junior syn. of Zelus speciosus.
Zelus mactans Stål, 1861, p. 148, orig. descr.; Stål, 1872, p. 88, cat. (subgenus Zelus); Uhler, 1886, p. 24, checklist; Lethierry and Severin, 1896, p. 152, cat.; Fracker, 1913, p. 239, 240, key and list (subgenus Zelus); Barber, 1923, p. 28, note; Wygodzinsky, 1949a, p. 49, checklist; Alayo, 1967, p. 36, key and note; Hart, 1986, p. 544, lectotype desig. and junior syn. of Z. longipes.
Diplodus mactans: Walker, 1873, p. 125, cat.
Velia agavis Blasguez, 1870, p. 289, 290, fig. 14, orig. descr.; Champion, 1898, p. 253, junior syn. of Z. rubidus; Kirkaldy, 1909, p. 32, junior syn. (as variety of Z. speciosus); Fracker, 1913, p. 240, junior syn. of Z. rubidus.
-
scientificName: Zelus longipes; family:Reduviidae; genus:Zelus; scientificNameAuthorship:(L., 1767); country:St. Thomas; sex:Adult Female; occurrenceRemarks:Bears the following labels: longipes / 65; institutionCode:Linnaean Society, London
-
scientificName: Zelus longipes; family:Reduviidae; genus:Zelus; scientificNameAuthorship:(L., 1767); country:CUBA; sex:Adult Male; occurrenceRemarks:Lectotype of Zelus mactans Stal, 1861 (designated by Hart, 1986), junior synonym of Zelus longipes (Linnaeus, 1767). Bears the following labels: Cuba / Stal / mactans Stal / Typus.; institutionCode:NHRS
-
scientificName: Zelus longipes; family:Reduviidae; genus:Zelus; scientificNameAuthorship:(L., 1767); country:CUBA; sex:Adult Female; occurrenceRemarks:Allolectotype of Zelus mactans Fabricius, 1803 (designated by Hart, 1986), junior synonym of Zelus mactans Stal, 1861, junior synonym of Zelus longipes (Linnaeus, 1767). Bears the following labels: Cuba / Stal / Stal / Paratypus.; institutionCode:NHRS
Description
Zelus longipes (L., 1767), habitus
b: Zelus longipes (L., 1767), male, lateral view (UCR_ENT 00046535, Chiapas, Mexico)
c: Zelus longipes (L., 1767), male, dorsal view (UCR_ENT 00046536, Baja California Sur, Mexico)
d: Zelus longipes (L., 1767), male, dorsal view (UCR_ENT 00017919, Copan, Honduras)
e: Zelus longipes (L., 1767), female, dorsal view (UCR_ENT 00025021, Baja California, Mexico)
f: Zelus longipes (L., 1767), female, lateral view (UCR_ENT 00025021, Baja California, Mexico)
Zelus longipes (L., 1767), male genitalic structures
b: Zelus longipes (L., 1767), phallus, dorsal view
Male: (
Female: (
Diagnosis
Although highly variable, the black and red coloration is distinctive of Z. longipes. The combination of size, coloration, rounded humeral angle, and raised anterior pronotal lobe serves to separate this species from any others that may cause confusions. Males can also be recognized by the long and slender medial process, the apex slightly folded posteriad, and the long paramere, exceeding apex of medial process. Among the Zelus longipes species group (
Distribution
Southern parts of US, Mexico, Central America, the Caribbean, Northern South America, Paraguay and Southern Brazil (
Taxon discussion
Zelus longipes is a highly variable species. In any given area there is a wide range of color and color pattern variations. The dorsal coloration can vary from nearly entirely orange brown, through various patterns of orange-brown and brownish-black, to almost completely black. The habitus images provided here only represent a subset of the range of variations. The most typical form is one with alternating orange and black areas on the dorsum, abdominal venter orange or reddish-brown, each segment black anteriorly, and legs black with two yellow rings medially.
Zelus luridus
Nomenclature
Zelus luridus Stål, 1862, p. 452, orig. descr.; Stål, 1872, p. 91, cat. (subgenus Diplodus) Lethierry and Severin, 1896, p. 152, cat.; Champion, 1898, p. 259–260, junior syn. of Z. exsanguis; Uhler, 1904, p. 364, list; Wirtner, 1904, p. 206, list; Torre-Bueno and Brimley, 1907, p. 437, list; Torre-Bueno, 1910, p. 32, note; Torre-Bueno and Engelhardt, 1910, p. 150, note; Torre-Bueno, 1913, p. 60, note (subgenus Diplodus); Barger, 1914, p. 506, list; Van Duzee, 1916, p. 30, junior syn. of Z. exsanguis; Hart, 1986, p. 537, redescription, lectotype desig, note, key, fig. and stat. rev.; Maldonado, 1990, p. 329, cat.
Diplodus luridus: Uhler, 1872a, p. 471, checklist; Uhler, 187213, p. 420, note; Walker, 1873, p. 124, cat.; Uhler, 1876, p. 61, note; Uhler, 1877, p. 429, note; Uhler, 1878, p. 427, note; Uhler, 1886, p. 24, checklist; Provancher, 1887, p. 181, note; Van Duzee, 1894, p. 183, list; Gillette and Baker, 1895, p. 60, list.
Diplocodus luridus: Van Duzee, 1912, p. 324, note.
Darbanus georgiae Provancher, 1872, p. 106, orig. descr.; Uhler, 1886, p. 24, checklist; Provancher, 1887, p. 181, note; Kelton, 1968, p. 1070, note; Hart, 1986, p. 53, junior syn. of Z. luridus.
Zelus georgiae: Lethierry and Severin, 1896, p. 152, cat.; Banks, 1910, p. 16, cat.; Van Duzee, 1916, p. 30, junior syn. of Z. exsanguis.
Zelus acanthogonius Say & Uhler, 1878, p. 427, manuscript name.
Evagoras viridis Uhler, 1878, p. 427, manuscript name
Darbanus palliatus Provancher, 1887, p. 182, orig. descr.; Kelton, 1968, 1070, note; Hart, 1986, p. 53, junior syn. of Z. luridus.
Zelus palliatus: Lethierry and Severin, 1896, p. 153, cat.; Banks, 1910, p. 16, cat.; Van Duzee, 1916, p. 30, junior syn. of Z. exsanguis.
Reduvius sp. Emmons, 1854, p. 168, P1. 7, fig. 3, note and fig.
Zelus exsanguis [misapplication of name due to Champion's synonymy of Z. luridus under Z. exsanguis]: Wirtner, 1904, p. 206, list; Barber, 1906, p. 285, list; Snow, 1907, p. 159, list; Parshley, 1914, p. 144, list; Hussey, 1922, p. 24, list; Britton, 1923, p. 687, list; Blatchley, 1926, p. 570–571, descr. and note (Diplodus); Readio, 1926, 167, P1. X, fig. 5, 6 and 7, note and fig.; Readio, 1927, p. 169, 171–177, P1. XIII, descr., notes and fig.; Leonard, 1928, p. 105, list; Brimley, 1938, p. 73, list; Harris, 1943, p. 151, list; Procter, 1946, p. 319, list; Elkins, 1951, p. 410, list; Sibley, 1951, p. 92, list; Hall, Downe, MacLellan and West, 1953; p. 199–204, note; Dome and West, 1954, p. 181–184, behavior; West and De Long, 1955, p. 97–101, biology; Davis, 1961, p. 351, note; Drew and Schaeffer, 1962, p. 106, list; Whitcomb and Bell, 1964, p. 22, list; Kelton, 1968, p. 1070– 1071, note; Yonke and Medler, 1970, p. 441–443, biology.
-
scientificName: Zelus luridus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:USA; stateProvince:North Carolina; county:unknown; locality:unknown; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00041007; occurrenceRemarks:Lectotype of Zelus luridus Stål, 1862 (designated by Hart, 1986). Verbatim label info: Germar / Carolina / luridus Stal / Lectotype Zelus luridus Stal / designated by E.R.Hart / Typus / NHRS-GULI 000000333; recordedBy:Stal; otherCatalogNumbers:NHRS-GULI 000000333; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:NHRS
-
scientificName: Zelus luridus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:USA; stateProvince:North Carolina; county:unknown; locality:unknown; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00041007; occurrenceRemarks:Paralectotype of Zelus luridus Stål, 1862 (Designated by Hart, 1986); recordedBy:Stal; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:NHRS
-
scientificName: Zelus luridus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:USA; stateProvince:Georgia; locality:unknown; eventDate:Macon; sex:Adult Male; occurrenceRemarks:Holotype of Darbanus georgiae Provancher, 1872, junior synonym of Zelus luridus Stål, 1862. Bears the following label: No. 114 Darbanus georgiae Prov. Zelus exsanguis (Stal).
-
scientificName: Zelus luridus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:CANADA; stateProvince:Ontario; eventDate:Ottawa; sex:Adult Male; occurrenceRemarks:Holotype of Darbanus palliatus Provancher, junior synonym of Zelus luridus Stål, 1862. Bears the following labels: No. 247, Darbanus palliatus Prov. Zelus exsanguis (Stal)
Description
Zelus luridus Stål, 1862, habitus
b: Zelus luridus Stål, 1862, male, lateral view (UCR_ENT 00047124, Iowa, USA)
c: Zelus luridus Stål, 1862, female, dorsal view (UCR_ENT 00039855, Texas, USA)
d: Zelus luridus Stål, 1862, female, lateral view (UCR_ENT 00039855, Texas, USA)
Zelus luridus Stål, 1862, male genitalic structures
b: Zelus luridus Stål, 1862, western population, pygophore, lateral and posterior views
c: Zelus luridus Stål, 1862, eastern population, phallus, dorsal view
d: Zelus luridus Stål, 1862, western population, phallus, dorsal view
Male: (
Female: (
Diagnosis
Recognized by the following combination of characters: Yellow-green to green-black; apices of femora with reddish or brown bands; disc elevated above humeral angle. As with other members of the Zelus luridus species group, the medial process is triangular, its base distinct from rest of the ventral rim of pygophore and apex without modification (
Distribution
North America (
Taxon discussion
This is one of the most commonly collected species in this genus.
Zelus mattogrossensis
Nomenclature
Zelus mattogrossensis Wygodzinsky, 1947, p. 431–434, orig. descr. and fig.; Wygodzinsky, 1948, p. 17, cat.; Wygodzinsky, 1949a, p. 49, checklist; Wygodzinsky, 1960, p. 308, note; Maldonado, 1990, p. 329, cat.
-
scientificName: Zelus mattogrossensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Wygodzinsky, 1947; country:BRAZIL; stateProvince:Mato Grosso; locality:Chavantina; decimalLatitude:-14.66667; decimalLongitude:-52.35; eventDate:1947-06; recordedBy:J.C.M. Carvalho; institutionCode:Instituto de Ecologia e Experimentacao Agricola, Rio de Janeiro
-
scientificName: Zelus mattogrossensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Wygodzinsky, 1947; country:BRAZIL; stateProvince:Goias; locality:Leopoldo Bulhoes; eventDate:1937-11; recordedBy:P. Wygodzinsky; institutionCode:collection of P. Wygodzinsky
-
scientificName: Zelus mattogrossensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Wygodzinsky, 1947; country:BRAZIL; stateProvince:Minas Gerais; locality:Carmo do Rio Claro; verbatimElevation:859 m; decimalLatitude:-20.9667; decimalLongitude:-46.1167; georeferenceSources:Gazetteer; eventDate:1944-12-13; sex:Adult Male; catalogNumber:UCR_ENT 00046752; recordedBy:J. C. M. Carvalho; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
Description
Zelus mattogrossensis Wygodzinsky, 1947, male genitalic structures
b: Zelus mattogrossensis Wygodzinsky, 1947, phallus, dorsal view
Male: (
Female: (
Diagnosis
Distinguished by the small size; the robust form, the humeral angle rounded, without projection; the profemur much longer than the metafemur (1.20x); the profemoral length being less than 20.0x the profemoral width (16.94x). The paramere base not distinctly constricted; the medial process slender, apex angulate and bearing subapical medial protrusion; the presence of blade-like process on dorsal phallotheca and the process not extending beyond mid-point.
Distribution
South America (
Zelus means
Nomenclature
Zelus means Fabricius, 1803, p. 282. orig. descr.; Stål, 1868, p. 107, descr.; Stål, 1872, p. 89, cat.; Walker, 1873, p. 134, cat; Lethierry and Severin, 1896, p.l62., cat.; Champion, 1898, p. 254, note; Wygodzinsky, 1949a, p. 49, checklist; Maldonado, 1990, p. 329, cat.
Eccagoras trimaculicollis Stål 1855, p. 189, orig. descr.
Zelus trimaculicollis Stål, 1866, p. 298, descr.; Stål, 1872, p. 89, cat.; Lethierry and Severin, 1896, p. 153, cat.; Champion, 1898, p. 254, note; Wygodzinsky, 1949a, p. 50, checklist; Maldonado, 1990, p. 331, cat. syn. nov. (current study).
Zelus trimaculatus Champion, 1898, p. 254, orig. descr.; Wygodzinsky, 1949a, p. 50, checklist; Maldonado, 1990, p. 331, cat. syn. nov. (current study).
-
scientificName: Zelus means; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Fabricius, 1803; locality:South America (American Meridonali); occurrenceRemarks:Lectotype of Zelus means Fabricius, 1803 (New Designation by Zhang, Har & Weirauch, 2016). Bears the following labels: Type / Z. means in Am. Mer. Schmidt; recordedBy:Schmidt; institutionCode:ZMUC
-
scientificName: Zelus means; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Fabricius, 1803; country:BRAZIL; stateProvince:unknown; locality:Unknown; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00041011; occurrenceRemarks:Lectotype of Zelus trimaculicollis Stal 1866 (New Designation by Zhang, Hart & Weirauch, 2016), now synonym of Zelus means Fabricius 1803. Verbatim label info: Brasil / Typus / trimaculicollis Stal / Lectotype Zelus trimaculicollis Stal / designated by E.R.Hart / Zelus means Fabricius det. E.R. Hart 1972 / NHRS-GULI 000000350; otherCatalogNumbers:NHRS-GULI 000000350; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:NHRS
-
scientificName: Zelus means; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Fabricius, 1803; country:BRAZIL; stateProvince:unknown; locality:Unknown; eventDate:No date provided; sex:Adult Female; occurrenceRemarks:Paralectotype of Zelus trimaculicollis Stal 1866 (New Designation by Zhang, Hart & Weirauch 2016), junior synonym of Zelus means Fabricius 1803. Verbatim label info: Brasil / Stal / Paratypus; institutionCode:NHRS
-
scientificName: Zelus means; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Fabricius, 1803; country:BRAZIL; stateProvince:unknown; locality:Unknown; eventDate:No date provided; sex:Adult Female; occurrenceRemarks:Paralectotype of Zelus trimaculicollis Stal 1866 (New Designation by Zhang, Hart & Weirauch, 2016), junior synonym of Zelus means Fabricius 1803. Verbatim label info: Brasil / Paratypus; institutionCode:NHRS
-
scientificName: Zelus means; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Fabricius, 1803; country:PANAMA; stateProvince:Chiriqui; eventDate:V. de Chiriqui; sex:Adult Female; occurrenceRemarks:Holotype of Zelus trimaculatus Champion 1898, junior synonym of Zelus means Fabricius 1803. Bears the following labels: Type/B.C.A. Rhyn. II. Zelus trimaculatus Ch / Sp. figured / V. de Chiriqui, 25-4000 ft., Champion; institutionCode:BMNH
Description
Zelus means Fabricius, 1803, habitus
b: Zelus means Fabricius, 1803, female, lateral view (UCR_ENT 00042088, Colombia)
c: Zelus means Fabricius, 1803, female, dorsal view (UCR_ENT 00002724, Ecuador)
d: Zelus means Fabricius, 1803, female, lateral view (UCR_ENT 00002724, Ecuador)
e: Zelus means Fabricius, 1803, female, dorsal view (adapted from Champion, 1898; as Zelus trimaculatus)
f: Zelus means Fabricius, 1803, female, dorsal view (UCR_ENT 00017819, Brazil)
Male: Unknown.
Female: (
Diagnosis
The humeral angle rounded; the pronotum with spine-like setae; and the colors usually consisting of yellow, orange, red and black. The anterior pronotal lobe is rather small, margins not laterally expanded, nearly continuous with lateral margins of posterior lobe, dorsally nearly flat, not bulging. The small anterior lobe together with the regularly sized posterior lobe gives the pronotum a triangular appearance. Most similar to Z. fuliginatus, but the postocular lobe is shorter.
Distribution
Southern Central America and South America (
Taxon discussion
On the basis of the spine-like setae on the head and pronotum and the rounded humeral angle, Zelus means would be placed in either the Zelus longipes species group or the Zelus vagans species group. Due to its close resemblance to Z. fuliginatus, this species is most likely part of the Zelus vagans species group.
This species appears to have a highly variable color pattern in all areas of its distribution. The clavus and corium range from entirely brownish-black to almost entirely light yellowish-brown. The dorsum of the pronotal lobe may be entirely brownish-black, but is usually variably patterned brownish-black and reddish-brown.
Zelus mimus
Nomenclature
Zelus mimus Stål, 1862, p. 451, orig. descr. (subgenus Diplodus); Stål, 1872, p. 91, cat. (subgenus Diplodus); Lethierry and Severin, 1896, p. 152, cat.; Champion, 1898, p. 257, 261, note, list and senior syn. of Z. umbratilis; Kuhlgatz, 1902, p. 266, note; Fracker, 1913, p. 239, 240, list (subgenus Diplodus); Williams, 1918, p. 163–173; Wygodzinsky, 1949a, p. 49, checklist; Maldonado, 1990, p. 329, cat.
Diplodus mimus: Walker, 1873, p. 124, cat.; Uhler, 1886, p. 24, checklist.
Zelus umbratilis Stål, 1862, p. 451, orig. descr. (subgenus Diplodus); Stål, 1872, p. 91, cat. (subgenus Diplodus); Lethierry and Severin, 1896, p. 153, cat.; Champion, 1898, p. 261, junior syn. of Z. mimus.
Diplodus umbratilis: Walker, 1873, p. 124, cat.; Uhler, 1886, p. 24, checklist.
-
scientificName: Zelus mimus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; stateProvince:None or Unknown; locality:unknown; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00075074; occurrenceRemarks:Lectotype of Zelus mimus Stål, 1862 (New Designation by Zhang, Hart & Weirauch, 2016). Verbatim label info: Mexico coll. Signoret / mimus det. Stal / B.C.A. Rhyn.II. Zelus mimus St. / Lectotype Zelus mimus Stal / designated by E. R. Hart / Lectotypus Zelus mimus STAL, 1862 etik. Hecher 1996 REDV. 480/1; recordedBy:Signoret; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:NHMW
-
scientificName: Zelus mimus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; stateProvince:None or Unknown; locality:unknown; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00041012; occurrenceRemarks:Lectotype of Zelus umbratilis Stål, 1862 (New Designation by Zhang, Hart & Weirauch, 2016), junior synonym of Zelus mimus Stål, 1862. Verbatim label info: Mexico / Salle / umbratilis Stal / Lectotype Zelus umbratilis Stal / designated by E.R.Hart / Zelus mimus Stal det. E.R. Hart 1972 / Typus / NHRS-GULI 000000351; recordedBy:salle; otherCatalogNumbers:NHRS-GULI 000000351; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:NHRS
Description
Zelus mimus Stål, 1862, habitus
b: Zelus mimus Stål, 1862, male, lateral view (UCR_ENT 00030644, Cartago, Costa Rica)
c: Zelus mimus Stål, 1862, female, dorsal view (UCR_ENT 00046735, Chiriqui, Panama)
d: Zelus mimus Stål, 1862, female, lateral view (UCR_ENT 00046735, Chiriqui, Panama)
e: Zelus mimus Stål, 1862, female, dorsal view (UCR_ENT 00046741, Chiriqui, Panama)
f: Zelus mimus Stål, 1862, female, dorsal view (UCR_ENT 00046739, Chiriqui, Panama)
Zelus mimus Stål, 1862, male genitalic structures
b: Zelus mimus Stål, 1862, phallus, dorsal view
Male: (
Female: (
Diagnosis
Dark brown coloration predominating dorsally in most specimens, posterior pronotal lobe laterally yellowish. Among species that have overlapping distributions (Southern Mexico and Central America), the coloration of Z. mimus is unique. Males can also be recognized by the paramere apically greatly projected dorsad (
Distribution
Southern Mexico and Central America (
Zelus minutus
Nomenclature
Zelus minutus Hart, 1987, p. 299-301, figs. 16-21, orig. descr., note, fig. and key; Maldonado, 1990, p. 330, cat.
-
scientificName: Zelus minutus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:SURINAME; stateProvince:Commewijne; locality:Pl. Leliendaal; decimalLatitude:5.86666; decimalLongitude:-55.03333; georeferenceSources:Gazetteer; eventDate:1963-03-17; sex:Adult Male; catalogNumber:UCR_ENT 00023689; recordedBy:P.H. van Doesburg, Jr; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:RMNH
-
scientificName: Zelus minutus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:SURINAME; stateProvince:Para; locality:Republiek; decimalLatitude:5.5; decimalLongitude:-55.2; georeferenceSources:Gazetteer; eventDate:1962-01-04; sex:Adult Female; recordedBy:P.H. van Doesburg, Jr; institutionCode:RMNH
-
scientificName: Zelus minutus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Magdalena; locality:Sevilla; decimalLatitude:10.76343; decimalLongitude:-74.13916; georeferenceSources:Gazetteer; eventDate:1926-07-24; sex:Adult Male; catalogNumber:UCR_ENT 00071201; recordedBy:F. W. Walker; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:TAMU
-
scientificName: Zelus minutus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PANAMA; stateProvince:Canal Zone; locality:Barro Colorado Island; decimalLatitude:9.16666; decimalLongitude:-79.83333; eventDate:1940-05-24; sex:Adult Male; catalogNumber:UCR_ENT 00009273; recordedBy:J. Zetek; otherCatalogNumbers:40-14769; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus minutus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PANAMA; stateProvince:Colon; locality:Barro Colorado Island, Canal Zone; decimalLatitude:9.15472; decimalLongitude:-79.84806; georeferenceSources:GeoLocate Software; eventDate:October; sex:Adult Male; catalogNumber:UCR_ENT 00017868; recordedBy:M. Bates; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus minutus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PANAMA; stateProvince:Colon; locality:Coco Solo; decimalLatitude:9.37; decimalLongitude:-79.8817; georeferenceSources:Gazetteer; eventDate:1944-04-27; sex:Adult Male; catalogNumber:UCR_ENT 00030177; occurrenceRemarks:'Planett 7144' and WW Wirth #1619 44-12740 on label; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus minutus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:TRINIDAD AND TOBAGO; stateProvince:Port-of-Spain; locality:Port-of-Spain, Department Agricultre Grounds; decimalLatitude:10.66615; decimalLongitude:-61.51613; georeferenceSources:Google Earth; eventDate:1918-11-23; sex:Adult Male; catalogNumber:UCR_ENT 00009272; recordedBy:Harold Morrison; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus minutus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:VENEZUELA; stateProvince:Portuguesa; locality:Guanare; verbatimElevation:173 m; decimalLatitude:9.05; decimalLongitude:-65.75; georeferenceSources:Gazetteer; eventDate:1957-09-10 to 1957-09-13; sex:Adult Male; catalogNumber:UCR_ENT 00019690; recordedBy:Borys Malkin; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:CAS
Description
Zelus minutus Hart, 1987, habitus
b: Zelus minutus Hart, 1987, male, lateral view (UCR_ENT 00017868, Panama)
c: Zelus minutus Hart, 1987, male, dorsal view (UCR_ENT 00009273, Panama)
d: Zelus minutus Hart, 1987, male, lateral view (UCR_ENT 00009273, Panama)
Male: (
Female: Similar to male, except for the following. Larger than male, total length 10.70 mm (
Diagnosis
This species can be readily recognized by the small size (<10.2 mm) and the disc of the posterior lobe with spinous tubercles. The medial process is broadly triangular, apex without modification (shared with the Zelus tetracanthus species group). Can be recognized among the Zelus tetracanthus species group by the relatively long paramere, exceeding the medial process.
Distribution
Southern Central America, South America and adjacent islands of the Caribbean (
Taxon discussion
Zelus minutus shows some variations through its range. The Panamanian specimens have noticeably shorter paramere and the apex of the dorsal phallothecal sclerite is somewhat less emarginate.
Zelus nigromaculatus
Nomenclature
Zelus nigromaculatus Champion, 1898, p. 261–262, Tab. XV, Fig. 26, orig. descr. and fig.; Wygodzinsky, 1949a, p. 49, checklist; Maldonado, 1990, p. 330, cat.
-
scientificName: Zelus nigromaculatus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Champion, 1899; country:PANAMA; stateProvince:Chiriqui; locality:Bugaba; verbatimElevation:457 m; decimalLatitude:8.4833; decimalLongitude:-82.6167; georeferenceSources:Gazetteer; eventDate:No date provided; sex:Adult Male; catalogNumber:UCR_ENT 00048757; occurrenceRemarks:Lectotype of Zelus nigromaculatus Champion, 1898 (New Designation by Zhang, Hart & Weirauch, 2016) Verbatim label info: Type / B.C.A.Rhyn.II. Zelus nigromaculatus Ch. / Sp. figured. / Bugaba, 800-1,500 ft. Champion. / Lectotype Zelus nigromaculatus Champion des. by E.R. Hart; recordedBy:G.C. Champion; identifiedBy:E. R. Hart; dateIdentified:1972; institutionCode:BMNH
-
scientificName: Zelus nigromaculatus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Champion, 1899; country:PANAMA; stateProvince:Chiriqui; locality:Bugaba; verbatimElevation:457 m; decimalLatitude:8.4833; decimalLongitude:-82.6167; georeferenceSources:Gazetteer; eventDate:No date provided; sex:Adult Female; occurrenceRemarks:Allolectotype of Zelus nigromaculatus Champion, 1898 (New Designation by Zhang, Hart & Weirauch, 2016) Verbatim label info: B.C.A.Rhyn.II. Zelus nigromaculatus Ch. / Bugaba, 800-1,500 ft. Champion. / Allolectotype Zelus nigromaculatus Champion des. by E.R. Hart; recordedBy:G.C. Champion; identifiedBy:E. R. Hart; dateIdentified:1972; institutionCode:BMNH
Description
Zelus nigromaculatus Champion, 1899, habitus
b: Zelus nigromaculatus Champion, 1899, male, lateral view (UCR_ENT 0001426, Limon, Costa Rica)
c: Zelus nigromaculatus Champion, 1899, female, dorsal view (UCR_ENT 00014444, Puntarenas, Costa Rica)
d: Zelus nigromaculatus Champion, 1899, female, lateral view (UCR_ENT 00014444, Puntarenas, Costa Rica
Zelus nigromaculatus Champion, 1899, male, genitalic structures
b: Zelus nigromaculatus Champion, 1899, phallus, dorsal view
Male: (
Female: (
Diagnosis
Recognized by the conspicuous black and yellow color pattern, resembling vespid wasp; and the meso- and metafemora with at least three dark bands three yellow bands. Among males of the Zelus panamensis group (
Distribution
Central America (
Zelus nugax
Nomenclature
Zelus nugax Stål, 1862, p. 450–451, orig. descr. (subgenus Diplodus); Stål, 1872, p. 91, cat. (subgenus Diplodus); Lethierry and Severin, 1896, p. 152, cat.; Champion, 1898, p. 257, 2, note and list; Kuhlgatz, 1902,, p. 266, note; Fracker, 1913, p. 239, 240, key and list (subgenus Diplodus); Fracker and Bruner, 1924, p. 171, list; Haviland, 1931, p. 137, 152, list and note; Leonard and Mills, 1931, p. 309–323, note; Wygodzinsky, 1947, p. 431, note; Wygodzinsky, 1949a, p. 49, checklist; Guagliumi, 1953, p. 16, note; Wygodzinsky, 1957, p. 268, junior syn. of Zelus obscuridorsis; Sucre, et. al., 1966, p. 31, note; Hart, 1986, p. 540, redescription, note, fig., key and lectotype desig.; Maldonado, 1990, p. 330, cat.
Diplodus nugax: Walker, 1873, p. 124, cat; Uhler, 1886, p. 24, checklist; Hart, 1986, p. 540, lectotype desig.
Zelus rufigeniculatus Haviland, 1931, p. 137, 148, 153–154, list, fig. and orig. descr. (subgenus Diplodus); Wygodzinsky, 1949a, p. 50, checklist; Hart, 1986, p. 540, junior syn. of Z. nugax.
-
scientificName: Zelus nugax; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; locality:unknown; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00041008; occurrenceRemarks:Lectotype of Zelus nugax Stål, 1862, designated by Hart (1986). Verbatim label info: Mexico / Salle / nugax Stal / Lectotype Zelus nugax Stal / designated by E.R.Hart / Typus / NHRS-GULI 000000336; recordedBy:Salle; otherCatalogNumbers:NHRS-GULI 000000336; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:NHRS
-
scientificName: Zelus nugax; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:Guyana; locality:Berbice; eventDate:1922-10; sex:Adult Female; occurrenceRemarks:Lectotype of Zelus rufigeniculatus Haviland, 1931, designated by Hart (1986). Bears following labels: Type / Berbice Brit. Guiana-October 1922-e. coll. M. D. Haviland-d.d. Collegium Newnhamense / Pres. By Mrs. Brindley-B.M. 1928-172 / Zelus rufigeniculatus Haviland; recordedBy:Haviland, M.D.; institutionCode:BMNH
-
scientificName: Zelus nugax; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:Guyana; locality:Berbice; eventDate:1922-10; sex:Adult Female; occurrenceRemarks:Paralectotype of Zelus rufigeniculatus Haviland, 1931, designated by Hart (1986). Bears following labels: Type / Berbice Brit. Guiana-October 1922-e. coll. M. D. Haviland-d.d. Collegium Newnhamense / Pres. By Mrs. Brindley-B.M. 1928-172 / Zelus rufigeniculatus Haviland; recordedBy:Haviland, M.D.; institutionCode:BMNH
Description
Zelus nugax Stål, 1862, habitus
b: Zelus nugax Stål, 1862, male, lateral view (UCR_ENT 00046940, Mexico)
c: Zelus nugax Stål, 1862, female, dorsal (UCR_ENT 00001214, French Guiana)
d: Zelus nugax Stål, 1862, female, lateral (UCR_ENT 00001214, French Guiana)
Zelus nugax Champion, 1899, male genitalic structures
b: Zelus nugax Champion, 1899, phallus, dorsal view
Male: (
Female: (
Diagnosis
The slender, cylindrical paramere and the laterally compressed medial process can separate males of this species from most other species of the genus. Difficult to distinguish from Z. pedestris, but the paramere apex is generally more truncate, the dorsal phallothecal sclerite usually without lateral indentation, and the basal plate arms are separate in most specimens (see taxon discussion).
Distribution
Mexico, Central America and South America (
Taxon discussion
This is by far the most widespread species of the Zelus nugax species group, and one of the most widespread members of the genus. In Ecuador, Mexico and Central America, Z. nugax is apparently among the most common species of reduviids, occurring in second growth foliage and tall grasses throughout its range. Other than a variation in color from yellowish-brown to dark brown in any given area and a more noticeably produced scutellar apex in South America, there are little readily apparent external variations in the species. In the internal male genitalia, however, we find some noticeable geographic variations. In South America the basal plate arms are apparently always separate, while there seems to be tendency toward fusion of these arms as one progresses northward to Mexico.
The following discussion on some of the diagnostic characters may be useful for separating species when confusions arise, but as we have not clearly defined the boundaries between Z. nugax and Z. pedestris, reliable identification may not be achieved at all times. We will discuss this in the next paragraph. The straight bladelike medial process of Z. nugax distinguishes it from Z. impar and Z. illotus, both of which have similar appearances to Z. nugax, but both have recurved medial processes. The less rounded pygophore and more blunted paramere separate this species externally from most male specimens of Z. pedestris. Although it is difficult to separate the females of Z. nugax from Z. illotus and Z. pedestris, there appears to be some differences among these species. The normal lack of erect setae on the dorsal surface of the posterior pronotal lobe of Z. illotus and lower posterior margin of the anterior pronotal lobe of Z. pedestris usually serve as diagnostic characters for someone with large series and a familiarization with all three species.
It remains unresolved if Z. nugax and Z. pedestris are distinct species. They are currently delimited based on several characters of the male genitalia, which, however, do not appear to be fixed. With regards to four characters (paramere apex shape, fusion of basal plate arms, lateral indentation on dorsal phallothecal sclerite, and basal plate arm extension) used in delimitation, the following combinations have been observed: (1) paramere apex acute, basal plate arms fused, phallothecal indentation present and basal plate arm extension present and expanded laterally (observations based on specimens from Santa Catarina, Brazil); (2) paramere apex truncate, slightly enlarged, somewhat diamond-shaped, basal plate arms fused, indentation absent and extension present, laterally expanded (La Molina, Peru); and (3) paramere apex truncate, slightly enlarged, basal plate arms separate, phallothecal indentation absent and extension absent or inconspicuous (El Valle, Panama). Following the phylogenetic species concept sensu Wheeler and Platnick (
Both the types of Z. nugax and Z. pedestris are females, further complicating the application of the names as we have not been able to distinguish females of the two species. The type of Z. nugax is from Mexico and that of Z. pedestris from South America (country not recorded). Zelus nugax and Z. pedestris overlap broadly in northern South America, but Z. nugax appears to be absent South of Peru and in most of Brazil and Z. pedestris not recorded from North and Central Americas. This geographic pattern currently serves as an additional means to apply the names.
Zelus panamensis , sp. n.
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Canal Zone; locality:Alhajuelo; eventDate:1911-04-05; sex:Adult Male; catalogNumber:UCR_ENT 00008001; recordedBy:A. Busck; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:NICARAGUA; stateProvince:Rio San Juan; locality:Refugio Bartola, nr. Indio Maíz Biological Reserve, ~6Km E of El Castillo; verbatimElevation:50; decimalLatitude:10.97252; decimalLongitude:-84.33916; georeferenceSources:Label; eventDate:2010-11-01 to 2010-11-06; sex:Adult Male; catalogNumber:UCR_ENT 00004645; occurrenceRemarks:Primary DNA voucher RCW_2806; recordedBy:J. Cryan, J. Urban, G. Svenson; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:UCR
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Meta; locality:Cano Grande; decimalLatitude:3.11171; decimalLongitude:-73.83689; georeferenceSources:Gazetteer; eventDate:1948-01-20; sex:Adult Male; catalogNumber:UCR_ENT 00007996; occurrenceRemarks:Designated as holotype for his new species Zelus cestartus [Mansucript name] by E. R. Hart (1972). Zelus panamensis and Z. cestartus were considered to be synonymic by G. Zhang. This specimen hence loses its holotype status (which is not official since the name was never published). The red holotype label affixed to the specimen by Hart, however, remains on the specimen for the purpose of recording this (informal) taxonomic history.; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Antioquia; locality:Carepa; decimalLatitude:7.7664; decimalLongitude:-76.6611; georeferenceSources:Gazetteer; eventDate:2001-09-13; sex:Adult Male; catalogNumber:UCR_ENT 00022969; recordedBy:Morales, Gilberto; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:MEFLG
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Antioquia; locality:San Luis, En Bosque; decimalLatitude:6.13562; decimalLongitude:-75.32785; georeferenceSources:Google Earth; eventDate:1986-01-01; sex:Adult Male; catalogNumber:UCR_ENT 00022970; recordedBy:R. Velez; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:MEFLG
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Antioquia; locality:Mutata, Villa Arteaga; decimalLatitude:7.25498; decimalLongitude:-76.43018; georeferenceSources:Google Earth; eventDate:1947-11-01; sex:Adult Male; catalogNumber:UCR_ENT 00022971; recordedBy:FL Gallego M; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:MEFLG
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Antioquia; locality:San Luis, Rio Claro; decimalLatitude:5.95906; decimalLongitude:-74.9418; georeferenceSources:Google Earth; eventDate:1988-09-01; sex:Adult Male; catalogNumber:UCR_ENT 00022972; recordedBy:R. Velez; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:MEFLG
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Choco; locality:Choco; verbatimElevation:500; decimalLatitude:5.75; decimalLongitude:-76.41667; georeferenceSources:Label; eventDate:1973-04-01; sex:Adult Female; catalogNumber:UCR_ENT 00017792; recordedBy:M. Madison; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Choco; locality:Tutunendo; decimalLatitude:5.75; decimalLongitude:-76.53333; georeferenceSources:Gazetteer; eventDate:1983-11-01; sex:Adult Female; catalogNumber:UCR_ENT 00022978; recordedBy:R. Velez; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:MEFLG
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Cordoba; locality:Monteria; decimalLatitude:8.7575; decimalLongitude:-75.89; georeferenceSources:Gazetteer; eventDate:1985-01-01; sex:Adult Male; catalogNumber:UCR_ENT 00009336; occurrenceRemarks:Drake Collection; recordedBy:A. Diaz; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Cordoba; locality:Monteria; verbatimElevation:25; decimalLatitude:8.7575; decimalLongitude:-75.89; georeferenceSources:Google Earth; eventDate:1983-05-01; sex:Adult Female; catalogNumber:UCR_ENT 00029351; occurrenceRemarks:Drake Collection. Previously determined to be Zelus cestartus [Manuscript name] in Hart (1972)'s dissertation. Zelus panamensis and Z. cestartus were considered to be synonymic by G. Zhang.; recordedBy:A. Dizz; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:unknown; locality:Laguayalana, W Colombia; eventDate:1956-08-08; sex:Adult Male; catalogNumber:UCR_ENT 00017032; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Alajuela; locality:Est. San Ramon Oeste; verbatimElevation:620; decimalLatitude:10.88327; decimalLongitude:-85.41354; georeferenceSources:Label; eventDate:1994-04-03 to 1994-04-19; sex:Adult Female; catalogNumber:UCR_ENT 00014395; recordedBy:F. Quesada; otherCatalogNumbers:CRI001776475; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Alajuela; locality:San Carlos; decimalLatitude:10.324; decimalLongitude:-84.427; georeferenceSources:Google Earth; eventDate:no date provided; sex:Adult Female; catalogNumber:UCR_ENT 00029358; occurrenceRemarks:Designated as allotype by Hart, unpublished. Changed to paratype in Zhang, Hart & Weirauch (2016); recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Alajuela; locality:San Carlos; decimalLatitude:10.324; decimalLongitude:-84.427; georeferenceSources:Google Earth; eventDate:no date provided; sex:Adult Female; catalogNumber:UCR_ENT 00029359; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Alajuela; locality:San Carlos; decimalLatitude:10.324; decimalLongitude:-84.427; georeferenceSources:Google Earth; eventDate:no date provided; sex:Adult Female; catalogNumber:UCR_ENT 00029360; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Alajuela; locality:San Carlos; decimalLatitude:10.324; decimalLongitude:-84.427; georeferenceSources:Google Earth; eventDate:no date provided; sex:Adult Female; catalogNumber:UCR_ENT 00029361; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Alajuela; locality:San Carlos; decimalLatitude:10.324; decimalLongitude:-84.427; georeferenceSources:Google Earth; eventDate:no date provided; sex:Adult Female; catalogNumber:UCR_ENT 00029362; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Guanacaste; locality:Estacion Pitilla, 9 Km S Santa Cecilla; verbatimElevation:700; decimalLatitude:10.98888; decimalLongitude:-85.42524; georeferenceSources:Label; eventDate:1994-04-01; sex:Adult Female; catalogNumber:UCR_ENT 00014248; recordedBy:C. Moraga; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Guanacaste; locality:Estacion Pitilla, 9 Km S Santa Cecilla; verbatimElevation:700; decimalLatitude:10.98888; decimalLongitude:-85.42524; georeferenceSources:Label; eventDate:1905-06-13; sex:Adult Male; catalogNumber:UCR_ENT 00014386; recordedBy:Unknown; otherCatalogNumbers:CRI000305674; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Guanacaste; locality:Est. Pitilla, 9 km S. Santa Cecilia, P.N. Guanacaste, A.C. Guanacaste; verbatimElevation:700; decimalLatitude:10.99261; decimalLongitude:-85.42948; georeferenceSources:Label; eventDate:1991-07-01; sex:Adult Male; catalogNumber:UCR_ENT 00014401; recordedBy:P.Rios; otherCatalogNumbers:CRI000336629; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Heredia; locality:Estacion Magsasay Parque Nacional Braullio Carrillo; verbatimElevation:200; decimalLatitude:10.39981; decimalLongitude:-84.04828; georeferenceSources:Label; eventDate:1990-07-01; sex:Adult Male; catalogNumber:UCR_ENT 00014258; recordedBy:G. Carballo; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Heredia; locality:Finca La Selva, near Puerto Viejo; verbatimElevation:48; decimalLatitude:10.43114; decimalLongitude:-84.00321; georeferenceSources:Google Earth; eventDate:1973-03-17 to 1973-03-19; sex:Adult Female; catalogNumber:UCR_ENT 00029350; recordedBy:D. C. Rentz; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Limon; locality:Amubri, Talamanca; verbatimElevation:70; decimalLatitude:9.51483; decimalLongitude:-82.95537; georeferenceSources:Label; eventDate:1992-10-12 to 1992-10-30; sex:Adult Male; catalogNumber:UCR_ENT 00014239; recordedBy:G. Gallardo; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Limon; locality:Amubri, Talamanca; verbatimElevation:70; decimalLatitude:9.51483; decimalLongitude:-82.95537; georeferenceSources:Label; eventDate:1992-10-12 to 1992-10-30; sex:Adult Female; catalogNumber:UCR_ENT 00014245; recordedBy:G. Gallardo; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Limon; locality:Rio Sardinas, R.N.S.F. Barra del Colorado; verbatimElevation:10; decimalLatitude:10.64405; decimalLongitude:-83.74201; georeferenceSources:Label; eventDate:1994-03-16 to 1994-03-20; sex:Adult Male; catalogNumber:UCR_ENT 00014257; recordedBy:F. Araya; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Limon; locality:R. B. Hitoy Cerere, Valle La Estrella; verbatimElevation:150; decimalLatitude:9.67177; decimalLongitude:-83.0277; georeferenceSources:Label; eventDate:1994-02-21 to 1994-03-08; sex:Adult Female; catalogNumber:UCR_ENT 00014387; recordedBy:G. Carballo; otherCatalogNumbers:CRI001734765; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Limon; locality:Rio Sardinas, R.N.S.F. Barra del Colorado; verbatimElevation:10; decimalLatitude:10.64405; decimalLongitude:-83.74201; georeferenceSources:Label; eventDate:1994-02-01 to 1994-02-14; sex:Adult Female; catalogNumber:UCR_ENT 00014391; recordedBy:E. Araya; otherCatalogNumbers:CRI001833288; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Limon; locality:Sector Cerro Cocori, Finca de E. Rojas; verbatimElevation:150; decimalLatitude:10.59281; decimalLongitude:-83.71456; georeferenceSources:Label; eventDate:1994-02-24; sex:Adult Female; catalogNumber:UCR_ENT 00014393; recordedBy:M. Epstein; otherCatalogNumbers:INB0003801591; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Limon; locality:Estacion Hitoy Cerere, R. Cerere, Res. Biol. Hitoy Cerere; verbatimElevation:100; decimalLatitude:9.67177; decimalLongitude:-83.0277; georeferenceSources:Label; eventDate:1992-03-05 to 1992-03-19; sex:Adult Female; catalogNumber:UCR_ENT 00014398; recordedBy:G. Carballo; otherCatalogNumbers:CRI000454403; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Limon; locality:Estacion Hitoy Cerere, R. Cerere, Res. Biol. Hitoy Cerere; verbatimElevation:100; decimalLatitude:9.67177; decimalLongitude:-83.0277; georeferenceSources:Label; eventDate:1992-03-27 to 1992-04-13; sex:Adult Female; catalogNumber:UCR_ENT 00014399; recordedBy:G. Carballo; otherCatalogNumbers:CRI000374162; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Limon; locality:Estacion Hitoy Cerere, R. Cerere, Res. Biol. Hitoy Cerere; verbatimElevation:100; decimalLatitude:9.67177; decimalLongitude:-83.0277; georeferenceSources:Label; eventDate:1992-03-27 to 1992-04-13; sex:Adult Female; catalogNumber:UCR_ENT 00014400; recordedBy:G. Carballo; otherCatalogNumbers:CRI000374161; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Limon; locality:Veragua Rainforest, Rio Victoria (Returu Station); verbatimElevation:250; eventDate:2008-08-01; sex:Adult Male; catalogNumber:UCR_ENT 00014402; occurrenceRemarks:Information on label: colecta libre; recordedBy:R. Villalobos; otherCatalogNumbers:INB000416412; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Rancho Quemado, Pen. de Osa, A. C. Osa; verbatimElevation:200; decimalLatitude:8.6791; decimalLongitude:-83.56671; eventDate:1993-07-04 to 1993-07-28; sex:Adult Male; catalogNumber:UCR_ENT 00014240; recordedBy:A. Gutierrez; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Parque Nacional Manuel Antonio, Quepos; verbatimElevation:80; decimalLatitude:9.39361; decimalLongitude:-84.12917; eventDate:1992-04-01; sex:Adult Male; catalogNumber:UCR_ENT 00014241; recordedBy:G. Varela; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Parque Nacional Manuel Antonio, Quepos; verbatimElevation:80; decimalLatitude:9.39361; decimalLongitude:-84.12917; eventDate:1992-10-01; sex:Adult Male; catalogNumber:UCR_ENT 00014242; recordedBy:G. Varela; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Estacion Sirena, Parque Nacional Corcovado; verbatimElevation:50; decimalLatitude:8.48005; decimalLongitude:-83.58935; georeferenceSources:Google Earth; eventDate:1992-04-01; sex:Adult Female; catalogNumber:UCR_ENT 00014244; recordedBy:G. Rodriguez; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Rancho Quemado, Peninsula de Osa; verbatimElevation:200; decimalLatitude:8.67776; decimalLongitude:-83.56478; georeferenceSources:Label; eventDate:1992-09-01; sex:Adult Female; catalogNumber:UCR_ENT 00014246; recordedBy:F. Quesada; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Rancho Quemado, Peninsula de Osa; verbatimElevation:200; decimalLatitude:8.67776; decimalLongitude:-83.56478; georeferenceSources:Label; eventDate:1992-08-01; sex:Adult Female; catalogNumber:UCR_ENT 00014247; recordedBy:M. Segura; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Rancho Quemado, Peninsula de Osa; verbatimElevation:200; decimalLatitude:8.67776; decimalLongitude:-83.56478; georeferenceSources:Label; eventDate:1992-12-01; sex:Adult Male; catalogNumber:UCR_ENT 00014359; recordedBy:F. Quesada; otherCatalogNumbers:INBIO CRI000 905864; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Parque Nacional Manuel Antonio, Quepos; verbatimElevation:80; decimalLatitude:9.39361; decimalLongitude:-84.12917; eventDate:1992-11-01; sex:Adult Male; catalogNumber:UCR_ENT 00014382; recordedBy:G. Varela; otherCatalogNumbers:CRI000823105; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Rancho Quemado, Peninsula de Osa; verbatimElevation:200; decimalLatitude:8.67776; decimalLongitude:-83.56478; georeferenceSources:Label; eventDate:1991-11-01; sex:Adult Male; catalogNumber:UCR_ENT 00014383; recordedBy:F. Quesada; otherCatalogNumbers:CRI000552719; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Estacion Sirena, Parque Nacional Corcovado; verbatimElevation:50; decimalLatitude:8.48005; decimalLongitude:-83.58935; georeferenceSources:Google Earth; eventDate:1993-06-01; sex:Adult Male; catalogNumber:UCR_ENT 00014384; recordedBy:G. Fonseca; otherCatalogNumbers:CRI001834042; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Estero de Guerra, Peninsula de Osa; verbatimElevation:50; eventDate:1993-01-01 to 1993-01-14; sex:Adult Male; catalogNumber:UCR_ENT 00014385; recordedBy:A. Marin; otherCatalogNumbers:CRI001690200; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Corcovado National Park Osa Peninsula; decimalLatitude:8.55516; decimalLongitude:-83.51755; georeferenceSources:Google Earth; eventDate:1997-08-02 to 1997-08-04; sex:Adult Female; catalogNumber:UCR_ENT 00014388; recordedBy:D. H. Janzen; otherCatalogNumbers:CRI001686899; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Golfito, Camino a las Torres; verbatimElevation:450; eventDate:2004-04-28; sex:Adult Female; catalogNumber:UCR_ENT 00014389; recordedBy:B. Gamboa; otherCatalogNumbers:INB0003836872; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Golfito, Camino a las Torres; verbatimElevation:450; eventDate:2004-04-28; sex:Adult Female; catalogNumber:UCR_ENT 00014390; recordedBy:D. Briceno; otherCatalogNumbers:INB0003838066; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Parque Nacional Manuel Antonio, Quepos; verbatimElevation:80; decimalLatitude:9.39361; decimalLongitude:-84.12917; eventDate:1992-08-01; sex:Adult Female; catalogNumber:UCR_ENT 00014392; recordedBy:G. Varela; otherCatalogNumbers:CRI000941360; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Golfito, Camino a las Torres; verbatimElevation:450; eventDate:2004-04-28; sex:Adult Female; catalogNumber:UCR_ENT 00014394; recordedBy:M. Moraga; otherCatalogNumbers:INB0003838274; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Estacion Sirena, ACOSA; verbatimElevation:50; decimalLatitude:8.48005; decimalLongitude:-83.58946; georeferenceSources:Google Earth; eventDate:1995-04-05 to 1995-04-24; sex:Adult Female; catalogNumber:UCR_ENT 00014396; recordedBy:B. Gamboa; otherCatalogNumbers:CRI002188028; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Estacion Sirena, Parque Nacional Corcovado; verbatimElevation:50; decimalLatitude:8.48005; decimalLongitude:-83.58935; georeferenceSources:Google Earth; eventDate:1992-04-01; sex:Adult Female; catalogNumber:UCR_ENT 00014397; recordedBy:G. Rodriguez; otherCatalogNumbers:CRI000545451; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Parque Nacional Manuel Antonio, Quepos; verbatimElevation:80; decimalLatitude:9.39361; decimalLongitude:-84.12917; eventDate:1992-04-01; sex:Adult Female; catalogNumber:UCR_ENT 00014456; recordedBy:C. Cano; otherCatalogNumbers:INBIOCRI001718402; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:INBIO
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Rincon, Osa Peninsula; verbatimElevation:1; decimalLatitude:8.7; decimalLongitude:-83.4833; georeferenceSources:Gazetteer; eventDate:1974-05-25 to 1974-05-28; sex:Adult Male; catalogNumber:UCR_ENT 00022662; recordedBy:E. Giesbert; otherCatalogNumbers:LACM ENT 264633; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:LACM
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Golfito; decimalLatitude:8.6407; decimalLongitude:-83.1686; georeferenceSources:Google Earth; eventDate:1957-07-30; sex:Adult Female; catalogNumber:UCR_ENT 00022666; recordedBy:Truxal & Menke; otherCatalogNumbers:LACM ENT 264680; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:LACM
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COSTA RICA; stateProvince:Puntarenas; locality:Hacienda Baru near Dominical; eventDate:2004-02-18; sex:Adult Male; catalogNumber:UCR_ENT 00072669; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:UCR
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Manabi; locality:Cojimies; decimalLatitude:0.36667; decimalLongitude:-80.03333; georeferenceSources:Gazetteer; eventDate:1947-11-09; sex:Adult Male; catalogNumber:UCR_ENT 00071200; recordedBy:W.C.M.; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:TAMU
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Pichincha; locality:Puerto Quito; decimalLatitude:0.1167; decimalLongitude:-79.2667; georeferenceSources:Gazetteer; eventDate:1978-02-01 to 1978-02-05; sex:Adult Male; catalogNumber:UCR_ENT 00009574; occurrenceRemarks:Drake Collection; recordedBy:J. J. Anderson; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Bocas del Toro; locality:2 km WSW Chiriqui Grande; decimalLatitude:8.94583; decimalLongitude:-82.13694; eventDate:1999-08-06; sex:Adult Male; catalogNumber:UCR_ENT 00071215; recordedBy:J. C. Schaffner; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:TAMU
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Canal Zone; locality:Rio Indio; eventDate:1936-12-27; sex:Adult Male; catalogNumber:UCR_ENT 00009288; recordedBy:S. W. Frost; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Canal Zone; locality:Pacific Slope; eventDate:1974-09-04; sex:Adult Male; catalogNumber:UCR_ENT 00017034; recordedBy:H. D. Engleman; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Canal Zone; locality:Coco Solo Hospital; decimalLatitude:9.35; decimalLongitude:-79.85; georeferenceSources:Label; eventDate:1972-03-09; sex:Adult Male; catalogNumber:UCR_ENT 00017035; recordedBy:Engleman; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Canal Zone; locality:Pipeline Road; eventDate:1974-06-30; sex:Adult Male; catalogNumber:UCR_ENT 00017036; recordedBy:D. Engleman; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Canal Zone; locality:Gatun Spillway; decimalLatitude:9.35055; decimalLongitude:-79.83145; georeferenceSources:Google Earth; eventDate:1971-07-12; sex:Adult Male; catalogNumber:UCR_ENT 00017037; recordedBy:D. Engleman; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Canal Zone; locality:Barro Colorado Island; decimalLatitude:9.15562; decimalLongitude:-79.84895; georeferenceSources:Google Earth; eventDate:1978-08-14; sex:Adult Female; catalogNumber:UCR_ENT 00017237; recordedBy:H. A. Hespenheide; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Canal Zone; locality:Escobal Road; decimalLatitude:9.21739; decimalLongitude:-79.95576; georeferenceSources:Google Earth; eventDate:1974-07-14; sex:Adult Female; catalogNumber:UCR_ENT 00017793; recordedBy:D. Engleman; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Canal Zone; locality:5 mi. NW of Gamboa; decimalLatitude:9.1629; decimalLongitude:-79.74871; georeferenceSources:Google Earth; eventDate:1974-05-01; sex:Adult Male; catalogNumber:UCR_ENT 00038443; recordedBy:H. P. Stockwell; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:UCB
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Canal Zone; locality:Coco Solo Hospital; decimalLatitude:9.35; decimalLongitude:-79.85; georeferenceSources:Label; eventDate:1973-10-14; sex:Adult Female; catalogNumber:UCR_ENT 00038451; recordedBy:D. Engleman; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:UCB
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Canal Zone; locality:Pina Road; decimalLatitude:9.25; decimalLongitude:-79.95; georeferenceSources:Label; eventDate:1973-01-30; sex:Adult Male; catalogNumber:UCR_ENT 00042954; recordedBy:D. Engleman; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CSUC
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Canal Zone; locality:5 mi NW of Gamboa; eventDate:1973-01-30; sex:Adult Female; catalogNumber:UCR_ENT 00042955; recordedBy:Strauch; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CSUC
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Canal Zone; locality:7 km W Margarita; decimalLatitude:9.33333; decimalLongitude:-79.96666; georeferenceSources:Label; eventDate:1978-07-29; sex:Adult Male; catalogNumber:UCR_ENT 00047063; recordedBy:H. A. Hespenheide; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Canal Zone; locality:Fort Clayton; verbatimElevation:419; decimalLatitude:9; decimalLongitude:-79.75; georeferenceSources:Gazetteer; eventDate:1944-04-01; sex:Adult Male; catalogNumber:UCR_ENT 00047960; recordedBy:K. E. Frick; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Canal Zone; locality:Fort Clayton; verbatimElevation:419; decimalLatitude:9; decimalLongitude:-79.75; georeferenceSources:Gazetteer; eventDate:1944-04-01; sex:Adult Male; catalogNumber:UCR_ENT 00047961; recordedBy:K. E. Frick; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Colon; locality:Pipeline Road, Gamboa; decimalLatitude:9.1167; decimalLongitude:-79.7; georeferenceSources:Gazetteer; eventDate:1972-07-22; sex:Adult Female; catalogNumber:UCR_ENT 00017791; recordedBy:D. Engleman; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Colon; locality:Barro Colorado Island, Canal Zone; decimalLatitude:9.15472; decimalLongitude:-79.84806; georeferenceSources:GeoLocate Software; eventDate:1964-12-11 to 1964-12-17; sex:Adult Female; catalogNumber:UCR_ENT 00029364; recordedBy:K. W. Cooper; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Colon; locality:Pipeline Road, 8 km NW Gamboa; eventDate:1981-01-12; sex:Adult Male; catalogNumber:UCR_ENT 00070010; recordedBy:C. D. Michener; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:KU
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Colon; locality:Pipeline Road, 8 km NW Gamboa; eventDate:1981-01-12; sex:Adult Female; catalogNumber:UCR_ENT 00070029; recordedBy:C. D. Michener; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:KU
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Panama; locality:Madden Forest, Canal Zone; decimalLatitude:9.08333; decimalLongitude:-79.58333; georeferenceSources:Label; eventDate:1972-10-23; sex:Adult Male; catalogNumber:UCR_ENT 00017038; recordedBy:D. Engleman; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Panama; locality:Old Gamboa Road; eventDate:1994-06-25; sex:Adult Male; catalogNumber:UCR_ENT 00037118; recordedBy:N. Smith, R. Kassabian; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:UCD
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Panama; locality:Madden Forest, Canal Zone; decimalLatitude:9.08333; decimalLongitude:-79.58333; georeferenceSources:Label; eventDate:1980-12-20; sex:Adult Male; catalogNumber:UCR_ENT 00046989; recordedBy:D. Engleman; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Panama; locality:Pipeline Road near Gamboa, Canal Zone; decimalLatitude:9.11811; decimalLongitude:-79.70019; georeferenceSources:Google Earth; eventDate:1987-02-09; sex:Adult Male; catalogNumber:UCR_ENT 00047959; recordedBy:E. S. Ross; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Panama; locality:Summit; decimalLatitude:9.04697; decimalLongitude:-79.64056; georeferenceSources:Google Earth; eventDate:1953-12-01; sex:Adult Male; catalogNumber:UCR_ENT 00017033; recordedBy:N. L. H. Krauss; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PANAMA; stateProvince:Panama; locality:Summit; decimalLatitude:9.04697; decimalLongitude:-79.64056; georeferenceSources:Google Earth; eventDate:1953-12-01; sex:Adult Female; catalogNumber:UCR_ENT 00017039; recordedBy:N. L. H. Krauss; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus panamensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:unknown; stateProvince:unknown; locality:Unknown; eventDate:1938-04-06; sex:Adult Female; catalogNumber:UCR_ENT 00029353; occurrenceRemarks:Drake Collection. Designated as allotype for his new species Zelus cestartus [Mansucript name] by E. R. Hart. Zelus panamensis and Z. cestartus were considered to be synonymic by G. Zhang. This specimen hence loses its allotype status. The allotype label affixed by Hart, however, remains attached to the pin.; recordedBy:unknown; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
Description
Zelus panamensis Zhang & Hart, sp. n., habitus
b: Zelus panamensis Zhang & Hart, sp. n., male, lateral view (UCR_ENT 00017034, Canal Zone, Panama)
c: Zelus panamensis Zhang & Hart, sp. n., female, dorsal view (UCR_ENT 00014248, Guanacaste, Costa Rica)
d: Zelus panamensis Zhang & Hart, sp. n., female, lateral view (UCR_ENT 00014248, Guanacaste, Costa Rica)
Zelus panamensis Zhang & Hart, sp. n., male genitalic structures
b: Zelus panamensis Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: (
Diagnosis
Recognized by the orangish or reddish head and the dark brown remainder of the body. The short, nearly straight medial process and the short paramere separate males of this species from all other species of the same species group. The yellowish or reddish ventral surface and the usually yellowish or reddish (blackish-brown in some specimens) lateral surface distinguishes females of this species from others in the same group.
Etymology
Named after the country Panama, where the holotype was collected.
Distribution
Southern Central America and northern South America (
Zelus paracephalus , sp. n.
-
scientificName: Zelus paracephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Meta; locality:Rio Guamal [Quamal?]; verbatimElevation:400 m; decimalLatitude:3.71667; decimalLongitude:-73.1667; georeferenceSources:Gazetteer; eventDate:1948-01-24; sex:Adult Male; catalogNumber:UCR_ENT 00057803; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus paracephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Meta; locality:San Martin: Reserva Natural El Caduceo; verbatimElevation:380 m; decimalLatitude:3.66593; decimalLongitude:-73.65773; georeferenceSources:GPS; eventDate:2010-10-21 to 2010-10-23; sex:Adult Male; catalogNumber:UCR_ENT 00004997; occurrenceRemarks:Primary DNA voucher RCW_2164; recordedBy:G. Zhang & J. Avendaño; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:UCR
-
scientificName: Zelus paracephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Orellana; locality:Reserva Etnica Waorani, 1 km S. Onkone Gare Camp, Transect Ent.; verbatimElevation:216 m; decimalLatitude:-0.65714; decimalLongitude:-76.453; georeferenceSources:Label; samplingProtocol:Fogging; eventDate:1995-02-10; sex:Adult Male; catalogNumber:UCR_ENT 00009475; occurrenceRemarks:LOT#992; Drake Collection; recordedBy:T. L. Erwin et al.; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus paracephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Orellana; locality:Reserva Etnica Waorani, 1 km S. Onkone Gare Camp, Transect Ent.; verbatimElevation:216 m; decimalLatitude:-0.65714; decimalLongitude:-76.453; georeferenceSources:Label; samplingProtocol:Fogging; eventDate:1996-10-02; sex:Adult Male; catalogNumber:UCR_ENT 00009476; occurrenceRemarks:Drake Collection; recordedBy:T. L. Erwin et al.; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus paracephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Orellana; locality:Reserva Etnica Waorani, 1 km S. Onkone Gare Camp, Transect Ent.; verbatimElevation:216 m; decimalLatitude:-0.65714; decimalLongitude:-76.453; georeferenceSources:Label; samplingProtocol:Fogging; eventDate:1996-10-02; sex:Adult Male; catalogNumber:UCR_ENT 00009477; occurrenceRemarks:Drake Collection; recordedBy:T. L. Erwin et al.; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus paracephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Orellana; locality:Reserva Etnica Waorani, 1 km S. Onkone Gare Camp, Transect Ent.; verbatimElevation:216 m; decimalLatitude:-0.65714; decimalLongitude:-76.453; georeferenceSources:Label; samplingProtocol:Fogging; eventDate:1996-06-26; sex:Adult Male; catalogNumber:UCR_ENT 00009479; occurrenceRemarks:Lot#1582 - Collection code moved to this field to prevent duplication.; recordedBy:T. L. Erwin et al.; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus paracephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Caqueta; locality:Valparaiso, Vda. Palastina Fca. La Ilusion; verbatimElevation:225 m; decimalLatitude:1.18333; decimalLongitude:-75.7; georeferenceSources:Label; eventDate:1997-01-24; sex:Adult Male; catalogNumber:UCR_ENT 00025329; recordedBy:G. Zambrano; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:UNAB
-
scientificName: Zelus paracephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km SW of Ariquemes, near Fzda. Rancho Grande; decimalLatitude:-10.32921; decimalLongitude:-63.46881; eventDate:1992-03-30 to 1992-04-10; sex:Adult Male; catalogNumber:UCR_ENT 00029302; occurrenceRemarks:Drake Collection; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus paracephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PERU; stateProvince:Ucayali; locality:Porvenir, Pulcal[l]pa; verbatimElevation:158 m; decimalLatitude:-8.3825; decimalLongitude:-74.5381; georeferenceSources:Gazetteer; eventDate:1992-07-01; sex:Adult Male; catalogNumber:UCR_ENT 00029304; occurrenceRemarks:Drake Collection; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus paracephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Vaupes; locality:Mitu; verbatimElevation:184 m; decimalLatitude:1.1983; decimalLongitude:-70.1733; georeferenceSources:Gazetteer; eventDate:1990-07-06 to 1990-07-17; sex:Adult Male; catalogNumber:UCR_ENT 00029305; occurrenceRemarks:Drake Collection; recordedBy:L. E. Peña; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus paracephalus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:unknown; locality:unknown; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00057802; occurrenceRemarks:Previously designated as 'allotype', a type status not used in the formal publication of this name (Zhang et al.); recordedBy:H. Sturm; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
Description
Zelus paracephalus Zhang & Hart, sp. n., habitus
b: Zelus paracephalus Zhang & Hart, sp. n., male, lateral view (UCR_ENT 00006068)
c: Zelus paracephalus Zhang & Hart, sp. n., female, dorsal view (UCR_ENT 00057802)
d: Zelus paracephalus Zhang & Hart, sp. n., female, lateral view (UCR_ENT 00057802)
Zelus paracephalus Zhang & Hart, sp. n., male genitalic structures
b: Zelus paracephalus Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: (
Diagnosis
The dorsal coloration nearly uniformly dark brown, the head reddish-brown, the membrane with indistinct iridescence are characteristic of this species. Most similar to Z. erythrocephalus and Z. russulumus; males can be distinguished from both by the rather wide medial process and the uniquely shaped paramere (
Distribution
South America (
Zelus pedestris
Nomenclature
Zelus pedestris Fabricius, 1803, p. 288, orig. descr.; Stål, 1872, p. 91, cat. (subgenus Diplodus); Lethierry and Severin, 1896, p. 151, cat.; Wygodzinsky, 1949a, p. 50, checklist; Maldonado, 1990, p. 330, cat.
Diplodus pedestris: Stål, 1868, p. 109, descr. and note; Walker, 1873, p. 125, cat.
Zelus dispar Fabricius, 1803, p. 291, orig. descr.; Stål, 1872, p. 92, cat. (subgenus Diplodus); Lethierry and Severin, 1896, p. 151, cat.; Wygodzinsky, 1949a, p. 49, checklist; Maldonado, 1990, p. 326, cat. syn. nov. (current study).
Diplodus dispar: Stål, 1868, p. 109–110, descr. and note; Walker, 1873, p. 125, cat.
Diplodus obscuridorsis Stål, 1860, p. 75, orig. descr.; Stål, 1868, p. 109, note; Walker, 1873, p. 125, cat.
Zelus obscuridorsis: Stål, 1872, p. 91, cat. and descr. (subgenus Diplodus); kthierry and Severin, 1896, p. 152, cat.; Wygodzinsky, 1949a, p. 50, checklist; Wygodzinsky, 1957, p. 26, note and senior syn. of Z. nugax, Z. illotus and Z. carvalhoi; Wygodzinsky, 1960, p. 307–308, list. syn. nov. (current study).
-
scientificName: Zelus pedestris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Fabricius, 1803; country:unknown; locality:America meridionali; sex:Adult female; occurrenceRemarks:Lectotype of Zelus pedestris Fabricius, 1803 (New Designation by Zhang, Hart & Weirauch, 2016). Bears the following labels: Type / Z. pedestris in Am. mer. Schmidt.; recordedBy:Schmidt; institutionCode:ZMUC
-
scientificName: Zelus pedestris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Fabricius, 1803; country:unknown; sex:Female; occurrenceRemarks:Paralectotype of Zelus pedestris Fabricius, 1803. (New Designation by Zhang, Hart & Weirauch, 2016). Bears only the 'Type' label.; institutionCode:ZMUC
-
scientificName: Zelus pedestris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Fabricius, 1803; country:unknown; locality:America meridionali; sex:Male; occurrenceRemarks:Lectotype of Zelus dispar Fabricius, 1803 (New Designation by Zhang, Hart & Weirauch (2016), junior synonym of Zelus pedestris Fabricius, 1803. Bears the following labels: Type / Z. pedestris in Am. mer. Schmidt.; recordedBy:Schmidt; institutionCode:ZMUC
-
scientificName: Zelus pedestris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Fabricius, 1803; country:unknown; sex:Male; occurrenceRemarks:Paralectotype of Zelus dispar Fabricius, 1803 (New Designation by Zhang, Hart & Weirauch, 2016), junior synonym of Zelus pedestris Fabricius, 1803. Bears only a "Type" label; institutionCode:ZMUC
-
scientificName: Zelus pedestris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Fabricius, 1803; country:BRAZIL; stateProvince:Rio de Janeiro; locality:unknown; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00041009; occurrenceRemarks:Lectotype of Zelus obscuridorsis Stål, 1860 (New Designation by Zhang, Hart & Weirauch, 2016). Verbatim label info: Rio Jan / Stal / Zelus pedestris Fabricius det E.R.Hart 1972 / Lectotype Zelus obscuridorsis Stal / designated by E.R.Hart / obscuridosis Stal / Typus / NHRS-GULI 000000345.; otherCatalogNumbers:NHRS-GULI 000000345; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:NHRS
-
scientificName: Zelus pedestris; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Fabricius, 1803; country:BRAZIL; locality:unknown; eventDate:No date provided; sex:Adult Female; occurrenceRemarks:Paralectotype of Zelus obscuridorsis Stål, 1860 (New Designation by Zhang, Hart & Weirauch, 2016); otherCatalogNumbers:NHRS-GULI 000000345; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:NHRS
Description
Zelus pedestris Fabricius, 1803, habitus
b: Zelus pedestris Fabricius, 1803, male, lateral view (UCR_ENT 00025985, Paraguay)
c: Zelus pedestris Fabricius, 1803, female, dorsal view (UCR_ENT 00047470, Brazil)
d: Zelus pedestris Fabricius, 1803, female, lateral view (UCR_ENT 00047470, Brazil)
Zelus pedestris Fabricius, 1803, male genitalic structures
b: Zelus pedestris Fabricius, 1803, phallus, dorsal view
Male: (
Female: (
Diagnosis
The slender, cylindrical paramere and the laterally compressed, blade-like medial process can separate this species from most other species of the genus. Different from Z. illotus and Z. impar by the straight medial process, contrasting with the recurved medial processes of the other two species. Difficult to distinguish from Z. nugax, but the paramere apex is generally rounded, the dorsal phallothecal sclerite usually with lateral indentation, the basal plate arms are fused and the basal plate arm extension is present and laterally expanded.
Distribution
South America and adjacent islands of the Caribbean (
Taxon discussion
Some helpful, although not consistently reliable, characters used to distinguish females of Z. pedestris from those of Z. nugax and Z. illotus are given in the taxon discussion section of Z. nugax. Uncertainties in species limits between Z. pedestris and Z. nugax are also discussed in that species. The application of the name Z. pedestris is restricted to specimens from South America.
The coloration of the posterior pronotal lobe is the most obvious variable character in the males. This varies from a very light to a medium reddish-brown in any given geographic area. This pronotal variation is absent in the females, the dorsal surface varying from a relatively uniform light to medium brown in any geographic area.
Zelus plagiatus
Nomenclature
Diplodus plagiatus Signoret, 1862, p. 584–585, orig. descr.; Walker, 1873, p. 126, cat.
Zelus plagiatus: Stål, 1872, p. 92, cat.; Lethierry and Severin, 1896, p. 153, cat.; Wygodzinsky, 1949a, p. 50, checklist; Maldonado, 1990, p. 330, cat.
-
scientificName: Zelus plagiatus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:(Signoret, 1852); country:PERU; stateProvince:unknown; locality:Unknown; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00075076; occurrenceRemarks:Verbatim label info: Peru coll. Signoret / Plagiat. det. Signoret. / Holotype / Zeuls plagiatus (Signoret) / Typus Diplodus plagiatus SIGNORET, 1862 etik. Hecher 1996 REDV. 484/1; recordedBy:Signoret; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:NHMW
Description
Zelus plagiatus (Signoret, 1852), habitus
b: Zelus plagiatus (Signoret, 1852), feamle, lateral view
Male: unknown.
Female: (
Diagnosis
The dorsum predominantly yellow with the posterior pronotal lobe partly black and two black spots on hemelytra is distinctive of this species.
Distribution
South America (
Zelus prolixus
Nomenclature
Euagoras prolixus Stål, 1860, p. 74, orig. descr.; Walker, 1873, p. 118, cat.
Zelus prolixus: Stål, 1872, p. 89, cat. (subgenus Zelus); Lethierry and Severin, 1896, p. 153, cat.; Champion, 1898, p. 255, note; Wygodzinsky, 1949a; p. 50, checklist; Elkins, 1954, p. 39, 40, note; Hart, 1987, p. 297-299, redescription, note, fig. and key; Maldonado, 1990, p. 330, cat.
-
scientificName: Zelus prolixus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:(Stål, 1860); country:BRAZIL; locality:unknown; sex:Adult Female; occurrenceRemarks:The specimen bears the following labels: Brasil / F. Sahib. / Typus / prolixus Stal / 167-53; recordedBy:F. Sahib.; identifiedBy:E.R. Hart; dateIdentified:1972; institutionCode:NHRS
Description
Zelus prolixus (Stål, 1860), habitus
b: Zelus prolixus (Stål, 1860), male, lateral view (UCR_ENT 00038433, Santa Catarina, Brazil)
c: Zelus prolixus (Stål, 1860), female, dorsal view (UCR_ENT 00038497, Santa Catarina, Brazil)
d: Zelus prolixus (Stål, 1860), female, lateral view (UCR_ENT 00038497, Santa Catarina, Brazil)
Zelus prolixus (Stål, 1860), male genitalic structures
b: Zelus prolixus (Stål, 1860), phallus, dorsal view
Male: (
Female: (
Diagnosis
Distinguished by the greenish coloration; the veins of membrane darker than the cells; the smallish size; the rather slender body and very delicate legs; the head somewhat dorsoventrally flattened; and the eye somewhat elongated. Males can be separated from most species of Zelus by the broadly triangular medial process. The short paramere not exceeding the medial process separates Z. prolixus from Z. minutus.
Distribution
South America and adjacent islands of the Caribbean (
Zelus puertoricensis
Nomenclature
Zelus puertoricensis Hart, 1987, p. 294, orig. descr., key and fig.; Maldonado, 1990, p. 330, cat.
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; locality:Coamo Springs; decimalLatitude:18.07996; decimalLongitude:-66.35794; georeferenceSources:Google Earth; eventDate:1914-07-17 to 1914-07-19; sex:Adult Male; catalogNumber:UCR_ENT 00057799; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; locality:Coamo Springs; decimalLatitude:18.07996; decimalLongitude:-66.35794; georeferenceSources:Google Earth; eventDate:1914-07-17 to 1914-07-19; sex:Adult Female; catalogNumber:UCR_ENT 00057798; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; stateProvince:Jayuya; locality:Jayuya; decimalLatitude:18.2186; decimalLongitude:-66.5916; georeferenceSources:Gazetteer; eventDate:1934-12-01; sex:Adult Male; catalogNumber:UCR_ENT 00016144; recordedBy:V. Biaggi; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; locality:Coamo Springs; verbatimElevation:126 m; decimalLatitude:18.07996; decimalLongitude:-66.35794; georeferenceSources:Google Earth; eventDate:1914-07-17 to 1914-07-19; sex:Adult Male; catalogNumber:UCR_ENT 00009438; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; locality:Cidra; decimalLatitude:18.17778; decimalLongitude:-66.16167; georeferenceSources:GeoLocate Software; eventDate:1948-06-04; sex:Adult Male; catalogNumber:UCR_ENT 00009439; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; locality:Cidra; decimalLatitude:18.17778; decimalLongitude:-66.16167; georeferenceSources:GeoLocate Software; eventDate:1948-06-04; sex:Adult Male; catalogNumber:UCR_ENT 00009440; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; locality:Cidra; decimalLatitude:18.17778; decimalLongitude:-66.16167; georeferenceSources:GeoLocate Software; eventDate:1948-06-04; sex:Adult Male; catalogNumber:UCR_ENT 00009441; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; locality:Arecibo; decimalLatitude:18.4744; decimalLongitude:-66.7161; eventDate:1915-05-10; sex:Adult Female; catalogNumber:UCR_ENT 00015108; recordedBy:A. H. Manee; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; locality:Coamo Springs; verbatimElevation:126 m; decimalLatitude:18.07996; decimalLongitude:-66.35794; georeferenceSources:Google Earth; eventDate:1914-07-17 to 1914-07-19; sex:Adult Male; catalogNumber:UCR_ENT 00016145; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; locality:Coamo Springs; verbatimElevation:126 m; decimalLatitude:18.07996; decimalLongitude:-66.35794; georeferenceSources:Google Earth; eventDate:1914-07-17 to 1914-07-19; sex:Adult Female; catalogNumber:UCR_ENT 00016146; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; locality:Coamo Springs; verbatimElevation:126 m; decimalLatitude:18.07996; decimalLongitude:-66.35794; georeferenceSources:Google Earth; eventDate:1914-07-17 to 1914-07-19; sex:Adult Female; catalogNumber:UCR_ENT 00016992; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; locality:Coamo Springs; verbatimElevation:126 m; decimalLatitude:18.07996; decimalLongitude:-66.35794; georeferenceSources:Google Earth; eventDate:1914-07-17 to 1914-07-19; sex:Adult Female; catalogNumber:UCR_ENT 00016993; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; locality:Coamo Springs; verbatimElevation:126 m; decimalLatitude:18.07996; decimalLongitude:-66.35794; georeferenceSources:Google Earth; eventDate:1914-07-17 to 1914-07-19; sex:Adult Female; catalogNumber:UCR_ENT 00016994; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; locality:Coamo Springs; verbatimElevation:126 m; decimalLatitude:18.07996; decimalLongitude:-66.35794; georeferenceSources:Google Earth; eventDate:1914-07-17 to 1914-07-19; sex:Adult Female; catalogNumber:UCR_ENT 00016995; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; locality:Coamo Springs; verbatimElevation:126 m; decimalLatitude:18.07996; decimalLongitude:-66.35794; georeferenceSources:Google Earth; eventDate:1914-07-17 to 1914-07-19; sex:Adult Female; catalogNumber:UCR_ENT 00016996; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; locality:Coamo Springs; verbatimElevation:126 m; decimalLatitude:18.07996; decimalLongitude:-66.35794; georeferenceSources:Google Earth; eventDate:1914-07-17 to 1914-07-19; sex:Adult Male; catalogNumber:UCR_ENT 00017655; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; stateProvince:Penuelas; locality:Penuelas; decimalLatitude:18.05833; decimalLongitude:-66.72194; georeferenceSources:GeoLocate Software; eventDate:1932-09-08; sex:Adult Female; catalogNumber:UCR_ENT 00009447; recordedBy:R. G. Oakley; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; stateProvince:San Juan; locality:Rio Piedras; verbatimElevation:29 m; decimalLatitude:18.3994; decimalLongitude:-66.0503; georeferenceSources:Gazetteer; eventDate:1931-06-01; sex:Adult Male; catalogNumber:UCR_ENT 00009437; recordedBy:M. D. Leonard; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; stateProvince:San Juan; locality:Rio Piedras; verbatimElevation:29 m; decimalLatitude:18.3994; decimalLongitude:-66.0503; georeferenceSources:Gazetteer; eventDate:1916-07-29; sex:Adult Female; catalogNumber:UCR_ENT 00009443; recordedBy:R.T. Cotton; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; stateProvince:San Juan; locality:Rio Piedras; verbatimElevation:29 m; decimalLatitude:18.3994; decimalLongitude:-66.0503; georeferenceSources:Gazetteer; eventDate:1916-07-29; sex:Adult Female; catalogNumber:UCR_ENT 00009444; recordedBy:R.T. Cotton; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; stateProvince:San Juan; locality:San Juan; decimalLatitude:18.46633; decimalLongitude:-66.10573; georeferenceSources:Google Earth; eventDate:1926-03-29; sex:Adult Female; catalogNumber:UCR_ENT 00009445; recordedBy:G. Bay; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; stateProvince:San Juan; locality:San Juan; decimalLatitude:18.46633; decimalLongitude:-66.10573; georeferenceSources:Google Earth; eventDate:1942-09-16; sex:Adult Male; catalogNumber:UCR_ENT 00030178; occurrenceRemarks:Bears labels 'On Zea mayais', 'San Juan No 8357', and 'Lot No 42-11687'; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; stateProvince:San Juan; locality:San Juan; decimalLatitude:18.46633; decimalLongitude:-66.10573; georeferenceSources:Google Earth; eventDate:1942-09-16; sex:Adult Female; catalogNumber:UCR_ENT 00030179; occurrenceRemarks:Bears labels 'On Zea mayais', 'San Juan No 8357', and 'Lot No 42-11687'; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus puertoricensis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PUERTO RICO; stateProvince:Vega Alta; locality:Vega Alta; verbatimElevation:100 m; decimalLatitude:18.4122; decimalLongitude:-66.3313; georeferenceSources:Gazetteer; eventDate:1917-01-26; sex:Adult Female; catalogNumber:UCR_ENT 00009442; recordedBy:R.T. Cotton; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
Description
Zelus puertoricensis Hart, 1987, habitus
b: Zelus puertoricensis Hart, 1987, male, lateral view (UCR_ENT 00015107, Dominican Republic)
c: Zelus puertoricensis Hart, 1987, female, dorsal view (UCR_ENT 00016146, Puerto Rico)
d: Zelus puertoricensis Hart, 1987, female, lateral view (UCR_ENT 00016146, Puerto Rico)
Zelus puertoricensis Hart, 1987, male genitalic structures
b: Zelus puertoricensis Hart, 1987, phallus, dorsal view
Male: (
Female: (
Diagnosis
The rather slender body form of Z. puertoricensis is characteristic of the Zelus puertoricensis species group (total length/width more than 8x). Both sexes of Z. puertoricensis have the dorsal and ventral surfaces of the postocular lobe nearly parallel through the anterior 2/3 of the lobe. This contrasts with the sloping configuration of the dorsal surface in Z. subimpressus.
Males can be recognized by the robust, posteriorly directed medial process, apex bent and the short, cylindrical paramere. This is smaller in Z. puertoricensis than in Z. subimpressus (
Distribution
The Caribbean, islands of Puerto Rico and Hispaniola (
Zelus renardii
Nomenclature
Zelus renardii Kolenati, 1857, p. 460, Tab. III. fig. 2, orig. descr. and fig.; Stål, 1872, p. 91, cat.; Kirkaldy, 1908, p. 195, list and senior syn. of Z. laevicollis Champion and Z. perigrinus Kirkaldy; Banks, 1910b, p. 16, cat.; Fracker, 1913, p. 239, 240, key and checklist; Van Duzee, 1914, p. 13, list; Van Duzee, 1916, p. 30, checklist (subgenus Diplocodus); Van Duzee, 1917, p. 261, cat. (subgenus Diplocodus); Muir, 1920, p. 285–286, note; Muir, 1921, p. 119, note; Horton, 1922, p. 385, note; Hawaiian Sugar Planter's Association, 1924, p, 29, note; Readio, 1927, p, 169, 178–179, key, descr. and note; Williams, 1931, p. 101, note; Haldaway and Look, 1942, p. 257–258, note; Ewing and Ivy, 1943, p, 604–606, note; Clancy, 1946, p. 326, note; Van Zwaluwenburg, 1946, p. 15, note; Zimmerman, 1948, p. 137–138 , note and fig.; Wygodzinsky, 1949a, p. 50, checklist; Elkins, 1951, p. 410, list; Sibley, 1951, p. 92, list; Nishida, 1955, p. 172, note; Atkins, et. al., 1957, p. 258, note; Nielsen and Henderson, 1959, p. 159, note; Wene and Sheets, 1962, p. 397, note; Butler, 1966, p. 1306–1307, note; Wygodzinsky, 1966, p. 66, note; Lingren, Ridgway and Jones, 1968, p. 615, note; Parencia, 1968, p. 276, note; Nutting and Spangler, 1969, p. 765, note; Hart, 1986, p. 540-542, redescription, note, fig. and key; Maldonado, 1990, p. 331, cat.
Diplodus renardii: Uhler, 1894, list.
Zelus laevicollis Champion, 1898, p. 260–261, Tab. XV. fig. 24, orig. descr. and fig.; Banks, 1910, p. 16, cat.; Fracker, 1913, p. 239, 240, key and list; Osborne and Drake, 1915, p. 531, note; Van Duzee, 1916, p. 30, checklist (subgenus Diplocodus); Van Duzee, 1917, p. 261, cat. (subgenus Diplocodus); Readio, 1927, p. 177–178, descr.; Wygodzinsky, 1949a, p. 49, checklist; Elkins, 1951, p. 410, list; Sibley, 1951, p. 92, list; Young and Sifuentes, 1960, p. 1109–1111, biology; Hart, 1986, p. 540, junior syn. of Z. renardii.
Zelus peregrinus Kirkaldy, 1902, p. 149–150, orig. descr.; Swezey, 1905, p. 232–234, biology; Kirkaldy, 1907a, p. 247, note; Kirkaldy, 1907b, p. 156–518, biology; Kirkaldy, 1908, p. 195, junior syn. of Z. renardii; Severin, et. al., 1914, p. 197, note; Fullaway, 1918, p. 12, note; Clausen, 1940, p. 589–590, note (sic. Zellus).
Diplocodus exsanguis: Van Duzee, 1914, p. 13, list (probable misidentification).
-
scientificName: Zelus renardii; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Kolenati, 1857; country:USA; stateProvince:California; locality:unknown; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00075077; occurrenceRemarks:Holotype of Zelus renardii Kolenati, 1857. Verbatim label info: California / Cyenaus [?] / Renardii det. Kolen. / Holotype / Zelus renardii Kolenati / Lectotypus Zelus renardii Kolenati, 1857 etik. Hecher 1996 REDV. 486/1; recordedBy:Unknown; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:NHMW
-
scientificName: Zelus renardii; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Kolenati, 1857; country:MEXICO; stateProvince:Mexico; locality:Milpas; verbatimElevation:1798 m; decimalLatitude:19.03; decimalLongitude:-99.9381; georeferenceSources:Gazetteer; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00048762; occurrenceRemarks:Holotype of Zelus laevicollis Champion, 1898, junior synonym of Zelus renardii Kolenati, 1857 (Hart, 1986). Verbatim label info: Type / B.C.A.Rhyn.II. Zelus laevicollis Ch. / Holotype / Sp. figured. / Milpas, Mex., 5900 ft. Forrer. / Zelus renardii Kolenati det. E.R.Hart 1972; recordedBy:Forrer; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:BMNH
Description
Zelus renardii Kolenati, 1857, habitus
b: Zelus renardii Kolenati, 1857, male, lateral view (UCR_ENT 00032058, Texas, USA)
c: Zelus renardii Kolenati, 1857, female, dorsal view (UCR_ENT 00040246, New Mexico, USA)
d: Zelus renardii Kolenati, 1857, female, lateral view (UCR_ENT 00040246, New Mexico, USA)
Zelus renardii Kolenati, 1857, male genitalic structures
b: Zelus renardii Kolenati, 1857, phallus, dorsal view
Zelus renardii Kolenati, 1857, specimen record map. Pacific islands (left) and American continent are not to the same scale.
Male: (
Female: (
Diagnosis
Can be recognized by the reddish corium; the remainder of the body surface greenish; the humeral angle with small subtuberculate projection. More robust than a very similar species, Z. cervicalis. Males can be recognized by the paramere apically greatly enlarged; the medial process apically curved ventrad, somewhat hooklike, more strongly than in Z. cervicalis, the only species that may be confused with; and the lateral margin of the dorsal phallothecal sclerite recurved.
Distribution
Western and Southwestern US, most of Mexico and Central America (
Taxon discussion
Zelus renardii is almost certainly sister species of Z. cervicalis. The two share two unique characters: the lateral margins of the dorsal phallothecal sclerite recurved and the medial process apically strongly hooked.
Zelus rosulentus , sp. n.
-
scientificName: Zelus rosulentus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Orellana; locality:Reserva Etnica Waorani, 1 km S. Onkone Gare Camp, Transect Ent.; verbatimElevation:216 m; decimalLatitude:-0.65714; decimalLongitude:-76.453; samplingProtocol:Fogging; eventDate:1996-09-30; sex:Adult Male; preparations:Pinned; catalogNumber:UCR_ENT 00009487; occurrenceRemarks:Lot#1669 - Collection code moved to this field to prevent duplication; Drake Collection; recordedBy:T. L. Erwin et al.; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus rosulentus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Orellana; locality:Reserva Etnica Waorani, 1 km S. Onkone Gare Camp, Transect Ent.; verbatimElevation:216 m; decimalLatitude:-0.65714; decimalLongitude:-76.453; eventDate:1996-10-04; sex:Adult Male; preparations:Pinned; catalogNumber:UCR_ENT 00009451; recordedBy:T. L. Erwin et al.; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus rosulentus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Orellana; locality:Reserva Etnica Waorani, 1 km S. Onkone Gare Camp, Transect Ent.; verbatimElevation:216 m; decimalLatitude:-0.65714; decimalLongitude:-76.453; eventDate:1995-02-12; sex:Adult Male; preparations:Pinned; catalogNumber:UCR_ENT 00009452; recordedBy:T. L. Erwin et al.; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus rosulentus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Orellana; locality:Reserva Etnica Waorani, 1 km S. Onkone Gare Camp, Transect Ent.; verbatimElevation:216 m; decimalLatitude:-0.65714; decimalLongitude:-76.453; eventDate:1995-07-02; sex:Adult Male; preparations:Pinned; catalogNumber:UCR_ENT 00009453; recordedBy:T. L. Erwin et al.; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus rosulentus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Orellana; locality:Reserva Etnica Waorani, 1 km S. Onkone Gare Camp, Transect Ent.; verbatimElevation:216 m; decimalLatitude:-0.65714; decimalLongitude:-76.453; eventDate:1994-10-09; sex:Adult Male; preparations:Pinned; catalogNumber:UCR_ENT 00009454; recordedBy:T. L. Erwin et al.; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus rosulentus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Orellana; locality:Reserva Etnica Waorani, 1 km S. Onkone Gare Camp, Transect Ent.; verbatimElevation:216 m; decimalLatitude:-0.65714; decimalLongitude:-76.453; samplingProtocol:Fogging; eventDate:1996-06-21; sex:Adult Male; preparations:Pinned; catalogNumber:UCR_ENT 00009484; occurrenceRemarks:Lot#1558 - Collection code moved to this field to prevent duplication; Drake Collection; recordedBy:T. L. Erwin et al.; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus rosulentus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Orellana; locality:Reserva Etnica Waorani, 1 km S. Onkone Gare Camp, Transect Ent.; verbatimElevation:216 m; decimalLatitude:-0.65714; decimalLongitude:-76.453; samplingProtocol:Fogging; eventDate:1995-02-10; sex:Adult Male; preparations:Pinned; catalogNumber:UCR_ENT 00009485; occurrenceRemarks:Lot#1007 - Collection code moved to this field to prevent duplication; Drake Collection; recordedBy:T. L. Erwin et al.; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus rosulentus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Orellana; locality:Reserva Etnica Waorani, 1 km S. Onkone Gare Camp, Transect Ent.; verbatimElevation:216 m; decimalLatitude:-0.65714; decimalLongitude:-76.453; samplingProtocol:Fogging; eventDate:1996-02-07; sex:Adult Male; preparations:Pinned; catalogNumber:UCR_ENT 00009486; occurrenceRemarks:Lot#1451 - Collection code moved to this field to prevent duplication; Drake Collection; recordedBy:T. L. Erwin et al.; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus rosulentus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Orellana; locality:Reserva Etnica Waorani, 1 km S. Onkone Gare Camp, Transect Ent.; verbatimElevation:216 m; decimalLatitude:-0.65714; decimalLongitude:-76.453; samplingProtocol:Fogging; eventDate:1995-02-12; sex:Adult Male; preparations:Pinned; catalogNumber:UCR_ENT 00009488; occurrenceRemarks:Lot#1043 - Collection code moved to this field to prevent duplication; Drake Collection; recordedBy:T. L. Erwin et al.; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
Description
Zelus rosulentus Zhang & Hart, sp. n., habitus
b: Zelus rosulentus Zhang & Hart, sp. n., male, lateral view (UCR_ENT 00009487, Ecuador)
Zelus rosulentus Zhang & Hart, sp. n., male genitalic structures
b: Zelus rosulentus Zhang & Hart, sp. n., phallus
Male: (
Female: unknown.
Diagnosis
The uniquely reddish coloration of the entire body makes this species easy to recognize. The medial process is highly reduced and rather indistinct, separating Z. rosulentus from other members of the Zelus tetracanthus species group (
Etymology
The specific epithet indicates the reddish-pink coloration of this species.
Distribution
South America (
Zelus ruficeps
Nomenclature
Zelus ruficeps Stål, 1862, p. 453–454, orig. descr.; Stål, 1872, p. 90, cat. (subgenus Diplodus); Lethierry and Severin, 1896, p. 153, cat.; Champion, 1898, p. 256, Tab. 15. fig. 15, note and fig.; Kuhlgatz, 1902, p. 266, note; Fracker, 1913, p. 239, 240, list (subgenus Diplodus); Wygodzinsky, 1949a, p. 50, checklist; Maldonado, 1990, p. 331, cat.
Diplodus ruficeps: Walker, 1873, p. 124, cat.; Uhler, 1886, p. 24, checklist.
-
scientificName: Zelus ruficeps; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; stateProvince:None or Unknown; locality:unknown; eventDate:No date provided; sex:Adult Male; catalogNumber:UCR_ENT 00041010; occurrenceRemarks:Lectotype of Zelus ruficeps Stål, 1862 (New Designation by Zhang, Hart & Weirauch, 2016). Verbatim label info: Mexico / Salle/ ruficeps Stal / Lectotype Zelus ruficeps Stal / designated by E.R.Hart / Paratypus / NHRS-GULI 000000347; recordedBy:salle; otherCatalogNumbers:NHRS-GULI 000000347; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:NHRS
-
scientificName: Zelus ruficeps; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; stateProvince:None or Unknown; locality:unknown; eventDate:No date provided; sex:Adult Female; occurrenceRemarks:Allolectotype of Zelus ruficeps Stål, 1862 (New Designation by Zhang, Hart & Weirauch, 2016); recordedBy:salle; institutionCode:NHRS
-
scientificName: Zelus ruficeps; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; stateProvince:None or Unknown; locality:unknown; eventDate:No date provided; sex:Adult Female; occurrenceRemarks:Paralectotype of Zelus ruficeps Stål, 1862 (New Designation by Zhang, Hart & Weirauch, 2016); recordedBy:salle; institutionCode:NHRS
-
scientificName: Zelus ruficeps; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; stateProvince:None or Unknown; locality:unknown; eventDate:No date provided; sex:Adult Male; occurrenceRemarks:Paralectotype of Zelus ruficeps Stål, 1862 (New Designation by Zhang, Hart & Weirauch, 2016); recordedBy:salle; institutionCode:NHRS
-
scientificName: Zelus ruficeps; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; stateProvince:None or Unknown; locality:unknown; eventDate:No date provided; sex:Adult Female; occurrenceRemarks:Paralectotype of Zelus ruficeps Stål, 1862 (New Designation by Zhang, Hart & Weirauch, 2016). Bears label: Mexico, coll. Signoret/ruficeps det. Stal; recordedBy:Signoret; institutionCode:NHMW
-
scientificName: Zelus ruficeps; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; stateProvince:None or Unknown; locality:unknown; eventDate:No date provided; sex:Adult Female; occurrenceRemarks:Paralectotype of Zelus ruficeps Stål, 1862 (New Designation by Zhang, Hart & Weirauch, 2016). Bears label: Mexico, coll. Signoret/ruficeps det. Stal; recordedBy:Signoret; institutionCode:NHMW
Description
Zelus ruficeps Stål, 1862, habitus
b: Zelus ruficeps Stål, 1862, male, lateral view (UCR_ENT 00037136, Chimaltenango, Guatemala)
c: Zelus ruficeps Stål, 1862, female, dorsal view (UCR_ENT 00030447, Veracruz, Mexico)
d: Zelus ruficeps Stål, 1862, female, lateral view (UCR_ENT 00030447, Veracruz, Mexico)
e: Zelus ruficeps Stål, 1862, male, dorsal view (UCR_ENT 00025688, Veracruz, Mexico)
Zelus ruficeps Stål, 1862, male genitalic structures
b: Zelus ruficeps Stål, 1862, phallus
Male: (
Female: (
Diagnosis
The combination of relatively large size, stout body, the reddish head and parts of body can separate both sexes of this species from other species of Zelus. Among males of the Zelus armillatus species group, Z. ruficeps has some of the most delicate medial process. Females may be confused with Z. grassans, but are much larger and the humeral angle is equipped with dentate process, contrasting to the nearly rounded humeral angle of Z. grassans.
Distribution
Southern Mexico to Northern South America (
Zelus russulumus , sp. n.
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Amazonas; locality:Manacapuru; decimalLatitude:3.3; decimalLongitude:-60.61667; georeferenceSources:Gazetteer; eventDate:1905-04-11; sex:Adult Male; catalogNumber:UCR_ENT 00069895; recordedBy:S.M. Klages; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:KU
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km S Ariquemes, Fazenda Rancho Grande; verbatimElevation:300 m; decimalLatitude:-10.3; decimalLongitude:-62.86666; eventDate:1992-03-30 to 1992-04-10; sex:Adult Male; catalogNumber:UCR_ENT 00009464; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km S Ariquemes, Fazenda Rancho Grande; verbatimElevation:300 m; decimalLatitude:-10.3; decimalLongitude:-62.86666; eventDate:1992-03-30 to 1992-04-10; sex:Adult Male; catalogNumber:UCR_ENT 00009465; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km S Ariquemes, Fazenda Rancho Grande; verbatimElevation:300 m; decimalLatitude:-10.3; decimalLongitude:-62.86666; eventDate:1996-12-03 to 1996-12-15; sex:Adult Male; catalogNumber:UCR_ENT 00009466; occurrenceRemarks:Drake collection; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km S Ariquemes, Fazenda Rancho Grande; verbatimElevation:300 m; decimalLatitude:-10.3; decimalLongitude:-62.86666; eventDate:1992-03-30 to 1992-04-10; sex:Adult Female; catalogNumber:UCR_ENT 00009467; occurrenceRemarks:Drake collection; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km S Ariquemes, Fazenda Rancho Grande; verbatimElevation:300 m; decimalLatitude:-10.3; decimalLongitude:-62.86666; eventDate:1996-12-03 to 1996-12-15; sex:Adult Female; catalogNumber:UCR_ENT 00009468; occurrenceRemarks:Drake collection; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km S Ariquemes, Fazenda Rancho Grande; verbatimElevation:300 m; decimalLatitude:-10.3; decimalLongitude:-62.86666; eventDate:1997-11-04 to 1997-11-16; sex:Adult Female; catalogNumber:UCR_ENT 00009469; occurrenceRemarks:Drake collection; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km S Ariquemes, Fazenda Rancho Grande; verbatimElevation:300 m; decimalLatitude:-10.3; decimalLongitude:-62.86666; eventDate:1997-11-04 to 1997-11-16; sex:Adult Female; catalogNumber:UCR_ENT 00009470; occurrenceRemarks:Drake collection; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km S Ariquemes, Fazenda Rancho Grande; verbatimElevation:300 m; decimalLatitude:-10.3; decimalLongitude:-62.86666; eventDate:1997-11-04 to 1997-11-16; sex:Adult Female; catalogNumber:UCR_ENT 00009471; occurrenceRemarks:Drake collection; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Napo; locality:Rio Blanco Ecological Reserve, 6 km E Puerto Misahuali, along Rio Napo; verbatimElevation:518 m; decimalLatitude:-1.05037; decimalLongitude:-77.62705; georeferenceSources:Google Earth; eventDate:1998-09-07; sex:Adult Female; catalogNumber:UCR_ENT 00009501; occurrenceRemarks:Drake Collection; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BOLIVIA; stateProvince:Pando; locality:Cachuela Esperanza; decimalLatitude:-10.5375; decimalLongitude:-65.5815; georeferenceSources:Google Earth; eventDate:no date provided; sex:Adult Female; catalogNumber:UCR_ENT 00029293; recordedBy:W. M. Mann; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Meta; locality:Villavicencio; decimalLatitude:4.15333; decimalLongitude:-73.635; georeferenceSources:Google Earth; eventDate:1946-05-11; sex:Adult Female; catalogNumber:UCR_ENT 00029294; occurrenceRemarks:Previously designated as 'allotype' of his manuscript name Zelus russulumus by Hart (1972), a type status not used in the formal publication of this new species name (Zhang et al., 2016); recordedBy:E.A. Chapin; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km SW of Ariquemes, near Fzda. Rancho Grande; decimalLatitude:-10.32921; decimalLongitude:-63.46881; eventDate:1992-03-30 to 1992-04-10; sex:Adult Male; catalogNumber:UCR_ENT 00029301; occurrenceRemarks:Drake Collection; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km SW of Ariquemes, near Fzda. Rancho Grande; decimalLatitude:-10.32921; decimalLongitude:-63.46881; eventDate:1992-03-30 to 1992-04-10; sex:Adult Male; catalogNumber:UCR_ENT 00029303; occurrenceRemarks:Drake Collection; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:COLOMBIA; stateProvince:Vaupes; locality:Mitu; verbatimElevation:184 m; decimalLatitude:1.1983; decimalLongitude:-70.1733; georeferenceSources:Gazetteer; eventDate:1990-07-06 to 1990-07-17; sex:Adult Female; catalogNumber:UCR_ENT 00029306; occurrenceRemarks:Drake Collection; recordedBy:L. E. Peña; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km S Ariquemes, RO Fazenda Rancho Grande; decimalLatitude:-10.46581; decimalLongitude:-63.0996; georeferenceSources:Google Earth; eventDate:1991-11-25; sex:Adult Male; catalogNumber:UCR_ENT 00037215; recordedBy:S.L. Heydon; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:UCD
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km S. Ariquemes, Fazenda Rancho Grande; verbatimElevation:187 m; decimalLatitude:-10.29801; decimalLongitude:-62.86806; georeferenceSources:Map - local/regional; eventDate:1990-12-06 to 1990-12-15; sex:Adult Male; catalogNumber:UCR_ENT 00071241; recordedBy:D. A. Rider and J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:TAMU
-
scientificName: Zelus russulumus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Goias; locality:Campinas; decimalLatitude:-16.66785; decimalLongitude:-49.29149; georeferenceSources:Google Earth; eventDate:2.1.936; sex:Adult Male; catalogNumber:UCR_ENT 00071254; recordedBy:Borgmeier and S. Lopes; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:TAMU
Description
Zelus russulumus Zhang & Hart, sp. n., habitus
b: Zelus russulumus Zhang & Hart, sp. n., male, lateral view (UCR_ENT 00009465, Rondônia, Brazil
c: Zelus russulumus Zhang & Hart, sp. n., female, dorsal view (UCR_ENT 00009501, Napo, Ecuador)
d: Zelus russulumus Zhang & Hart, sp. n., female, lateral view (UCR_ENT 00009501, Napo, Ecuador)
Zelus russulumus Zhang & Hart, sp. n., male genitalic structures
b: Zelus russulumus Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: (
Diagnosis
The dorsal coloration nearly uniformly dark brown, the head reddish-brown, and the membrane with blue or green iridescence. Most similar to Z. erythrocephalus and Z. paracephalus; can be distinguished by the medial process wider than that in Z. erythrocephalus and narrower than Z. paracephalus. Females of Z. erythrocephalus, Z. paracephalus and Z. russulumus are difficult to separate.
Etymology
The species epithet refers to the reddish-brown area on the membrane.
Distribution
South America (
Zelus spatulosus , sp. n.
-
scientificName: Zelus spatulosus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BELIZE; stateProvince:Toledo; locality:Rio Grande; decimalLatitude:16.13333; decimalLongitude:-88.75; georeferenceSources:Gazetteer; eventDate:1931-07-12; sex:Adult Male; catalogNumber:UCR_ENT 00008002; occurrenceRemarks:Additional labels: J C Lutz Collection 1961, Property USNM; recordedBy:J. J. White; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
Description
Zelus spatulosus Zhang & Hart, sp. n., habitus
b: Zelus spatulosus Zhang & Hart, sp. n., male, dorsal view (UCR_ENT 00008002)
Zelus spatulosus Zhang & Hart, sp. n., male genitalic structures
b: Zelus spatulosus Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: unknown.
Diagnosis
Zelus spatulosus has a rather distinctly enlarged apical part of the paramere (
Etymology
The specific epithet is from spatula, referring to the rather broad apical part of the paramere.
Distribution
Central America (
Taxon discussion
The primary basis for placing Z. spatulosus in the Zelus luridus group is the expanded paramere (
Zelus sphegeus
Nomenclature
Zelus sphegeus Fabricius, 1803, p. 287, orig. descr.; Stål, 1872, p. 91, cat.; Lethierry and Severin, 1896, p. 153, cat.; Haviland, 1931, p. 137, 153, list and note; Wygodzinsky, 1949a, p. 50, checklist; Maldonado, 1990, p. 331, cat.
Diplodus sphegeus, Stål, 1868, p. 109, descr.; Walker, 1873, p. 125, cat.
-
scientificName: Zelus sphegeus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Fabricius, 1803; country:unknown; stateProvince:unknown; locality:Habitat in America meridionali; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00075108; recordedBy:Dom. Smidt; otherCatalogNumbers:ZMUC 103083; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:ZMUC
Description
Zelus sphegeus Fabricius, 1803, habitus
b: Zelus sphegeus Fabricius, 1803, female, lateral view (UCR_ENT 00019688, Cuyuni-Mazaruni, Guyana)
Male: unknown.
Female: (
Diagnosis
This species can be recognized by the pronotum bicolorous, anterior lobe yellowish and posterior lobe dark brown; the humeral angle with long spinous process. Similar to females of Z. truxali, but the legs are not banded and the anterior pronotal lobe appears to be more humped than that in Z. truxali.
Distribution
Central America and South America (
Zelus subimpressus
Nomenclature
Zelus subimpressus Stål, 1872, p. 91, orig. descr. (subgenus Diplodus); Lethierry and Severin, 1896, p. 153, cat.; Fracker, 1913, p. 239, 240, key and list (subgenus Diplodus); Fracker and Bruner, 1924, p. 170, note; Bruner 1926, p. 78, 79, key and note; Leonard, 1933, p. 319, note; Wolcott, 1950, p. 212, note; Wygodzinsky, 1949a, p. 50, checklist; Alayo, 1967, p. 37, note; Hart, 1987, p. 294, redescription, note, fig. and key; Maldonado, 1990, p. 331, cat.
Diplodus subimpressus: Uhler, 1886, p. 24, checklist; Gundlach, 1894, p. 598, checklist.
-
scientificName: Zelus subimpressus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stĺl, 1872; country:CUBA; sex:Adult female; occurrenceRemarks:Bears the following label: Cuba / Stal. Subimpressus Stal / Typus; institutionCode:NHRS
Description
Zelus subimpressus Stål, 1872, habitus
b: Zelus subimpressus Stål, 1872, male, lateral view (UCR_ENT 00017843, Ciego de Avila, Cuba)
c: Zelus subimpressus Stål, 1872, female, dorsal view (UCR_ENT 00017850, Ciego de Avila, Cuba)
d: Zelus subimpressus Stål, 1872, female, lateral view (UCR_ENT 00017850, Ciego de Avila, Cuba)
Zelus subimpressus Stål, 1872, male genitalic structures
b: Zelus subimpressus Stål, 1872, phallus, dorsal view
Male: (
Female: (
Diagnosis
The rather slender body form of Z. subimpressus is characteristic of the Zelus puertoricensis species group (total length/width more than 8x). Both sexes are readily distinguished from Z. puertoricensis by having a sloping dorsal surface on the postocular lobe. Males can be recognized by the robust, posteriorly directed medial process, apex bent and the short, cylindrical paramere. This is larger in Z. subimpressus than in Z. puertoricensis (
Distribution
The Caribbean, the islands of Cuba and Hispaniola (
Zelus sulcicollis
Nomenclature
Zelus sulcicollis Champion, 1898, p. 258–259, Tab. XV. fig. 21, orig. descr. and fig.; Fracker, 1913, p. 239, 240, key and list (subgenus Diplodus); Wygodzinsky, 1949a, p. 50, checklist; Maldonado, 1990, p. 331, cat.
-
scientificName: Zelus sulcicollis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Champion, 1899; country:GUATEMALA; stateProvince:Baja Verapaz; locality:San Geronimo; decimalLatitude:15.13333; decimalLongitude:-90.18333; georeferenceSources:Publication; eventDate:No date provided; sex:Adult Female; catalogNumber:UCR_ENT 00048760; occurrenceRemarks:Lectotype of Zelus sulcicollis Champion, 1899 (New Designation by Zhang, Hart & Weirauch, 2016) Verbatim label info: Type / B.C.A.Rhyn.II. Zelus sulcicollis Ch. / Sp. figured. / S. Geronimo, Guatemala. Champion. / Lectotype Zelus sulcicollis Champion des. by. E.R. Hart; recordedBy:G.C. Champion; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:BMNH
-
scientificName: Zelus sulcicollis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Champion, 1899; country:GUATEMALA; stateProvince:Baja Verapaz; locality:San Geronimo; decimalLatitude:15.13333; decimalLongitude:-90.18333; georeferenceSources:Publication; eventDate:No date provided; sex:Adult Male; occurrenceRemarks:Allolectotype of Zelus sulcicollis Champion, 1899 (New Designation by Zhang, Hart & Weirauch, 2016) Verbatim label info: S. Geronimo, Guatemala. Champion. / B.C.A.Rhyn.II. Zelus sulcicollis Ch. / Allolectotype Zelus sulcicollis Champion des. by. E.R. Hart; recordedBy:G.C. Champion; identifiedBy:E.R. Hart; dateIdentified:1972; institutionCode:BMNH
-
scientificName: Zelus sulcicollis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Champion, 1899; country:MEXICO; stateProvince:Guerrero; locality:l. Tepic; georeferenceSources:Publication; eventDate:No date provided; sex:Adult Female; occurrenceRemarks:Paralectotype of Zelus sulcicollis Champion, 1899 (New Designation by Zhang, Hart & Weirauch, 2016) Verbatim label info: l. Tepic, Mex., July, Schumann / B.C.A.Rhyn.II. Zelus sulcicollis Ch., Omilteme, Guerrero, 8000 ft., July, H. H. Smith / B.C.A. Rhyn. II. Zelus sulcicollis Ch. / Paralectotype Zelus sulcicollis Champion des. by. E.R. Hart; recordedBy:H.H. Smith; identifiedBy:E.R. Hart; dateIdentified:1972; institutionCode:BMNH
-
scientificName: Zelus sulcicollis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Champion, 1899; country:MEXICO; stateProvince:Guerrero; locality:l. Tepic; georeferenceSources:Publication; eventDate:No date provided; sex:Adult Male; occurrenceRemarks:Paralectotype of Zelus sulcicollis Champion, 1899 (New Designation by Zhang, Hart & Weirauch, 2016) Verbatim label info: l. Tepic, Mex., July, Schumann / B.C.A.Rhyn.II. Zelus sulcicollis Ch., Omilteme, Guerrero, 8000 ft., July, H. H. Smith / B.C.A. Rhyn. II. Zelus sulcicollis Ch. / Paralectotype Zelus sulcicollis Champion des. by. E.R. Hart; recordedBy:G.C. Champion; identifiedBy:E.R. Hart; dateIdentified:1972; institutionCode:BMNH
Description
Zelus sulcicollis Champion, 1899, habitus
b: Zelus sulcicollis Champion, 1899, male, lateral view (UCR_ENT 00010792, Guerrero, Mexico)
c: Zelus sulcicollis Champion, 1899, female, dorsal view (UCR_ENT 00030233, Hidalgo, Mexico)
d: Zelus sulcicollis Champion, 1899, female, dorsal view (UCR_ENT 00030233, Hidalgo, Mexico)
Zelus sulcicollis Champion, 1899, male genitalic structures
b: Zelus sulcicollis Champion, 1899, phallus, dorsal view
Male: (
Female: (
Diagnosis
Recognized by the dorsal coloration nearly uniformly brown, somewhat reddish and the posterior pronotal lobe medially depressed. Among males of the Zelus armillatus species group occurring in the same geographic range, the medial process and paramere of Z. sulcicollis are longer than that in Z. litigiosus, Z. ruficeps and Z. janus.
Distribution
Southern Mexico and Northern Central America (
Zelus tetracanthus
Nomenclature
Zelus tetracanthus Stål , 1862, p. 454, orig. descr. (subgenus Pindus) ; Stål , 1872, p. 92, cat. (subgenus Pindus); Lethierry and Severin, 1896, p. 153, cat.; Champion, 1898, p. 362, Tab. XV, fig. 27, note and fig.; Fracker, 1913, p. 240, key and list (subgenus Pindus); Wygodzinsky, 1949a, p. 50, checklist; Hart, 1986, p. 536-537, redescription, note, fig. and key; Maldonado, 1990, p. 331, cat.
Diplodus tetracanthus: Walker, 1873, p. 124, cat.; Uhler, 1886, p. 24, checklist.
Pindus socius Uhler, 1872b, p. 420, orig. descr.; Uhler, 186, p. 62, list (reprint); Uhler, 1877, p. 1329, list; Uhler, 1894, p. 284, list.; Hart, 1986, p. 536, junior syn. of Z. tetracanthus.
Diplodus socius: Uhler, 1886, p. 24, checklist; Gillette and Baker, 1895, p. 60, list.
Zelus socius: Lethierry and Severin, 1896, p. 153 cat.; Barber, 1906, p. 28,6, list; Hart, 1907, p. 237, list; Torre-Bueno, 1908, p. 235, list (subgenus Pindus); Van Duzee, 1909, p. 177, list; Banks, 1910b, p. 16, cat.; Fracker, 1913, p. 240, key and list (subgenus Pindus); Torre-Bueno, 1913, p. 60, list (subgenus Pindus); Barber, 1914, p. 506, list; Parshley, 1914, p. 144, list; Van duzee, 1916, p. 30, checklist (subgenus Pindus); Van Duzee, 1917, p. 261, cat. (subgenus Pindus); Parshley, 1921, p. 5, list; Anonymous, 1923, p. 120-135, note; Blatchley, 1926, p. 569, 571, key and descr. (subgenus Pindus); Readio, 1926, p. 168, 177, note and fig.; Readio, 1927, p. 169, 179-181, P1. XIV. fig. 1,2,5, descr., notes and fig.; Leonard, 1928, p. 105, list; Essig, 1929, p. 357, note; Knowlton, 1932, p. 12, note; Harris, 1937, p. 174, list; Brimley, 1938, p. 73, list; Procter, 1946, p. 319, list; Wygodzinsky, 1949a, p. 50, checklist; Elkins, 1951, p. 410, list; Sibley, 1951, p. 92, list; Elkins, 1954, p. 47, note; Atkins, et. al., 1957, p. 251-259, note; Drew and Schaeffer, 1962, p. 106, list; Wene and Sheets, 1962, p. 395-398, note; Whitcomb and Bell, 1964, p. 22, list; Butler, 1966, p. 1306-1307, note; Nutting and Spangler, 1969, p. 763-769, note and photo.
Diplocodus socius: Van Duzee, 1914, p. 13, list and note.
Zelus audax Banks, 1910, p. 325, orig. descr.; Fracker, 1913, p. 240, note; Van Duzee, 1916, p. 30 checklist (subgenus Pindus); Van Duzee, 1917, p. 261, cat. (subgenus Pindus); Britton, 1923, p. 687, list (subgenus Pindus); Blatchley, 1926, p. 569, 571-572, key and descr. (subgenus Pindus); Readio, 1927, p. 169, 181, descr.; Leonard, 1928, p. 105, list; Wygodzinsky, 1949a, p. 48, checklist; Hart, 1986, p. 536, junior syn. of Z. tetracanthus and lectotype desig.
Zelus occiduus Torre-Bueno, 1913a, p. 22, orig. descr. (subgenus Pindus); Van Duzee, 1916, p. 30, checklist; Van Duzee, 1917, p. 261, cat. (subgenus Pindus) ; Readio, 1927, p. 169, 181-182, key and descr.; Wygodzinsky, 1949a, p. 50, checklist; Elkins, 1951, p. 410, list; Hart, 1986, p. 536, junior syn. of Z. tetracanthus and lectotype desig.
Zelus angustatus Hussey, 1925, p. 66-67, orig. descr.; Blatchley, 1926, p. 569, 572, descr. and note (subgenus Pindus); Readio, 1927, p. 169, 182., key and descr.; Blatchley, 1928, p. 6, note (subgenus Pindus); Wygodzinsky, 1949a, p. 48, checklist; Elkins, 1951, p. 410, list; Hussey, 1953, p. 9-11, note; Hart, 1986, p. 536, junior syn. of Z. tetracanthus.
-
scientificName: Zelus tetracanthus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:MEXICO; stateProvince:None or Unknown; locality:unknown; eventDate:No date provided; sex:Adult Male; catalogNumber:UCR_ENT 00075078; occurrenceRemarks:Verbatim label info: Mexico Coll. Signoret / Tetracanth. det. Stal / B.C.A. Rhyn. II. Zelus tetracanthus St. / Holotype / Zelus tetracanthus Stal / Lectotypus Zelus tetracanthus STAL, 1862 etik. Hecher 1996 REDV. 488/1; recordedBy:Signoret; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:NHMW
-
scientificName: Zelus tetracanthus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:USA; stateProvince:New York; county:Nassau; locality:Sea Cliff, Long Island; verbatimElevation:5 m; decimalLatitude:40.84944; decimalLongitude:-73.65233; georeferenceSources:Google Earth; eventDate:no date provided; sex:Adult Female; catalogNumber:UCR_ENT 00057796; occurrenceRemarks:Lectotype of Zelus audax Banks, 1910, designated by Hart (1986), junior synonym of Zelus tetracanthus Stal; recordedBy:N. Banks; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus tetracanthus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:USA; stateProvince:California; county:Santa Clara; locality:Salt Marshes, Palo Alto; decimalLatitude:37.44188; decimalLongitude:-122.14302; georeferenceSources:GeoLocate Software; eventDate:1906-04-20; sex:Adult Female; catalogNumber:UCR_ENT 00057797; occurrenceRemarks:Lectotype of Zelus occiduus Torre-Bueno, designated by Hart (1986), junior synonym of Zelus tetracanthus Stal. Additional label information: M.C.Z Paratype 27979, Zelus (Pindus) occiduus 776 Bueno, LECTOTYPE Zelus occiduus Torre-Bueno des. by E. R. Hart; recordedBy:J.M.A.; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus tetracanthus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:USA; stateProvince:New York; county:Nassau; locality:Sea Cliff, Long Island; verbatimElevation:5 m; decimalLatitude:40.84944; decimalLongitude:-73.65233; georeferenceSources:Google Earth; eventDate:no date provided; sex:Adult Male; catalogNumber:UCR_ENT 00017636; occurrenceRemarks:Paralectotype of Zelus audax Banks, 1911, designated by Hart 1986, junior synonym of Zelus tetracanthus Stal; recordedBy:N. Banks; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus tetracanthus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:USA; stateProvince:Virginia; county:Falls Church; locality:Falls Church; decimalLatitude:38.88222; decimalLongitude:-77.17138; eventDate:1900-08-29; sex:Adult Female; catalogNumber:UCR_ENT 00046742; occurrenceRemarks:Paralectotype of Zelus audax Banks, 1911, designated by Hart 1986, junior synonym of Zelus tetracanthus Stal; recordedBy:N. Banks; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus tetracanthus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Stål, 1862; country:USA; stateProvince:Florida; locality:Gainesville; eventDate:1923-12; sex:Adult Male; occurrenceRemarks:Holotype of Zelus angustatus Hussey, 1925, junior synonym of Zelus tetracanthus Stal. Type specimen information extracted from literature. Location of actual specimen could not be determined (Hart, 1986); recordedBy:T.H. Hubbell; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:Unknown
Description
Zelus tetracanthus Stål, 1862, habitus
b: Zelus tetracanthus Stål, 1862, female, dorsal view (UCR_ENT 00001117, Arizona, USA)
c: Zelus tetracanthus Stål, 1862, female, lateral view (UCR_ENT 00001117, Arizona, USA)
Zelus tetracanthus Stål, 1862, male genitalic structures
b: Zelus tetracanthus Stål, 1862, southern and highland population, pygophore, lateral and posterior views
c: Zelus tetracanthus Stål, 1862, northern and lowland population, phallus, dorsal view
d: Zelus tetracanthus Stål, 1862, southern and highland population, phallus, dorsal view
Male: (
Female: (
Diagnosis
Recognized by the disc of the posterior pronotal lobe with large conspicuous tubercles and the greyish-black coloration. Males can also be recognized by the medial process broadly triangular; the paramere not exceeding medial process; and the dorsal phallothecal sclerite apically with deep emargination.
Distribution
North America, Central America and parts of South America (
Taxon discussion
Besides Z. tetracanthus, two other species, Z. minutus and Z. lewisi also exhibit tuberculate processes on posterior pronotal lobe, but these species can be easily separated on the basis of coloration and body shape. Zelus tetracanthus is a rather widely distributed species, found nearly throughout North America, ranging from southern Canada to parts of Central America. A few records are from Brazil and Paraguay. As the collecting events of these specimens are unrelated, it is highly unlikely these specimens are mislabelled (unless they were each independently mislabelled, which is not likely). Further investigations should examine if the South Amerian populations are native or introduced.
Zelus truxali , sp. n.
-
scientificName: Zelus truxali; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PERU; stateProvince:Pasco; locality:Chontilla 22 km. SE of Iscozazin; decimalLatitude:-10.3357; decimalLongitude:-75.11004; georeferenceSources:Google Earth; eventDate:1961-07-20; sex:Adult Male; catalogNumber:UCR_ENT 00022668; recordedBy:F. S. Truxal; otherCatalogNumbers:LACM ENT 160234; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:LACM
-
scientificName: Zelus truxali; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BOLIVIA; stateProvince:Cochabamba; locality:Villa Tunari, Chapare; verbatimElevation:500 m; decimalLatitude:-16.91666; decimalLongitude:-65.36667; eventDate:1958-01-09; sex:Adult Male; catalogNumber:UCR_ENT 00046974; recordedBy:Wygodzinsky and Monros; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus truxali; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BOLIVIA; stateProvince:Cochabamba; locality:Villa Tunari, Chapare; verbatimElevation:500 m; decimalLatitude:-16.91666; decimalLongitude:-65.36667; eventDate:1958-01-09; sex:Adult Female; catalogNumber:UCR_ENT 00047331; recordedBy:Wygodzinsky and Monros; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus truxali; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Para; locality:Jacareacanga; verbatimElevation:88 m; decimalLatitude:-6.2667; decimalLongitude:-57.65; georeferenceSources:Gazetteer; eventDate:1969-10-01; sex:Adult Male; catalogNumber:UCR_ENT 00046896; recordedBy:F.R. Barbosa; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus truxali; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BRAZIL; stateProvince:Rondonia; locality:62 km SW Ariquemes, near Fzda. Rancho Grande; decimalLatitude:-10.3081; decimalLongitude:-63.44383; georeferenceSources:Google Earth; eventDate:1996-12-03 to 1996-12-15; sex:Adult Male; catalogNumber:UCR_ENT 00009317; occurrenceRemarks:Additional Labels C.J. Drake Fund Accession 1997; recordedBy:J. E. Eger; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus truxali; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:ECUADOR; stateProvince:Sucumbios; locality:Limoncocha; verbatimElevation:274 m; decimalLatitude:-0.43333; decimalLongitude:-76.63333; georeferenceSources:Label; eventDate:1974-03-23 to 1974-03-31; sex:Adult Male; catalogNumber:UCR_ENT 00015109; recordedBy:Dodge Engleman; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus truxali; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PERU; stateProvince:Pasco; locality:Chontilla 22 km. SE of Iscozazin; decimalLatitude:-10.3357; decimalLongitude:-75.11004; georeferenceSources:Google Earth; eventDate:1961-07-20; sex:Adult Male; catalogNumber:UCR_ENT 00010797; recordedBy:F. S. Truxal; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:LACM
-
scientificName: Zelus truxali; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PERU; stateProvince:Pasco; locality:Chontilla 22 km. SE of Iscozazin; decimalLatitude:-10.3357; decimalLongitude:-75.11004; georeferenceSources:Google Earth; eventDate:1961-07-20; sex:Adult Female; catalogNumber:UCR_ENT 00010798; recordedBy:F. S. Truxal; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:LACM
-
scientificName: Zelus truxali; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PERU; stateProvince:Pasco; locality:Chontilla 22 km. SE of Iscozazin; decimalLatitude:-10.3357; decimalLongitude:-75.11004; georeferenceSources:Google Earth; eventDate:1961-07-20; sex:Adult Female; catalogNumber:UCR_ENT 00010799; recordedBy:F. S. Truxal; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:LACM
-
scientificName: Zelus truxali; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PERU; stateProvince:Pasco; locality:Chontilla 22 km. SE of Iscozazin; decimalLatitude:-10.3357; decimalLongitude:-75.11004; georeferenceSources:Google Earth; eventDate:1961-07-20; sex:Adult Female; catalogNumber:UCR_ENT 00010800; recordedBy:F. S. Truxal; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:LACM
-
scientificName: Zelus truxali; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PERU; stateProvince:Pasco; locality:Chontilla 22 km. SE of Iscozazin; decimalLatitude:-10.3357; decimalLongitude:-75.11004; georeferenceSources:Google Earth; eventDate:1961-07-20; sex:Adult Female; catalogNumber:UCR_ENT 00010801; recordedBy:F. S. Truxal; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:LACM
-
scientificName: Zelus truxali; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PERU; stateProvince:Pasco; locality:Chontilla 22 km. SE of Iscozazin; decimalLatitude:-10.3357; decimalLongitude:-75.11004; georeferenceSources:Google Earth; eventDate:1961-07-20; sex:Adult Female; catalogNumber:UCR_ENT 00022671; occurrenceRemarks:Previously designated as 'allotype' of his manuscript name Zelus truxali by Hart, a type status not used in the formal publication of this name (Zhang et al., 2016).; recordedBy:F. S. Truxal; otherCatalogNumbers:LACM ENT 160235; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:LACM
Description
Zelus truxali Zhang & Hart, sp. n., habitus
b: Zelus truxali Zhang & Hart, sp. n., male, lateral view (UCR_ENT 00015109, Sucumbios, Ecuador)
c: Zelus truxali Zhang & Hart, sp. n., female, dorsal view (UCR_ENT 00010800, Pasco, Peru)
d: Zelus truxali Zhang & Hart, sp. n., female, lateral view (UCR_ENT 00010800, Pasco, Peru)
Zelus truxali Zhang & Hart, sp. n., male genitalic structures
b: Zelus truxali Zhang & Hart, sp. n., phallus, dorsal
Male: (
Female: (
Diagnosis
The uniquely slender, recurved medial process can distinguish this species among the members of the Zelus panamensis species group (Fig. 12). In females the anterior pronotal lobe yellowish and posterior lobe brown; the lateral and ventral surface of the body yellowish is unique among females of all species. Females are very similar to Z. sphegeus, but are separate from that species by the banded legs and the anterior pronotal lobe nearly flat, not distinctly elevated.
Etymology
Named after F. S. Truxal, the collector of the type specimen.
Distribution
South America (
Zelus umbraculoides , sp. n.
-
scientificName: Zelus umbraculoides; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:BOLIVIA; stateProvince:La Paz; locality:Tres Esteros, Guanay; decimalLatitude:-15.4833; decimalLongitude:-67.8833; eventDate:1989-08-19 to 1989-08-25; sex:Adult Male; catalogNumber:UCR_ENT 00026160; occurrenceRemarks:Drake Collection; recordedBy:L. Pena; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus umbraculoides; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2017; country:BRAZIL; stateProvince:Parana; locality:Caviuna; decimalLatitude:-23.2; decimalLongitude:-51.36666; georeferenceSources:GeoLocate Software; eventDate:1947-08-01; sex:Adult Male; catalogNumber:UCR_ENT 00017040; recordedBy:A. Maller; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus umbraculoides; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PARAGUAY; stateProvince:Alto Parana; locality:Alto Parana; decimalLatitude:-25.60752; decimalLongitude:-54.96117; georeferenceSources:GeoLocate Software; eventDate:1990-11-12 to 1990-11-16; sex:Adult Male; catalogNumber:UCR_ENT 00029367; occurrenceRemarks:Drake Collection; recordedBy:G. Arriagada; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus umbraculoides; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PERU; stateProvince:Madre de Dios; county:Tambopata; locality:Rio Tambopata Reserve, 30 air km SW Pto. Maldonado; verbatimElevation:290 m; decimalLatitude:-12.74338; decimalLongitude:-69.49339; georeferenceSources:Google Earth; eventDate:1979-11-16 to 1979-11-20; sex:Adult Male; catalogNumber:UCR_ENT 00009314; recordedBy:J. B. Heppner; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
Description
Zelus umbraculoides Zhang & Hart, sp. n., habitus
b: Zelus umbraculoides Zhang & Hart, sp. n., male, lateral view
Zelus umbraculoides Zhang & Hart, sp. n., male genitalic structures
b: Zelus umbraculoides Zhang & Hart, sp. n., phallus, dorsal
Male: (
Female: Unknown.
Diagnosis
Recognized by the body surface greenish-brown; the area of hemelytron adjacent to the quadrate cell dark brown, inversely U-shaped in appearance; the head short and stout (L/W=<2.1) ; the ocellus situated on conspicuous elevation (the same set of characters are also present in Z. umbraculus). Characters separating Z. umbraculoides and Z. umbraculus are discussed in the diagnosis of the latter.
Etymology
The specific epithet indicates its close resemblance to Z. umbraculus, another new species described in the current study.
Distribution
South America (
Taxon discussion
Zelus umbraculus , sp. n.
-
scientificName: Zelus umbraculus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PERU; stateProvince:Lambayeque; locality:94 mi E of Olmos; decimalLatitude:-5.98497; decimalLongitude:-78.3751; georeferenceSources:Google Earth; eventDate:1955-01-18; sex:Adult Male; catalogNumber:UCR_ENT 00047973; recordedBy:E. I. Schlinger & E. S. Ross; otherCatalogNumbers:CAS Type No. 12717; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus umbraculus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2018; country:ECUADOR; stateProvince:Orellana; locality:Coca (on Rio Napo); decimalLatitude:-0.4183; decimalLongitude:-76.9857; georeferenceSources:GeoLocate Software; eventDate:1965-05-01; sex:Adult Male; catalogNumber:UCR_ENT 00017041; recordedBy:L. E. Peña; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:AMNH
-
scientificName: Zelus umbraculus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:PERU; stateProvince:Lambayeque; locality:94 mi E of Olmos; decimalLatitude:-5.98497; decimalLongitude:-78.3751; georeferenceSources:Google Earth; eventDate:1955-01-18; sex:Adult Male; catalogNumber:UCR_ENT 00006067; recordedBy:E. I. Schlinger & E. S. Ross; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus umbraculus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2019; country:PERU; stateProvince:Lambayeque; locality:94 mi E of Olmos; decimalLatitude:-5.98497; decimalLongitude:-78.3751; georeferenceSources:Google Earth; eventDate:1955-01-18; sex:Adult Male; catalogNumber:UCR_ENT 00019692; recordedBy:E. I. Schlinger & E. S. Ross; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
-
scientificName: Zelus umbraculus; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2020; country:PERU; stateProvince:Lambayeque; locality:94 mi E of Olmos; decimalLatitude:-5.98497; decimalLongitude:-78.3751; georeferenceSources:Google Earth; eventDate:1955-01-18; sex:Adult Male; catalogNumber:UCR_ENT 00019694; recordedBy:E. I. Schlinger & E. S. Ross; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:CAS
Description
Zelus umbraculus Zhang & Hart, sp. n., habitus
b: Zelus umbraculus Zhang & Hart, sp. n., male, lateral view (UCR_ENT 00019694, Lambayeque, Peru)
Zelus umbraculus, Zhang & Hart, sp. n., male genitalic structures
b: Zelus umbraculus, Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: Unknown.
Diagnosis
Recognized by the body surface greenish-brown; the area of hemelytron adjacent to the quadrate cell dark brown, inversely V-shaped in appearance; the head short and stout (L/W=<2.1) ; the ocellus situated on conspicuous elevation (the same set of characters are also present in Z. umbraculus). Zelus umbraculus can be separated from Z. umbraculoides by several characters listed below (
Etymology
The specific epithet is from Latin "umbra", referring to the shadow in the anterior part of the membrane.
Distribution
South America (
Zelus vagans
Nomenclature
Zelus vagans Fabricius, 1803, p. 284, orig. descr.; Stål, 1868, p. 108, descr. and note; Stål, 1872, p. 88, cat.; Walker, 1873, p. 134, cat.; Lethierry and Severin, 1896, p. 153, cat.; Wygodzinsky, 1949a, p. 50, checklist; Maldonado, 1990, p. 332, cat.
-
scientificName: Zelus vagans; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Fabricius, 1803; country:unknown; stateProvince:unknown; locality:Habitat in America meridionali; eventDate:No date provided; sex:Adult Male; catalogNumber:UCR_ENT 00075107; recordedBy:Dom. Smidt; otherCatalogNumbers:ZMUC 102689; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:ZMUC
Description
Zelus vagans Fabricius, 1803, habitus
b: Zelus vagans Fabricius, 1803, male, lateral view (UCR_ENT 00037121, Rondônia, Brazil)
Zelus vagans Fabricius, 1803, male genitalic structures
b: Zelus vagans Fabricius, 1803, phallus, dorsal view
Male: (
Female: Unknown.
Diagnosis
Can be easily identified by the unique coloration pattern, the posterior pronotal lobe medially black and laterally orange. Distinguished among members of the Zelus vagans species group by the smaller size; the postocular lobe covered with recumbent setae. The paramere is similar to that in Z. championi in showing ventrally directed curvature, but is shorter than in Z. championi and reaching to only about mid-point of medial process.
Distribution
South America (
Zelus varius
Nomenclature
Euagoras varius Herrich-Schaeffer, 1853, p. 122, orig. descr.
Zelus varius: Stål , 1872, p. 92, cat . (subgenus Diplodus); Lethierry and Severin, 1896, p. 153, cat.; Wygodzinsky 1949a, p. 50, checklist; Maldonado, 1990, p. 332, cat.
Diplodus varius: Walker, 1873, p. 126, cat.
-
scientificName: Zelus varius; family:Reduviidae; genus:Zelus; scientificNameAuthorship:(Herrich-Schaeffer, 1853); country:GUYANA; stateProvince:Cuyuni-Mazaruni Region; locality:Bartica; decimalLatitude:5.78765; decimalLongitude:-57.62801; eventDate:No date provided; sex:Adult Male; catalogNumber:UCR_ENT 00069896; occurrenceRemarks:Neotype of Zelus varius (Herrich-Schaeffer, 1853) (New Designation by Zhang, Hart & Weirauch, 2016); recordedBy:J. R. de la Torre-Bueno; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:KU
Description
Zelus varius (Herrich-Schaeffer, 1853), habitus
b: Zelus varius (Herrich-Schaeffer, 1853), male, lateral view (UCR_ENT 00009478, Napo, Ecuador)
Zelus varius (Herrich-Schaeffer, 1853), male genitalic structures
b: Zelus varius (Herrich-Schaeffer, 1853), phallus, dorsal view
Male: (
Female: Similar to male, except for the following. Larger than male, total length 13.71–14.03 mm (mean 13.87 mm,
Diagnosis
Recognized by the following combination of characters: The posterior margin of posterior pronotal lobe with expansion laterad to scutellum; the rather long, spinous process on humeral angle; the smallish body size; the paramere short; the apical part of medial process compressed laterally, anterior side ridge-like; and the apex of medial process re-expanded, not acute.
Distribution
South America (
Zelus versicolor
Nomenclature
Euagoras versicolor Herrich-Schaeffer, 1848, p. 46-47, Tab. CCLXIV, fig. 820, orig. descr. and fig.; Herrich-Schaeffer, 1853, p. 92, cat.; Walker, 1873, p. 118, note.
Diplodus versicolor , Stål, 1860, p. 75, cat.
Zelus versicolor: Stål, 1862, p. 451, note; Stål, 1872, p. 92, cat. (subgenus Diplodus); Lethierry and Severin, 1896, p. 153, cat.; Wygodzinsky, 1949a, p. 50, checklist; Maldonado, 1990, p. 332, cat.
Euagoras nigrispinus Herrich-Schaeffer, 1848, p. 47-48, Tab. CCLXII, fig. 816, orig. descr. and fig.; Herrich-Schaeffer, 1853, p. 92, cat. Gil-Santana, 2008, p. 48, Figs 15-21, junior syn. of Zelus versicolor (Herrich-Schaeffer, 1848).
Diplodus nigrispinus: Stål, 1860, p. 75, cat.; Stål, 1868, p. 109, note; Walker, 1873, p. 126, cat.
Zelus nigrispinus: Mayr, 1866, p. 139, cat.; Stål, 1872, p. 91, cat. (subgenus Diplodus); Berg, 1879, p. 154; Lethierry and Severin, 1896, p. 152, cat.; Wygodzinsky, 1949a, p. 49, checklist; Maldonado, 1990, p. 330, cat; Gil-Santana, 2008, p. 48, junior syn. of Z. versicolor.
Zelus personatus Berg, 1879, p. 150-151, orig. descr. (subgenus Zelus); Lethierry and Severin, 1896, p. 153, cat.; Wygodzinsky, 1949a, p. 50, checklist; Wygodzinsky, 1957, p. 264, 268-269, list and note; Maldonado, 1990, p. 330, cat. syn. nov. (current study).
-
scientificName: Zelus versicolor; family:Reduviidae; genus:Zelus; scientificNameAuthorship:(Herrich-Schaeffer, 1848); country:ARGENTINA; stateProvince:Misiones; eventDate:no date provided; sex:Adult Male; occurrenceRemarks:Holotype of Zelus personatus Berg, 1879, junior synonym o Zelus versicolor (Herrich-Schaeffer, 1848). Bears the following labels: Typus / Misiones / Zelus personatus Berg / Zelus personatus Berg, Wygodzinsky det./1555; identifiedBy:E. R. Hart; dateIdentified:1972; institutionCode:Universidad Nacional de La Plata, La Plata
Description
Zelus versicolor (Herrich-Schaeffer, 1848), habitus
b: Zelus (Herrich-Schffer, 1848), male, lateral view (UCR_ENT 00017744, Rio de Janeiro, Brazil)
c: Zelus versicolor (Herrich-Schaeffer, 1848), female, dorsal view (UCR ENT 00017755, Santa Catarina, Brazil)
d: Zelus versicolor (Herrich-Schaeffer, 1848), female, lateral view (UCR ENT 00017755, Santa Catarina, Brazil)
Zelus versicolor (Herrich-Schaeffer, 1848), male genitalic structures
b: Zelus versicolor (Herrich-Schaeffer, 1848), phallus, dorsal view
Male: (
Female: Similar to male, except for the following. Larger than male, total length 14.43–15.84 mm (mean 15.01 mm, Table 4.2). Dorsum dark brown, a large number of specimens with anterior 1/2 of posterior pronotal lobe yellow and some also with entire anterior pronotal lobe yellow; lateral surface and abdomen yellowish. Hemelytron slightly surpassing apex of abdomen.
Diagnosis
Recognized by the following combination of characters: the posterior pronotal lobe usually lighter than the anterior pronotal lobe, orangish or reddish-brown; the paramere bulbous, basally constricted, curved towards medial process; the medial process triangular, apex with pair of processes; the dorsal phallothecal sclerite with submedial ridge-like dorsad projection continuous from basal arm; and the basal plate arms subparallel and strongly curved. In females the posterior pronotal lobe is often bicolorous, anterior portion yellowish and posterior portion brown.
Distribution
South America (
Taxon discussion
The type specimens of Z. versicolor and Z. nigrispinus were destroyed during World War II.
Zelus vespiformis
Nomenclature
Zelus vespiformis Hart, 1987, p. 301, figs. 22-27, orig. descr., note, fig. and key; Maldonado, 1990, p. 332, cat.
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PANAMA; stateProvince:Canal Zone; locality:Barro Colorado Island, Drayton Trail; decimalLatitude:9.15472; decimalLongitude:-79.84806; georeferenceSources:GeoLocate Software; eventDate:1930-11-11; sex:Adult Male; catalogNumber:UCR_ENT 00057794; occurrenceRemarks:The institution code on the barcode (USI) lable is AM ENT, referring to the American Museum of Natural History. This is an error. The correct form should be AMNH ENT. Also the USI number is UCR_ENT 57794, which is lacking three '0' before '57794'.; recordedBy:H. F. Schwarz; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PANAMA; stateProvince:Canal Zone; locality:Barro Colorado Island, Drayton Trail; decimalLatitude:9.15472; decimalLongitude:-79.84806; georeferenceSources:GeoLocate Software; eventDate:1930-11-11; sex:Adult Female; catalogNumber:UCR_ENT 00057795; recordedBy:H. F. Schwarz; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Amazonas; locality:Rio Cotuhe, Tatapaca; decimalLatitude:-2.88331; decimalLongitude:-69.73332; georeferenceSources:Google Earth; eventDate:1946-09-12; sex:Adult Female; catalogNumber:UCR_ENT 00071181; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:TAMU
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Cundinamarca; locality:Fusagasuga; verbatimElevation:1750 m; eventDate:1940-01-02; sex:Adult Female; catalogNumber:UCR_ENT 00009411; recordedBy:F. Jotaya; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Cundinamarca; locality:El Colegio; decimalLatitude:4.58472; decimalLongitude:-74.94944; georeferenceSources:Gazetteer; eventDate:1946-05-23; sex:Adult Female; catalogNumber:UCR_ENT 00009413; recordedBy:E.A. Chapin; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Cundinamarca; locality:Fusagasuga; decimalLatitude:4.3439; decimalLongitude:-74.3678; georeferenceSources:Gazetteer; eventDate:1944-06-05; sex:Adult Female; catalogNumber:UCR_ENT 00040461; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:TAMU
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Cundinamarca; locality:Fusagasuga; decimalLatitude:4.3439; decimalLongitude:-74.3678; georeferenceSources:Gazetteer; eventDate:1944-06-05; sex:Adult Female; catalogNumber:UCR_ENT 00071173; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:TAMU
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Magdalena; locality:Rio Frio; verbatimElevation:716 m; decimalLatitude:10.92912; decimalLongitude:-74.13301; georeferenceSources:Google Earth; eventDate:1925-07-20; sex:Adult Female; catalogNumber:UCR_ENT 00040462; recordedBy:F. W. Walker; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:TAMU
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Magdalena; locality:Sevilla; decimalLatitude:10.76343; decimalLongitude:-74.13916; georeferenceSources:Gazetteer; eventDate:1926-08-14; sex:Adult Female; catalogNumber:UCR_ENT 00071180; recordedBy:F. W. Walker; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:TAMU
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Meta; locality:Rio Guamal [Quamal?]; verbatimElevation:400 m; decimalLatitude:3.7987; decimalLongitude:-73.66261; georeferenceSources:Google Earth; eventDate:1948-01-24; sex:Adult Female; catalogNumber:UCR_ENT 00009415; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Meta; locality:Cano Grande, Selba del Llano; verbatimElevation:450 m; eventDate:1948-01-20; sex:Adult Female; catalogNumber:UCR_ENT 00009416; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Meta; locality:Rio Guayeriba, a triburary of Rio Meta; eventDate:1948-01-24; sex:Adult Male; catalogNumber:UCR_ENT 00017877; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Meta; locality:Rio Guayeriba, a triburary of Rio Meta; eventDate:1948-01-24; sex:Adult Female; catalogNumber:UCR_ENT 00017879; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Meta; locality:Rio Guayuriba; verbatimElevation:400 m; decimalLatitude:4.01978; decimalLongitude:-73.60807; georeferenceSources:Google Earth; eventDate:1944-09-06; sex:Adult Male; catalogNumber:UCR_ENT 00040463; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:TAMU
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Meta; locality:Cano Grande, Selba del Llano; verbatimElevation:450 m; eventDate:1944-03-06; sex:Adult Male; catalogNumber:UCR_ENT 00040464; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:TAMU
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Meta; locality:Rio Guayuriba; verbatimElevation:400 m; decimalLatitude:4.01978; decimalLongitude:-73.60807; georeferenceSources:Google Earth; eventDate:1947-09-06; sex:Adult Female; catalogNumber:UCR_ENT 00071175; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:TAMU
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Meta; locality:Cano Grande, Selba del Llano; verbatimElevation:450 m; eventDate:1947-12-02; sex:Adult Female; catalogNumber:UCR_ENT 00071176; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:TAMU
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Meta; locality:Cano Grande, Selba del Llano; verbatimElevation:450 m; eventDate:1948-02-20; sex:Adult Female; catalogNumber:UCR_ENT 00071177; recordedBy:Richter; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:TAMU
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Santander; locality:Vista Nieve; decimalLatitude:6.98944; decimalLongitude:-73.66972; georeferenceSources:Gazetteer; eventDate:1926-08-07; sex:Adult Female; catalogNumber:UCR_ENT 00071174; recordedBy:F. W. Walker; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:TAMU
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:unknown; locality:unknown; decimalLatitude:5.02584; decimalLongitude:-74.02986; georeferenceSources:Google Earth; eventDate:no date provided; sex:Adult Male; catalogNumber:UCR_ENT 00009405; recordedBy:B. Losada; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:unknown; locality:unknown; decimalLatitude:5.02584; decimalLongitude:-74.02986; georeferenceSources:Google Earth; eventDate:no date provided; sex:Adult Female; catalogNumber:UCR_ENT 00009412; recordedBy:B. Losada; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Valle del Cauca; locality:Palmira; decimalLatitude:3.5364; decimalLongitude:-76.3036; georeferenceSources:Gazetteer; eventDate:1942-01-01; sex:Adult Male; catalogNumber:UCR_ENT 00009408; recordedBy:B. Losada; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Valle del Cauca; locality:Cali; decimalLatitude:3.4372; decimalLongitude:-76.5225; georeferenceSources:Gazetteer; eventDate:1943-07-01; sex:Adult Female; catalogNumber:UCR_ENT 00009414; recordedBy:B. Losada; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Valle del Cauca; locality:Palmira; decimalLatitude:3.5364; decimalLongitude:-76.3036; georeferenceSources:Gazetteer; eventDate:1955-07-17; sex:Adult Female; catalogNumber:UCR_ENT 00009420; recordedBy:M. Benavides; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Valle del Cauca; locality:Cali District; verbatimElevation:994 m; eventDate:1935-02-07; sex:Adult Male; catalogNumber:UCR_ENT 00017878; recordedBy:Severo Quintero; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:COLOMBIA; stateProvince:Valle del Cauca; locality:Cali District; verbatimElevation:994 m; eventDate:1935-02-07; sex:Adult Female; catalogNumber:UCR_ENT 00017882; recordedBy:Severo Quintero; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:AMNH
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:EL SALVADOR; stateProvince:La Libertad; locality:Santa Tecla; decimalLatitude:13.6769; decimalLongitude:-89.2797; eventDate:1953-01-24; sex:Adult Male; catalogNumber:UCR_ENT 00009402; recordedBy:Salazar; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PANAMA; stateProvince:Canal Zone; locality:Barro Colorado Island; decimalLatitude:9.15562; decimalLongitude:-79.84895; georeferenceSources:Google Earth; eventDate:1946-12-01 to 1947-02-01; sex:Adult Male; catalogNumber:UCR_ENT 00009400; recordedBy:J. Zelek; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:PANAMA; stateProvince:Canal Zone; locality:Barro Colorado Island; decimalLatitude:9.15562; decimalLongitude:-79.84895; georeferenceSources:Google Earth; eventDate:1929-04-03; sex:Adult Male; catalogNumber:UCR_ENT 00009401; recordedBy:S. W. Frost; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:TRINIDAD AND TOBAGO; stateProvince:Trinidad; locality:Saint Joseph; eventDate:1953-03-06; sex:Adult Female; catalogNumber:UCR_ENT 00009423; recordedBy:F.J. Simmonds; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:VENEZUELA; stateProvince:Distrito Federal; locality:La Florida; decimalLatitude:10.50171; decimalLongitude:-66.86585; georeferenceSources:Google Earth; eventDate:1938-04-16; sex:Adult Male; catalogNumber:UCR_ENT 00009406; recordedBy:C.H. Ballou; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:VENEZUELA; stateProvince:Distrito Federal; locality:Caracas; decimalLatitude:10.5; decimalLongitude:-66.9; georeferenceSources:GeoLocate Software; eventDate:1905-04-21; sex:Adult Male; catalogNumber:UCR_ENT 00009407; recordedBy:Anthonias; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:VENEZUELA; stateProvince:Distrito Federal; locality:Florida, Caracas; decimalLatitude:10.50395; decimalLongitude:-66.87425; georeferenceSources:Google Earth; eventDate:1938-11-08; sex:Adult Female; catalogNumber:UCR_ENT 00009417; recordedBy:C.H. Ballou; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:VENEZUELA; stateProvince:Distrito Federal; locality:Caracas; decimalLatitude:10.5; decimalLongitude:-66.9; georeferenceSources:GeoLocate Software; eventDate:1905-04-21; sex:Adult Female; catalogNumber:UCR_ENT 00009421; recordedBy:Anthonias; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:VENEZUELA; stateProvince:Distrito Federal; locality:Berg Avila, Caracas; decimalLatitude:10.48801; decimalLongitude:-66.87919; georeferenceSources:Gazetteer; eventDate:no date provided; sex:Adult Female; catalogNumber:UCR_ENT 00071171; recordedBy:P. Cor. Vogl; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:TAMU
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:VENEZUELA; stateProvince:Distrito Federal; locality:Berg Avila, Caracas; decimalLatitude:10.48801; decimalLongitude:-66.87919; georeferenceSources:Gazetteer; eventDate:no date provided; sex:Adult Female; catalogNumber:UCR_ENT 00071172; recordedBy:P. Cor. Vogl; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:TAMU
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:VENEZUELA; stateProvince:unknown; locality:El Valle; eventDate:1939-02-01; sex:Adult Female; catalogNumber:UCR_ENT 00009424; recordedBy:C.H. Ballou; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:VENEZUELA; stateProvince:Vargas; locality:Mama, near La Guayra; decimalLatitude:10.6; decimalLongitude:-66.9333; georeferenceSources:Gazetteer; eventDate:1927-02-01; sex:Adult Female; catalogNumber:UCR_ENT 00009410; recordedBy:H.E. Hox; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:VENEZUELA; stateProvince:Vargas; locality:Mama, near La Guayra; decimalLatitude:10.6; decimalLongitude:-66.9333; georeferenceSources:Gazetteer; eventDate:1927-02-01; sex:Adult Female; catalogNumber:UCR_ENT 00009419; recordedBy:H.E. Hox; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:VENEZUELA; stateProvince:Zulia; locality:Perija; decimalLatitude:9.74202; decimalLongitude:-72.97539; georeferenceSources:Google Earth; eventDate:1918-01-18; sex:Adult Male; catalogNumber:UCR_ENT 00009403; recordedBy:Tejera; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:VENEZUELA; stateProvince:Zulia; locality:Perija; decimalLatitude:9.74202; decimalLongitude:-72.97539; georeferenceSources:Google Earth; eventDate:1918-01-18; sex:Adult Male; catalogNumber:UCR_ENT 00009404; recordedBy:Tejera; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:VENEZUELA; stateProvince:Zulia; locality:Maracaibo; decimalLatitude:10.6317; decimalLongitude:-71.6406; georeferenceSources:Gazetteer; eventDate:no date provided; sex:Adult Female; catalogNumber:UCR_ENT 00009418; recordedBy:Tejera; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
-
scientificName: Zelus vespiformis; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Hart, 1987; country:VENEZUELA; stateProvince:Zulia; locality:Perija; decimalLatitude:9.74202; decimalLongitude:-72.97539; georeferenceSources:Google Earth; eventDate:1918-01-18; sex:Adult Female; catalogNumber:UCR_ENT 00009422; recordedBy:Tejera; identifiedBy:G. Zhang; dateIdentified:2012; institutionCode:USNM
Description
Zelus vespiformis Hart, 1987, habitus
b: Zelus vespiformis Hart, 1987, male, lateral view (UCR_ENT 00022501, Santander, Colombia)
c: Zelus vespiformis Hart, 1987, female, dorsal view (UCR_ENT 00023006, Antioquia, Colombia)
d: Zelus vespiformis Hart, 1987, female, lateral view (UCR_ENT 00023006, Antioquia, Colombia)
e: Zelus vespiformis Hart, 1987, female, dorsal view (UCR_ENT 00014266, Guanacaste, Costa Rica)
f: Zelus vespiformis Hart, 1987, female, lateral view (UCR_ENT 00011313, Guanacaste, Costa Rica)
Zelus vespiformis Hart, 1987, male genitalic structures
b: Zelus vespiformis Hart, 1987, phallus, dorsal view
Male: (
Female: Similar to male, except for the following. Larger than male, total length 16.48–18.29 mm (mean 17.47 mm,
Diagnosis
The generally wasp-like coloration pattern can separate this species from most other species of the Zelus, but not from a few species that may also display a wasp-like appearance. Males can be recognized by the rather slender and short medial process, it being much shorter than that in Zelus errans, the only species that may cause confusion.
Distribution
Central America and Northern South America (
Zelus xouthos , sp. n.
-
scientificName: Zelus xouthos; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:GUATEMALA; stateProvince:Izabal; locality:Cayuga; verbatimElevation:25 m; decimalLatitude:15.5333; decimalLongitude:-88.7; georeferenceSources:Gazetteer; eventDate:1915-05-01; sex:Adult Male; catalogNumber:UCR_ENT 00008003; recordedBy:W. M. Schaus; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
-
scientificName: Zelus xouthos; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Zhang and Hart, 2016; country:GUATEMALA; stateProvince:Peten; locality:Tikal; decimalLatitude:17.225; decimalLongitude:-89.6133; georeferenceSources:Gazetteer; eventDate:1956-05-12; sex:Adult sex unknown; catalogNumber:UCR_ENT 00029352; occurrenceRemarks:Abdomen missing. Designated as allotype by Hart, unpublished. This status is not used in Zhang et al., which formally publishes this species.; recordedBy:T. H. Hubbell; identifiedBy:G. Zhang; dateIdentified:2013; institutionCode:USNM
Description
Zelus xouthos Zhang & Hart, sp. n., habitus
b: Zelus xouthos Zhang & Hart, sp. n., male, lateral view (UCR_ENT 00008003, Izabal, Guatemala
Zelus xouthos Zhang & Hart, sp. n., male genitalic structures
b: Zelus xouthos Zhang & Hart, sp. n., phallus, dorsal view
Male: (
Female: Similar to male, except for the following. Larger than male, total length 14.69 mm (n=1,
Diagnosis
Recognized by the following combination of characters: the slender body and delicate legs, the dorsal coloration somewhat reddish; the humeral angle elevated nearly to the level of and nearly continuous with pronotal disc; the paramere uniquely shaped, long, exceeding medial process, apex somewhat obliquely truncate; and the medial process apically not compressed.
Etymology
From Greek xutho, meaning yellowish-brown, referring to the yellowish coloration.
Distribution
Central America (
Taxon discussion
This species appears to be the most divergent of the Zelus panamensis species group. Although three other species within the same geographical range of this species, Z. janus, Z. exsanguis, Z. ambulans, also have an elevated humeral angle, their much greater sizes and features of the male genitalia should eliminate any confusion in recognizing Z. xouthos.
Zelus zayasi
Nomenclature
Zelus zayasi Bruner and Barber, 1937, p. 186–188, orig. descr. and fig. (subgenus Zelus); Wygodzinsky, 1949a, p. 50, checklist; Zayas, 1960, p. 125, 126, note; Alayo, 1967, p. 36, 37, key and list.; Hart, 1987, p. 296, key and note; Maldonado, 1990, p. 332, cat.
-
scientificName: Zelus zayasi; family:Reduviidae; genus:Zelus; scientificNameAuthorship:Bruner & Barber, 1937; country:CUBA; stateProvince:Guantanamo; locality:El Yunque, Baracoa; decimalLatitude:20.35237; decimalLongitude:-74.57356; georeferenceSources:Google Earth; eventDate:1935-07-10; sex:Adult Female; catalogNumber:UCR_ENT 00007933; occurrenceRemarks:bears label 'Type No. 51844 U.S.N.M' and 'E.E.A. Cuba Ento No.10644'; recordedBy:F. de Zayas; otherCatalogNumbers:USNM Type No. 51844; identifiedBy:Burner & Barber; dateIdentified:1937; institutionCode:USNM
Description
Zelus zayasi Bruner and Barber, 1937, habitus
b: Zelus zayasi Bruner and Barber, 1937, female, lateral view (UCR_ENT 00007933, Baracoa, Cuba)
Male: unknown.
Female: (
Diagnosis
The extremely slender body form separates this species from most other species of Zelus. Can be distinguished from Z. puertoricensis by the lack of conspicuous lateral process on the humeral angle and from Z. subimpressus by the parallel dorsal and ventral surfaces on the anterior 2/3 of the postocular lobe. The dorsal surface in Z. subimpressus is sloping.
Distribution
The Caribbean. Known only from Cuba (
Taxon discussion
Based on the description and figure of Z. bruneri, it is possible that Z. bruneri is the male of Z. zayasi. Because the specimens of Z. bruneri were not physically examined, we restrain from making this association and a formal synonymy between these two species.
Identification keys
|
Key to species groups of Zelus (males only) |
||
| 1 | Apex of medial process without ventrally or posteriorly directed hook, projection or fold ( |
2 |
| – | Apex of medial process with ventrally or posteriorly directed hook, projection or fold ( |
5 |
| 2 | Medial process triangular, broad at base, if slender, paramere apically enlarged; anteroposteriorly compressed ( |
3 |
| – | Medial process with lateral margins subparallel or narrowly triangular; base not conspicuously broadened; laterally compressed or blade-like throughout or in apical 1/2 ( |
4 |
| 3 | Medial process basally nearly continuous with rest of pygophore, base not protruding posteriorly. Paramere apically not enlarged or slightly enlarged ( |
Zelus tetracanthus species group |
| – | Medial process basally readily separable from rest of pygophore; base prominent and protruding posteriorly. Paramere expanded apically in some species ( |
Zelus luridus species group |
| 4 | Medial process slender, laterally compressed, dorsally oriented, at about forty-five degree angle to body axis (except in Z. grassans) ( |
Zelus nugax species group |
| – | Medial process stout, posteriorly oriented, nearly parallel to body axis ( |
Zelus vagans species group |
| 5 | Apex of medial process strongly curved ventrally to form hook-like process or lip-like fold ( |
6 |
| – | Apex of medial process not curved or weakly curved ventrally ( |
7 |
| 6 | Medial process laterally compressed; apex strongly curved ventrally, clearly hook-like. Paramere apically expanded, slightly curved dorsally ( |
Zelus renardii species group |
| – | Medial process cylindrical or broad at base; not laterally compressed; apex curved, lip-like rather than hook-like ( |
Zelus mimus species group |
| 7 | Apex of medial process with distinct, acute, hook-like process or ridge-like elevation ( |
8 |
| – | Apex of medial process with indistinct process or weak curvature, not forming sharp projection ( |
9 |
| 8 | Medial process dorsally directed and apical 1/3 not curving posteriorly; usually anteroposteriorly compressed; base broad in some species; ridge-like elevation on posterior surface, usually extending ventrally and elevated, removed from apex and appearing as pair of projections in some species ( |
Zelus erythrocephalus species group |
| – | Medial process long, slender, stalk-like; somewhat laterally compressed; lateral margins subparallel, base not broadened or slightly broadened; gradually curving and directed posteriorly in apical 1/3 or 1/2; apex with short, acute, hook-like process or sharp fold ( |
Zelus panamensis species group |
| 9 | Medial process extremely slender, usually long ( |
Zelus longipes species group |
| – | Medial process stout, relatively short ( |
10 |
| 10 | Large (>13 mm), robust. Apex of medial process with pair of minute projections ( |
Zelus armillatus species group |
| – | Medium-sized (<12 mm), elongate. Apex of medial process with inconspicuous folding ( |
Zelus puertoricensis species group |
|
Key to males of the Zelus tetracanthus species group |
||
| 1 | Posterior pronotal lobe disc bears pair of tubercles. | 2 |
| – | Posterior pronotal lobe disc without tubercles. | 3 |
| 2 | Smallish, 7.8-10.1 mm. Legs delicate. Head and parts of pronotum orangish-brown. Paramere clearly exceeds medial process ( |
Zelus minutus ( |
| – | Length 11.3-13.7 mm. Greyish-brown to brownish-black. Paramere removed from or reaching medial process, never exceeding ( |
Zelus tetracanthus ( |
| 3 | Entire surface reddish-brown ( |
Zelus rosulentus |
| – | Pale brown ( |
Zelus prolixus |
|
Key to males of the Zelus luridus species group |
||
| 1 | Humeral angle raised to level of, and usually continuous with, disc of posterior pronotal lobe. | 2 |
| – | Humeral angle clearly below level of, and not continuous with, disc. | 3 |
| 2 | Medial longitudinal sulcus of anterior pronotal lobe with dark brown area near posterior margin. Femora usually with dark apical bands. Parameres in fresh or relaxed specimens not achieving apex of medial process ( |
Zelus ambulans ( |
| – | Medial longitudinal sulcus of anterior pronotal lobe same color as surrounding area at posterior margin, any dark areas present very small. Femora apically reddish-brown, not forming distinct bands. Paramere in fresh or relaxed specimens achieving or surpassing apex of medial process ( |
Zelus exsanguis ( |
| 3 | Paramere spatulate apically, compressed; apical portion about 2x width of cylindrical basal portion ( |
Zelus spatulosus ( |
| – | Paramere slightly expanded apically, apical portion less than 2x width of base. Medial process triangular, base broad and protruding posteriorly. | 4 |
| 4 | Eyes prominent, extending below ventral surface of head. |
Zelus grandoculus ( |
| – | Eyes moderate, not extending below ventral surface of head. | 5 |
| 5 | Anterior pronotal lobe usually 1/2 or greater of length of posterior pronotal lobe; if slightly less than 1/2, profemoral length less than 20x profemoral diameter. Protrusion at base of medial process moderate ( |
Zelus luridus ( |
| – | Anterior pronotal lobe less than 1/2 length of posterior pronotal lobe; profemoral length 20x or more profemoral diameter (C.A.). Protrusion at base of medial process more prominent ( |
Zelus antiguensis ( |
|
Key to males of the Zelus nugax species group |
||
| 1 | Paramere curved strongly medially and recurved apically ( |
Zelus grassans ( |
| – | Paramere straight or only slightly curved. Medial process dorsally directed. Brown, abdomen without banding. | 2 |
| 2 | Medial process exhibiting pronounced curvature posteriorly about 1/3 of distance from base, then recurved toward dorsum about 3/4 distance from base. | 3 |
| – | Medial process blade-like, straight; any curvature toward posterior weak. | 4 |
| 3 | Labial segment I and coxae medium brown to brownish-black. Paramere nearly straight, any curvature weak ( |
Zelus impar ( |
| – | Labium and coxae light yellowish-brown. Paramere medially curved ventrally ( |
Zelus illotus ( |
| 4 | Basal plate arms fused. Pygophore as in |
Zelus pedestris ( |
| – | Basal plate arms not fused, separate in specimens from S.A., tendency to fuse in C.A. and Mexico. Pygophore as in |
Zelus nugax ( |
|
Key to males of the Zelus vagans species group |
||
| 1 | Dorsal surface entirely black. Abdomen brown, yellow or red. | 2 |
| – | Dorsal surface with orange and dark brown areas on pronotum or hemelytron. Abdomen typically orange or reddish with terminal segments dark brown; variations exist. | 4 |
| 2 | Abdomen brown, not brightly yellow or red. Cells of membrane conspicuously less pigmented than veins. Postocular lobe with longitudinal lateral patch of whitish recumbent setae. |
Zelus aithaleos ( |
| – | Abdomen brightly yellow or red. Cells of membrane same color as veins. Postocular lobe without lateral whitish setae. | 3 |
| 3 | Abdomen red. Paramere medially curved ventrad ( |
Zelus championi ( |
| – | Abdomen yellow. Paramere slightly bent near base, straight in remaining part ( |
Zelus fuliginatus ( |
| 4 | Dorsal surface of pronotum medially dark brown, laterally orange-brown. Paramere removed from apex of medial process, medially curved ventrad ( |
Zelus vagans ( |
| – | Coloration of pronotum not as described above; anterior lobe dark brown and posterior lobe orange-brown in some specimens. Paramere straight, apex oblique ( |
Zelus gracilipes ( |
|
Key to males of the Zelus renardii species group |
||
| 1 | Relatively robust. Humeral angle of pronotum widened; body length 5.5x or less of width through humeral angles. Apical hook of paramere more prominent ( |
Zelus renardii ( |
| – | Very slender. Humeral angle not conspicuously widened; body length greater than 5.5x width through humeral angles. Apical hook of paramere less prominent ( |
Zelus cervicalis ( |
|
Key to males of the Zelus mimus species group |
||
| 1 | Medial process long and slender ( |
Zelus mimus ( |
| – | Medial process short and broad ( |
Zelus inconstans ( |
|
Key to males of the Zelus erythrocephalus species group |
||
| 1 | Head reddish-brown. Dorsal surface of pronotum and corium brownish-black. Membrane of hemelytron pale brown or blue or green iridescent. Legs brownish-black, without banding or with inconspicuous bands. | 2 |
| – | Head brown, yellow, or black, sometimes with stripes. Dorsal surface uniformly brown or with light-colored areas, mainly on pronotum or corium. Membrane brown, not iridescent. Legs with or without banding. | 4 |
| 2 | Medial process very slender, diameter less than that of paramere ( |
Zelus erythrocephalus ( |
| – | Medial process broad, diameter greater than that of paramere. | 3 |
| 3 | Medial process twice as broad as paramere ( |
Zelus paracephalus ( |
| – | Medial process only slightly broader than paramere. Paramere bent ventrally near base, not recurved ( |
Zelus russulumus ( |
| 4 | Medial process very broad; lateral margins parallel or converging basally. | 5 |
| – | Medial process triangular; lateral margins converging apically, base broader than apex. | 6 |
| 5 | Length less than 11.4 mm. Medial process apex broader than base, apex acute, medially not notched ( |
Zelus laticornis ( |
| – | Length more than 12 mm. Medial process lateral margins parallel, apex notched in middle ( |
Zelus casii ( |
| 6 | Posterior surface of medial process with ridge-like elevation, extending ventrally, not across width of medial process ( |
7 |
| – | Posterior surface of medial process with hook-like process, across width of medial process; if extending ventrally, as pair of dentate processes. | 8 |
| 7 | Posterior margin of posterior pronotal lobe yellowish, much lighter than remaining surface of the lobe. Paramere strongly curved downward, width more or less uniform, abruptly constricted at base ( |
Zelus kartaboides ( |
| – | Posterior pronotal lobe uniformly colored, dark brown. Paramere with basal 1/2 nearly straight, only curved in apical 1/2, gradually expanded toward apex ( |
Zelus kartabensis ( |
| 8 | Humeral angle raised to level of, and nearly continuous with, disc. Short, light, shining recumbent setae dorsally on head and pronotum. Medial process long, longer than parameres; base very broad ( |
Zelus auralanus ( |
| – | Pronotal disc clearly elevated above humeral angle. Medial process narrowly triangular, shorter or subequal in length to paramere. Paramere constricted at base and somewhat bent ventrad. | 9 |
| 9 | Humeral angle rounded. Medial process long, upright ( |
Zelus mattogrossensis ( |
| – | Humeral angle with spinous or dentate process. Medial process relatively short, semi-erect. | 10 |
| 10 | Medial process posterior surface with pair of processes near apex ( |
Zelus versicolor ( |
| – | Medial process apex hook-like, entire, not discontinuous as pair of projections ( |
Zelus chamaeleon ( |
|
Key to males of the Zelus panamensis species group |
||
| 1 | Paramere achieving or surpassing apex of medial process. | 2 |
| – | Paramere not achieving apex of medial process. | 3 |
| 2 | Reddish-brown. Femora without banding. Paramere curved ventrally, apex recurved slightly dorsally ( |
Zelus xouthos ( |
| – | Anterior pronotal lobe dark brown, posterior lobe yellowish-brown. Femora dark brown, with two yellowish bands. Paramere straight ( |
Zelus banksi ( |
| 3 | Medial process shorter than or at most subequal in length to paramere. | 4 |
| – | Medial process at least 1.1x length of paramere. | 6 |
| 4 | Head orangish or reddish. |
Zelus panamensis ( |
| – | Head brown or dark brown. | 5 |
| 5 | Postocular lobe with longitudinal yellowish stripe. Medial process about as long as paramere ( |
Zelus truxali ( |
| – | Postocular lobe without stripe. Medial process less than 0.8x length of paramere ( |
Zelus korystos ( |
| 6 | Paramere diameter constant through apical 3/4 or only slightly expanding. | 7 |
| – | Paramere expanded apically, enlarged portion in lateral view much greater than diameter of medial process. | 9 |
| 7 | Medial process bent in middle ( |
Zelus filicauda ( |
| – | Medial process nearly straight, curving gradually. Paramere diameter constant or weakly expanding. | 8 |
| 8 | Medial process posteriorly directed, at less than forty-five degree angle to horizontal axis ( |
Zelus gilboventris ( |
| – | Medial process erect, at larger than forty-five degree angle to horizontal axis ( |
Zelus nigromaculatus ( |
| 9 | Medial process narrowed apically in lateral view ( |
Zelus cordazulus ( |
| – | Medial process slightly expanded subapically in lateral view ( |
Zelus varius ( |
|
Key to males of the Zelus armillatus species group |
||
| 1 | General coloration greenish or yellowish pale brown, rather uniform. Legs without banding. | 2 |
| – | Not as described above, consisting of typically two or more different colors; if uniformly colored, then dark brown or blackish-brown. Legs typically banded; if unbanded, then uniformly blackish-brown. | 4 |
| 2 | Paramere long, achieving or surpassing apex of medial process ( |
Zelus amblycephalus ( |
| – | Paramere removed from apex of medial process. | 3 |
| 3 | Disc of posterior pronotal lobe strongly bulging. Paramere slender; diameter of basal 1/2 less than that of medial process in lateral view ( |
Zelus umbraculoides ( |
| – | Disc slightly bulging, nearly flat. Diameter of paramere greater than that of medial process in lateral view ( |
Zelus umbraculus ( |
| 4 | Disc of posterior pronotal lobe depressed in middle. | 5 |
| – | Disc bulging or flat. | 6 |
| 5 | Dorsal coloration predominantly brownish-black, with various reddish areas; venter reddish; white waxy exudation usually conspicuous dorsally and laterally (Southern S.A.). |
Zelus leucogrammus ( |
| – | Entire surface testaceous-brown (Mexico and C.A.). |
Zelus sulcicollis ( |
| 6 | Paramere long, more than 2x length of median process. | 7 |
| – | Paramere short, less than 2x length of median process. | 8 |
| 7 | Dorsal coloration brownish-black; femora without or with few indistinct bands. Disc of posterior pronotal lobe bears pair of tubercles (C.A.). |
Zelus lewisi ( |
| – | Dorsal coloration yellow-green; legs conspicuously annulated. Disc without tubercles (Northern S.A.). |
Zelus annulosus ( |
| 8 | Humeral angle raised nearly to level of, and almost continuous with, disc. |
Zelus janus ( |
| – | Humeral angle clearly below level of disc. | 9 |
| 9 | Medial process relatively robust. | 10 |
| – | Medial process delicate. | 11 |
| 10 | As viewed posteriorly, diameter of medial process less than 1.5x ocellar diameter, slightly larger than that of paramere ( |
Zelus armillatus ( |
| – | Medial process broad, diameter near middle at least 1.5x ocellar diameter ( |
Zelus conjungens ( |
| 11 | Head reddish-brown. Paramere very delicate ( |
Zelus ruficeps ( |
| – | Head dark brown, sometimes with stripes. Apical portion of paramere obviously thicker than medial process ( |
Zelus litigiosus ( |
|
Key to males of the Zelus longipes species group |
||
| 1 | Paramere more than 2x length of medial process in lateral view, surpassing medial process by a moderately large margin. | 2 |
| – | Paramere less than 2x length of medial process, achieving or slightly surpassing medial process. | 3 |
| 2 | Coloration consisting of reddish-brown and brownish-black, locations and relative amounts highly variable. Paramere diameter more or less constant ( |
Zelus longipes ( |
| – | Coloration mainly of yellow and dark brown, posterior pronotal lobe orangish-brown. Paramere medially slightly constricted ( |
Zelus bahiaensis ( |
| 3 | Body elongated. Medial process long ( |
Zelus errans ( |
| – | Body not as elongated as that of Zelus errans. Medial process short ( |
Zelus vespiformis ( |
|
Key to males of the Zelus puertoricensis species group Zelus bruneri and Zelus zayasi are not included in the key. No physical specimens were examined for the former and male is not known for the latter. |
||
| 1 | Postocular lobe with dorsal and ventral surfaces nearly parallel through anterior 2/3; height at middle of lobe and through ocelli subequal. Medial process short ( |
Zelus puertoricensis ( |
| – | Dorsal surface sloping downward from ocelli, height at middle of lobe less than 0.9x that through ocelli. Medial process moderately long ( |
Zelus subimpressus ( |
|
Key to females of Zelus This key is not organized by species group, as females do not readily display characters placing them to species groups. It is especially challenging or sometimes impossible to distinguish females of species in several species groups. Large series of co-occurring males and females will be helpful in these cases. Coloration is heavily used to key out females, and readers are reminded that some species exhibit a wide range of color variations and the exemplar habitus images provided in this work are not inclusive of all variants. |
||
| 1 | Anterior pronotal lobe dorsally with short, dense, erect, spine-like setae on well-defined setal tracts. Humeral angle usually rounded, some armed. | 2 |
| – | Anterior pronotal lobe dorsally with fine setae or nearly bare, any spine-like setae sparse; setal tracts not necessarily well-defined. Humeral angle usually armed, some rounded. | 8 |
| 2 | Humeral angle armed with spinous process. Body elongate. | Zelus annulosus |
| – | Humeral angle rounded, without lateral process. | 3 |
| 3 | Dorsal surface of head, pronotum, scutellum and hemelytron nearly unicolorous, dark brown to black. Cells of membrane occasionally lighter in color than veins. | 4 |
| – | Dorsal surface with reddish-brown areas on pronotum and/or scutellum, often with yellowish-brown to reddish-brown areas on clavus and corium and/or basal 1/2 of membrane. | 5 |
| 4 | Veins and cells of membrane same color; cranial setae dark (northern S. A.). |
Zelus fuliginatus ( |
| – | Cells of membrane conspicuously less pigmented than veins; postocular lobe with longitudinal lateral patch of whitish recumbent setae (Bolivia, Peru, Paraguay, southern Brazil). | Zelus aithaleos |
| 5 | Profemoral length less than 20x profemoral diameter. Quadrate cell short and broad; Pcu of quadrate cell less than 1/2 length of Cu. |
Zelus means ( |
| – | Profemoral length at least 20x profemoral diameter. Quadrate cell elongate, if broad, then Pcu more than 1/2 length of Cu. | 6 |
| 6 | Body elongated. Cu and Pcu of quadrate cell subparallel, Cu-Pcu2 (posterior cross vein) less than 1/2x length of Cu. | 7 |
| – | Body not as elongated as that of Zelus errans or Z. gracilipes. Cu and Pcu of quadrate cell somewhat converging anteriorly, Cu-Pcu2 (posterior cross vein) more than 1/2x length of Cu. |
Zelus vespiformis ( |
| 7 | Entire membrane colored or opaque. |
Zelus gracilipes ( |
| – | Anterior 1/2 of membrane clear or semi-translucent. |
Zelus errans ( |
| 8 | Compound eyes extending below ventral surface of head. | Zelus grandoculus |
| – | Compound eyes not extending below ventral surface of head. | 9 |
| 9 | Posterior margin of pronotal disc with two tubercles. Humeral angle with spinous process. | 10 |
| – | Posterior margin of pronotal disc unarmed. Humeral angle armed or unarmed. | 12 |
| 10 | Tubercles of pronotal disc pronounced. More than 13 mm in length. | 11 |
| – | Tubercles very small, minute in some specimens. Thirteen mm or less in length. (Southern C.A. and Northern S.A.) | Zelus minutus |
| 11 | Large, 22-25 mm. Head, pronotum and abdomen yellow or reddish with dark spots or irregularly shaped patches (C.A.). |
Zelus lewisi ( |
| – | Medium-sized, 13-16 mm. Pronotum and abdomen more or less unicolorous, greyish (N.A., C.A., southern S.A.). |
Zelus tetracanthus ( |
| 12 | Posterior pronotal lobe, as viewed from behind, with posterior margin sloping sharply downward on either side of scutellum (northern S.A.). | Zelus varius |
| – | Posterior pronotal lobe, with posterior margin straight or sloping gradually (not appreciably more than forty-five degrees) downward on either side of scutellum. | 13 |
| 13 | Head surface reddish-brown, any darker cranial markings with indistinct outlines; remainder of dorsal surface of body primarily dark brown to brownish-black. 13.0-18.5 mm in length. | 14 |
| – | Coloration other than above. Length variable. | 17 |
| 14 | Membrane and clavus with bluish iridescence. |
Zelus erythrocephalus ( |
| – | Membrane shining, but not showing bluish iridescence. | 15 |
| 15 | Veins of corium anterior to membrane reddish-brown. Postocular lobe with dark semierect to erect setae dorsally. Scutellum bearing light recumbent setae dorsally. |
Zelus russulumus ( |
| – | At least one of the characters not as described above. | 16 |
| 16 | Postocular lobe with light-colored, inconspicuous setae dorsally (northern S.A.). |
Zelus paracephalus ( |
| – | Postocular lobe with conspicuous dark semi-erect to erect setae (southern C.A. and northern S.A.). |
Zelus panamensis ( |
| 17 | Length of postocular lobe less than 0.77x width of head through compound eyes. Postocular lobe with long erect setae over surface. Metafemoral length less than 20x metafemoral width (northern S.A.). |
Zelus chamaeleon ( |
| – | Length of postocular lobe at least 0.80x width of head through compound eyes; if long setae present on postocular lobe, then not over entire surface. | 18 |
| 18 | Anterior portion of posterior pronotal lobe broadly sulcate on dorsal surface; at least 18.5 mm in length. | 19 |
| – | Posterior pronotal lobe not conspicuously depressed medially; if slight depression present, not larger than 17.0 mm in length | 20 |
| 19 | General coloration brown; female profemur approximately 0.95x or more diameter of mesofemur (Mexico and C.A.). |
Zelus sulcicollis ( |
| – | Dorsal coloration reddish-brown and brownish-black; white waxy exudation usually conspicuous dorsally; profemur approximately 0.90x or less diameter of mesofemur (southern and central S.A.). |
Zelus leucogrammus ( |
| 20 | Humeral angle of pronotum rounded; dorsal surface, except dorsum of abdomen, reddish-brown and brownish-black, pattern variable; erect setae predominating on dorsum of pronotum; posterior portion of anterior pronotal lobe conspicuously raised above level of anterior margin of posterior lobe (N.A. C.A., S.A. and Caribbean). |
Zelus longipes ( |
| – | At least one of the characters not as described above. | 21 |
| 21 | Length at least 5x width. Profemoral diameter equal to or greater than that of mesofemur. Mesofemoral diameter less than 1.2x that of metafemur. Tuberculate to small spinous lateral processes on humeral angle. Interocular distance less than 1.15x interocellar distance. | 22 |
| – | At least one of the characters not as described above. | 23 |
| 22 | Postocular lobe with dorsal and ventral surfaces nearly parallel through anterior 2/3; height at middle of lobe and through ocelli subequal (Greater Antilles). |
Zelus puertoricensis ( |
| – | Dorsal surface sloping downward from ocelli; height at middle of lobe less than 0.9x that through ocelli (Greater Antilles). |
Zelus subimpressus ( |
| 23 | Length at least 5.5x width. | 24 |
| – | Length less than 5.5x width. | 26 |
| 24 | Length 12.5 mm or greater (Southern U.S., Mexico, C.A. and northwestern S.A.) |
Zelus cervicalis ( |
| – | Length less than 12.5 mm (9.11-12.00 mm). | 25 |
| 25 | Anteocular lobe at least 1.25x length of postocular lobe. Body length at least 7.2x width (Cuba). | Zelus bruneri |
| – | Anteocular lobe less than 1.25x length of postocular lobe. Body length less than 7.2x width (S.A.) |
Zelus prolixus ( |
| 26 | Humeral angle rounded. Less than 14 mm in length. | 27 |
| – | Humeral angle with at least small tubercles or spines; if tubercles and spines not readily evident, at least 14 mm in length. | 28 |
| 27 | Profemoral length at least 20x profemoral width (S.A. and southern C.A.). |
Zelus inconstans ( |
| – | Profemoral length less than 20x profemoral width (Brazil, Bolivia and Paraguay). |
Zelus mattogrossensis ( |
| 28 | Length usually less than 4.0x width; if length greater than 4.0x and less than 4.5x width, profemoral diameter greater than that of mesofemur. | 29 |
| – | Length usually at least 4.0x width; if length less than 4.0x width, mesofemoral diameter greatest. | 34 |
| 29 | Length usually at least 4.0x width; if length less than 4.0x width, mesofemur enlarged, diameter greatest. |
Zelus conjungens ( |
| – | Mesofemoral diameter less than that of profemur, if mesofemoral diameter greatest, then never exceeding 1.1x that of profemoral diameter. | 30 |
| 30 | Length of anteocular lobe at least 1.1x that of postocular lobe. Rostral segment II less than 1.35x length of segment I. Dorsal coloration uniform, pale brown, somewhat greenish. Legs not banded (Mexico, C.A. and S.A.). |
Zelus amblycephalus ( |
| – | Anteocular lobe less than 1.1x length of postocular lobe. Rostral segment II at least 1.35x length of segment I. Dorsal surface is either variously patterned, with contrasting pale and dark regions or uniformly colored, dark brown to black. Legs usually with bands or annulations, or uniformly dark brown to black. | 31 |
| 31 | Humeral angle raised to level of, and nearly continuous with, disc (Mexico and C.A.). |
Zelus janus ( |
| – | Humeral angle below level of disc; disc clearly elevated. | 32 |
| 32 | Setal tracts of anterior pronotal lobe and dorsum of posterior lobe with conspicuous light-colored shining recumbent setae, especially dorsolateral area of lobe; some erect setae also present; setal tracts usually of contrasting color. Length:width ratio approximately 4:1 (Mexico and C.A.). |
Zelus litigiosus ( |
| – | Setal tracts and dorsum of posterior pronotal lobe with few, if any, recumbent setae; tracts not usually of contrasting color. Length:width ratio usually less than 3.5:1. | 33 |
| 33 | Pubescence distributed evenly over lateral surface of abdominal segments (S.A.). |
Zelus armillatus ( |
| – | Pubescence, except for some scattered setae, restricted to anterior 1/2 of lateral surface of abdominal segments (Mexico, C.A., and northern S.A.). |
Zelus ruficeps ( |
| 34 | Dorsal surface dark brown to brownish-black. Golden, shining, short recumbent setae dorsally, especially on head and pronotum. Humeral angle raised to level of, and nearly continuous with, disc (central and southern S.A.). |
Zelus auralanus ( |
| – | Dorsal surface usually not dark brown, but if so, then setae not as above. If humeral angle raised, coloration not as dark above. | 35 |
| 35 | Humeral angle raised to level of, and nearly continuous with, disc. General dorsal coloration yellowish-brown. | 36 |
| – | Humeral angle below level of disc. If dorsal coloration yellowish-brown as above, then disc clearly differentiated. | 38 |
| 36 | Profemoral length at least 22x profemoral width. | Zelus xouthos |
| – | Profemoral length less than 22x profemoral width. | 37 |
| 37 | Medial sulcus of anterior pronotal lobe with dark brown areas near posterior margin (Mexico and C.A.). |
Zelus ambulans ( |
| – | Medial sulcus of anterior pronotal lobe same color as surrounding area of lobe near posterior margin (Mexico and C.A.). |
Zelus exsanguis ( |
| 38 | Length of rostral segment II less than 1.8x that of segment I. Profemoral length less than 22x profemoral width. Pronotum covered with erect setae dorsally, some setae subequal in length to diameter of shaft of antennal segment I. Humeral angle with very small tuberculate or subtuberculate lateral process (Mexico, C.A., and northern S.A.). |
Zelus grassans ( |
| – | At least one of the characters not as described above. | 39 |
| 39 | Length of rostral segment II less than 2x that of segment I. Profemoral and mesofemoral lengths less than 20x and 11x that of respective widths. Length of posterior pronotal lobe greater 2.4x that of anterior lobe. |
Zelus laticornis ( |
| – | At least one of the characters not as described above. | 40 |
| 40 | Profemoral length less than 17x that of profemoral width. Length of rostral segment II less than 2.1x that of segment I. | 41 |
| – | Profemoral length at least 17x of width. Rostral segment II usually greater than 2.1x that of segment I. | 43 |
| 41 | Humeral angle with short, inconspicuous subtuberculate to nearly dentate lateral processes, usually same color as surrounding area. Posterior pronotal lobe nearly smooth (Guatemala, Mexico and western and southwestern U.S.). |
Zelus renardii ( |
| – | Humeral angle with short to moderate, conical, spinous lateral processes, usually darker than surrounding area. Posterior pronotal lobe noticeably rugulose. | 42 |
| 42 | Dorsum of postocular lobe with long erect setae on posterior 1/2, some longer than ocular-ocellar distance (C.A.). |
Zelus antiguensis ( |
| – | Any erect setae on dorsum of postocular lobe shorter than ocular-ocellar distance (Canada, U.S. and Mexico). |
Zelus luridus ( |
| 43 | Length greater than 4.5x width. Humeral angle with very short inconspicuous, spinous lateral processes. Dorsum predominately dark brown to brownish-black except for light to dark reddish-brown to brown posterior pronotal lobe, but lighter than anterior lobe (Mexico and C.A.). |
Zelus mimus ( |
| – | At least one of the characters not as described above. | 44 |
| 44 | Posterior pronotal lobe with single broad dark brown transverse band posteriorly or pair of bands anteriorly and posteriorly, remaining dorsal surface of pronotum yellowish, with or without band. | 45 |
| – | Coloration of pronotum not as described above, brown or stramineous coloration predominating dorsally, anterior pronotal lobe same color as or lighter than posterior lobe. | 49 |
| 45 | Anterior pronotal lobe and anterior part of posterior lobe with dark bands; wasp-like appearance. |
Zelus nigromaculatus ( |
| – | Anterior pronotal lobe and anterior portion of posterior pronotal lobe of same yellowish color. | 46 |
| 46 | Transverse dark band on pronotum does not cover posterior margin of posterior pronotal lobe, which is instead yellowish. | 47 |
| – | Transverse dark band on pronotum covers posterior margin of posterior pronotal lobe. | 48 |
| 47 | Femoral yellowish-brown with only small darker markings near apices (central and northern S.A.) |
Zelus kartabensis ( |
| – | Femora yellowish-brown on basal 1/3 to 1/2; remainder dark brown to brownish-black, usually with one or two narrow yellowish bands (S.A.). |
Zelus plagiatus ( |
| 48 | Pubescence of lateral surface of posterior pronotal lobe consisting almost entirely of erect setae, many longer than diameter of shaft of antennal segment II (S.A.). |
Zelus versicolor ( |
| – | Majority of setae on lateral surface of posterior pronotal lobe semi-erect to recumbent, no erect setae as long as diameter of shaft of antennal segment II (southern C.A. and northern S.A.). |
Zelus fasciatus ( |
| 49 | Length >14 mm. Anterior pronotal lobe lighter than or same color as posterior lobe. | 50 |
| – | Length <13 mm. Anterior and posterior pronotal lobes same color. |
Zelus illotus ( |
| 50 | Anterior pronotal lobe yellowish, lighter than posterior lobe. | 51 |
| – | Anterior pronotal lobe brown, same color as posterior lobe. | 52 |
| 51 | Femora unicolorous or with indistinct single bands. Anterior pronotal lobe elevated. |
Zelus sphegeus ( |
| – | Meso- and Metafemora with two or three dark bands. Anterior pronotal lobe nearly flat, not elevated. |
Zelus truxali ( |
| 52 | Meso- and metafemora apical half more or less continuously reddish-brown, not broken into bands. Abdominal sternites each bearing a small black spot. |
Zelus cordazulus ( |
| – | Meso- and metafemora apical half with three indistinct but visible reddish-brown bands; abdominal sternites yellow, without black spots. |
Zelus gilboventris ( |
Acknowledgements
Financial support is provided primarily by the NSF grant “Partnership in Enhancing Expertise in Taxonomy” (PEET) #0933853 awarded to C. Weirauch. Additional financial support comes from the Robert van den Bosch Scholarship (Center for Biological Control, University of California, Berkeley) and the Dissertation Year Fellowship (University of California, Riverside) awarded to G. Zhang. The following individuals assisted with imaging, databasing or measurements: Tracy Castaneda, Nick Duncan, Isaac Esquivel, Rochelle Hoey-Chamberlain, Erika Olson, Elizabeth Romero, Kaleigh Russell, Laura Soto, Amanda Villareal and Haris Yu. Jean-Michel Bérenger kindly provided the habitus images of Z. couturieri. The editorial and technical staff (Lyubomir Penev and Teodor Georgiev) of Pensoft provided critical technical support.
References
-
Feeding behavior of an assassin bug, Zelus renardii.Annals of the Entomological Society of America71:476‑478. DOI: 10.1093/aesa/71.4.476
-
Effect of temperature on development and survival of Zelus renardii.Environmental Entomology7:889‑890. DOI: 10.1093/ee/7.6.889
-
Histoire naturelle des insectes. Hemipteres.Fain et Thunot,Paris,lxxi + 681pp. DOI: 10.5962/bhl.title.8471
-
A new synonymy in the genus Zelus (Heteroptera, Reduviidae, Harpactorinae).Boletín de la Sociedad Entomológica Aragonesa (S.E.A.)47:362. URL: www.sea-entomologia.org/Publicaciones/PDF/BOLN_47/362BSEA47COMPLETO-40.pdf
-
Iquitozelus couturieri, nouveaux genre et nouvelle espèce d’Harpactorinae du Pérou (Heteroptera, Reduviidae).Nouvelle Revue d’Entomologie (N. S.)20:23‑27. [InFrench]. URL: www.researchgate.net/publication/264862392_Iquitozelus_couturieri_nouveau_genre_et_nouvelle_espece_d%27Harpactorinae_du_Perou_Heteroptera_Reduviidae
-
Hemiptera Argentina Enumeravit Speciesque Novas Descripsit.Pauli E. Coni,Buenos Aires,316pp. DOI: 10.5962/bhl.title.36493
-
Darwin Core Terms: A quick reference guide. http://rs.tdwg.org/dwc/terms/
-
Histoire naturelle des Insectes. Orthopteres, Neuropteres, Hemipteres, Hymenopteres, kpidopteres et Dipteres.P. Duménil,Paris,672pp. DOI: 10.5962/bhl.title.59226
-
Heteroptera or True Bugs of Eastern North America.The Nature Publishing Company,Indianapolis,1116pp. DOI: 10.5962/bhl.title.6871
-
Histoire naturelle des Insects (Coleopteres, Orthopteres et Hemipteres), traitant de leur organization et de leurs moeurs en général.FD Pillot,Paris,415pp.
-
Handbuch der Entomologie. Zweiter Band: II Ordnung Rhynchota.Theod. Ehr. Friedr. Enslin,Berlin,400pp.
-
Insecta. Rhynchota. Hemiptera-Heteroptera. Biologia Centrali Americana. Vol. 2.Taylor and Francis,London,466pp. DOI: 10.5962/bhl.title.730
-
Changes in the foraging behavior, within-plant vertical distribution, and microhabitat selection of a generalist insect predator: an age analysis.Environmental Entomology27(4):949‑957. DOI: 10.1093/ee/27.4.949
-
Influence of prey size on predation success by Zelus longipes L. (Het., Reduviidae).Journal of Applied Entomology126:74‑78. DOI: 10.1046/j.1439-0418.2002.00593.x
-
Relative prey weight influences handling time and biomass extraction in Sinea confusa and Zelus renardii (Heteroptera: Reduviidae).Environmental Entomology26:559‑565. DOI: 10.1093/ee/26.3.559
-
Presencia de Zelus renardii Kolenati (Heteroptera: Reduviidae) en Chile.Boletin de la S.E.A.34:163‑165.
-
A general system for coding taxonomic descriptions.Taxon29:41‑46. DOI: 10.2307/1219595
-
DEscription Language for TAxonomy (DELTA). Release date:1999 1 01. URL: http://delta-intkey.com/
-
Contributions to morphology and phylogeny of Reduvioidea (Hemiptera - Heteroptera). Part III. The male and female genitalia.Annals of the Entomological Society of America59:911‑924. DOI: 10.1093/aesa/59.5.911
-
. Entomological Magazine147:157‑162.
-
Review of the genus Namadytes Hesse, 1969 (Insecta: Diptera: Mydidae: Syllegomydinae).Biodiversity Data Journal2:e1071. DOI: 10.3897/bdj.2.e1071
-
Zelus cervicalis Stål (Hemiptera: Reduviidae: Harpactorinae), aporte neártico a la entomofauna introducida de Chile.Gayana (Concepción)68(1):133‑136.
-
A new American Harpactorine genus.Texas Reports on Biology and Medicine12:39‑48.
-
Systema entomologiae.Flensburgi et Lipsiae,Korte,832pp. DOI: 10.5962/bhl.title.36510
-
Systema Rhyngotorum Secundum Ordines, Genera, Species, Adjectis Synonymis, Locis Observationibus, Descriptionibus.Apud Carolum Reichard,Brunsvigae,314pp. DOI: 10.5962/bhl.title.11644
-
. Entomologica Americana118(1):278‑284. DOI: 10.1664/12-ra-021.1
-
Comparative genitalic morphology in the New World resin bugs Apiomerini (Hemiptera, Heteroptera, Reduviidae, Harpactorinae).Deutsche Entomologische Zeitschrift59:5‑41. DOI: 10.1002/mmnd.201200001
-
A systematic outline of the Reduviidae of North America.Proceedings of the Iowa Academy of Sciences19:217‑252. URL: books.google.com/books?id=RegoAAAAYAAJ&pg=PA215&lpg=PA215&dq=A+systematic+outline+of+the+Reduviidae+of+North+America&source=bl&ots=wefNIJywMn&sig=gZ2ipSw0yl0O-w1lauAhuIXuWgw&hl=en&sa=X&ved=0ahUKEwjciYSD86DNAhUJ9GMKHRI0AAgQ6AEIKjAC#v=onepage&q=A%20systematic%20outline%20of%20the%20Reduviidae%20of%20North%20America&f=false
-
Association between Zelus versicolor (Herrich-Schäffer) (Hemiptera, Reduviidae, Harpactorinae) and Bidens rubifolia Kunth (Asterales, Asteraceae).EntomoBrasilis. 2011;4(1)30-324(1):30‑32. URL: http://www.periodico.ebras.bio.br/ojs/index.php/ebras/article/view/95
-
Aristathlus imperatorius Bergroth, a newly recognized synonym of Reduvius iopterus Perty, with the new combination Aristathlus iopterus (Perty, 1834) (Hemiptera: Reduviidae: Harpactorinae).Biodiversity Data Journal3:e5152. DOI: 10.3897/bdj.3.e5152
-
New records, and nomenclatural and biological notes on Reduviidae (Hemiptera: Heteroptera) from Bolivia and Brazil.Zootaxa1785:43‑53. URL: www.researchgate.net/publication/288272090_New_records_and_nomenclatural_and_biological_notes_on_Reduviidae_Hemiptera_Heteroptera_from_Bolivia_and_Brazil
-
A Systematic Revision of the Genus Zelus Fabricius (Hemiptera: Reduviidae). Ph.D dissertation.Texas A&M University,College Station,595pp.
-
Genus Zelus Fabricius in the United States, Canada, and Northern Mexico (Hemiptera: Reduviidae).Annals of the Entomological Society of America79(3):535‑548. DOI: 10.1093/aesa/79.3.535
-
The Genus Zelus Fabricius in the West Indies (Hemiptera: Reduviidae).Annals of the Entomological Society of America80(2):293‑305. DOI: 10.1093/aesa/80.2.293
-
The Reduviidae of Kartabo, Bartica. District, British Guiana.Zoologica7:129-154.
-
Die Wanzenartigen Insekten. Vol. 8.Lotzbeck,Nürnberg,130pp. DOI: 10.5962/bhl.title.11547
-
Die Wanzenartigen Insekten. Vol. 9.Lotzbeck,Nürnberg,210pp. DOI: 10.5962/bhl.title.11547
-
Description morphologique et approche genetique d’un nouveau Reduviidae (Harpactorinae): Zelus josephpaulusi, n. sp.Lambillionea102(3):303‑328. [InFrench].
-
Bibliographical and nomenclatorial notes on the Rhynchota. No 1.The Entomologist33:238‑243. DOI: 10.5962/bhl.part.3888
-
On the nomenclature of the genera of the Rhynchota, Heteroptera and Auchenorrynchous Homoptera.The Entomologist33:25‑28.
-
On the nomenclature of the genera of the Rhynchota, Heteroptera and Auchenorrpchous Homoptera (cont.).The Entomologist34:176‑179.
-
Hemiptera. Fauna Hawaiiensis. Vol. 3. Pt. 2.Cambridge University Press,London,93-174pp.
-
On the nomenclature of the genera of the Rhynchota, Heteroptera and Auchenorrynchous Homoptera (cont.).The Entomologist36:213‑216.
-
Electronic publications need registration in ZooBank to be available.Bulletin of Zoological Nomenclature72(3):245‑251. URL: http://iczn.org/node/40562
-
Essai d'une classification systematique de l'ordre des Hemipteres (Hemipteres-Heteropteres, Latr.).Magazin de Zoologie (Guérin)1:1‑88. DOI: 10.5962/bhl.title.15830
-
Sticky Substance on Eggs Improves Predation Success and Substrate Adhesion in Newly Hatched <I>Zelus renardii</I> (Hemiptera: Reduviidae) Instars.Annals of the Entomological Society of America103(5):771‑774. DOI: 10.1603/an09143
-
Hemiptera Heteroptera. In: Olivier AG (Ed.)Dictionnaire des Insectes.10.Encyclopédie Méthodique,Paris,270-273pp.
-
Catalogue General des Hemipteres.Tome 3.R. Friedlander und Fils,Berlin,275pp. DOI: 10.5962/bhl.title.15830
-
Systema naturæ, Tom. I. Pars II. Editio duodecima reformata.Holmiae: Impensis Direct. Laurentii Salvii,Stockholm,533–1327pp. URL: bibdigital.rjb.csic.es/ing/Libro.php?Libro=1682
-
Systematic Catalogue of the Reduviidae of the World. Caribbean Journal of Science, Special publication No. 1.University of Puerto Rico, Mayagüez,Puerto Rico,694pp.
-
Incidence of arthropod predators in different soybean cropping systems.Environmental Entomology11(3):685‑689. DOI: 10.1093/ee/11.3.685
-
Delectus animalium articulatorum.Autor,Munich,125-224pp. DOI: 10.5962/bhl.title.102991
-
First record of the Nearctic Zelus renardii (Heteroptera, Reduviidae, Harpactocorinae) in Europe.Entomologia Hellenica20:75‑81. URL: www.entsoc.gr/journaleee/first-record-of-the-nearctic-zelus-renardii-heteroptera-reduviidae-harpactocorinae-in-europe/
-
Description de plusierurs Hemipteres nouveaus.le Nat. Canadien4:350‑352. URL: www.biodiversitylibrary.org/part/15003#/summary
-
Studies on the biology of the Reduviidae of America north of Mexico.University of Kansas Science Bulletin17:5‑291.
-
An Assassin among Predators: The Relationship between Plant-Ants, Their Host Myrmecophytes and the Reduviidae Zelus annulosus.PLoS ONE5(10):e13110. DOI: 10.1371/journal.pone.0013110
-
Integrating specimen databases and revisionary systematics.ZooKeys209:255‑267. DOI: 10.3897/zookeys.209.3288
-
Description d’Hemipteres nouveaux de Jurimagnas et Moyabamba (Perou).Annales de la Société Entomologique de France4:579‑588.
-
Nya Hemiptera.Öfversigt af Kongliga Vetenskaps-Akademiens Förhandlingar12:181‑192.
-
Till kaennedomen om Reduvini.Öfversigt af Kongliga Vetenskaps-Akademiens Förhandlingar16:175‑204.
-
Bidrag till Rio Janeiro-traktens Hemipter-fauna.Kongliga Svenska Vetenskaps-Akademiens Handlingar2(7):1‑84.
-
Miscellanea hemipterologica.Stettiner Entomologische Zeitung22:129‑153.
-
Hemiptera mexicana enumeravit speciesque novas descripsit (continuation).Stettiner Entomologische Zeitung23:437‑462.
-
Bidrag till Reduviidernas kaennedom.Öfversigt af Kongliga Vetenskaps-Akademiens Förhandlingar23:235‑302.
-
Enumeratio Hemipterorum. Bidrag till en förteckning öfver alla hittils kånda Hemiptera, jemte systematiska meddelanden 2.Kongliga Svenska Vetenskaps-Akademiens Handlingar10(4):1‑159.
-
Natuurlyke en naar't leeven naauwkeurig gekleurde Afbeeldingen en Beschryvingen der Cicaden en Wantzen in alle vier Waerelds deelen Europa, Asia, Africa en America huishoudende, by een verzameld en beschreeven.2.Jan Christiaan Sepp,Amsterdam,172pp.
-
A list of Hemiptera collected in eastern Colorado and northeastern New Mexico, by C. Thomas, during the expedition of 1869. In: Hayden FV (Ed.)Preliminary Report of the U.S. Geological Survey of Wyoming and Portions of Contiguous Territories.Government Printing Office,Washington,471-472pp. URL: www.biodiversitylibrary.org/item/124638#page/477/mode/1up
-
Notices of the Hemiptera of the western territories of the United States, chiefly from the surveys of Dr. F. V. Hayden. In: Hayden FV (Ed.)Preliminary Report of U.S. Geological Survey of Montana and Portions of Adjacent Territories.Government Printing Office,Washington,392-423pp. URL: archive.org/details/preliminaryrepor00geol
-
Check-list of the Hemiptera Heteroptera of North America.Brooklyn Entomological Society,Brooklyn,i + 32pp. DOI: 10.5962/bhl.title.33049
-
Ein aktueller Nachweis von Zelus renardii (Kolenati, 1856) auf Kreta/Griechenland (Hemiptera: Heteroptera: Reduviidae: Harpactorinae).BV News Publicaciones Científicas4(52):55‑59. [InGerman]. URL: www.biodiversidadvirtual.org/taxofoto/sites/default/files/ein_aktueller_nachweis_von_zelus_renardii_kolenati_1856_auf_kreta_griechenland.pdf
-
First record of Zelus armillatus (Lepeletier & Serville, 1825) in Costa Rica, with notes on taxonomic problems regarding the genus Zelus Fabricius, 1803 (Hemiptera: Heteroptera: Reduviidae: Harpactorinae: Harpactorini).Arquivos Entomoloxicos12:85‑90. URL: www.researchgate.net/publication/264978058_First_record_of_Zelus_armillatus_Lepeletier_Serville_1825_in_Costa_Rica_with_notes_on_taxonomic_problems_regarding_the_genus_Zelus_Fabricius_1803_Hemiptera_Heteroptera_Reduviidae_Harpactorinae_Harpact
-
Check List of the Hemiptera (Excepting the Aphididae, Aleurodidae, and Coccidae) of America north of Mexico.New York Entomological Society,New York,xi + 111pp. URL: www.biodiversitylibrary.org/item/33088#page/9/mode/1up
-
Catalogue of the Hemiptera of America North of Mexico.University of California,Berkeley,xiv + 902pp. DOI: 10.5962/bhl.title.29381
-
Preliminary observations on Zelus obscuridorsis (Stål) (Hemiptera: Reduviidae) as predator of the corn leafhopper (Hemiptera: Cicadellidae) in Argentina.Insects6(2):508‑513. DOI: 10.3390/insects6020508
-
Primera cita en España de la especie Zelus renardii (Kolenati, 1857) (Heteroptera: Reduviidae) que representa la segunda cita en Europa.BV News Publicaciones Científicas1(6):34‑40. [InSpanish]. URL: www.biodiversidadvirtual.org/taxofoto/sites/default/files/primera_cita_en_espana_de_la_especie_zelus_renardii_kolenati_1857_heteroptera_reduviidae_que_representa_la_segunda_cita_en_europa.pdf
-
Cladistic analysis of Reduviidae (Heteroptera: Cimicomorpha) based on morphological characters.Systematic Entomology33(2):229‑274. DOI: 10.1111/j.1365-3113.2007.00417.x
-
From four- to three-segmented labium in Reduviidae (Hemiptera: Heteroptera).Acta Entomologica Musei Nationalis Pragae48:331‑344. URL: www.aemnp.eu/PDF/48_2/48_2_331.pdf
-
Zelus renardii and Z. tetracanthus (Hemiptera: Reduviidae): Biological attributes and the potential for dispersal in two assassin bug species.Florida Entomologist95(3):641‑649. DOI: 10.1653/024.095.0315
-
The phylogenetic species concept (Sensu Wheeler and Platnick). In: Wheeler Q, Meier R (Eds)Species concepts and phylogenetic theory.Columbia University Press,New York,55-69pp. URL: www.researchgate.net/publication/247609844_Species_Concepts_and_Phylogenetic_Theory_A_Debate
-
Elenco sistemático de los Reduviiformes americanos.Instituto de Medicina Regional de la Universidad Nacional de Tucumán, Monografia1:1‑102.
-
Nueva especie de Reduvido Cubano.Memorias de la Sociedad Cubana de Historia Natural24:125‑126.
-
Sticky predators: a comparative study of sticky glands in harpactorine assassin bugs (Insecta: Hemiptera: Reduviidae).Acta Zoologica94(1):1‑10. DOI: 10.1111/j.1463-6395.2011.00522.x
-
Molecular phylogeny of Harpactorini (Insecta: Reduviidae): correlation of novel predation strategy with accelerated evolution of predatory leg morphology.Cladistics30(4):339‑351. DOI: 10.1111/cla.12049
-
Insects of Hawaii. Vol. 3, Heteroptera.University of Hawaii Press,265pp.
Supplementary materials
This file contains specimen records of species of Zelus, including type and non-type materials examined by Zhang et al. (Biodiversity Data Journal) or recorded from literature.
Download file (3.49 MB)
Measurements of species of Zelus Fabricius, 1803.
Download file (2.07 MB)