|
Biodiversity Data Journal :
Single Taxon Treatment
|
|
Corresponding author: Salvatore S Anzaldo (sanzaldo@asu.edu)
Academic editor: Jennifer C. Girón Duque
Received: 18 Apr 2022 | Accepted: 30 Aug 2022 | Published: 30 Sep 2022
© 2022 Salvatore Anzaldo, Valentina Díaz-Grisales
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Anzaldo SS, Díaz-Grisales V (2022) Heilipus squamosus (LeConte, 1824): clarification of the correct name for the “avocado tree girdler” with updates on its biology and distribution (Coleoptera, Curculionidae, Molytinae, Hylobiini). Biodiversity Data Journal 10: e85499. https://doi.org/10.3897/BDJ.10.e85499
|
|
Abstract
Background
A single species of the genus Heilipus Germar, 1824 is native to the south-eastern United States and was a pest of avocado in Florida in the mid-20th century. Two names—Heilipus apiatus (Olivier, 1807) and H. squamosus (LeConte, 1824)—have both recently been used as the valid name for this species, with H. apiatus also being recently used as the valid name for a species from French Guiana. Confusion surrounding the correct application of these names and the distribution of these species traces back to an erroneous distribution given in Olivier's 1807 description of H. apiatus and, although some authors clarified this previously, there continues to be confusion concerning the correct name. Outside of avocado-producing areas, this species was rarely collected and little was known about its biology. Recent observations on iNaturalist and BugGuide suggest the species is more widely distributed in the United States and less rare than it was previously thought to be.
New information
Heilipus squamosus (LeConte, 1824) is recognised as the valid name for the sole species of Heilipus occurring in the United States, while Heilipus apiatus (Olivier, 1807) is a very different species from French Guiana. Heilipus squamosus was previously recorded from eight States in the south-eastern United States and, after examining records from natural history collections, iNaturalist, BugGuide and literature sources, the species is newly recorded from an additional seven States: Arkansas, Kansas, Kentucky, Louisiana, Missouri, Oklahoma and Texas. Though native host plants have been unconfirmed by rearing records, the evidence indicating the possible host plants in the plant family Lauraceae is reviewed.
Keywords
weevil, Lauraceae, host associations, new state record, Persea
Introduction
The genus Heilipus Germar, 1824 (
On three occasions, the confusion surrounding the distribution and valid names for these species appeared to have been resolved, but was overlooked by subsequent authors. First,
Since the latest clarification by
Materials and methods
One hundred and thirty-five occurrence records of H. squamosus were analysed (Suppl. material
- ABS – Archbold Biological Station Arthropod Collection, Lake Placid, FL
- ASUCOB – Arizona State University Charles W. O’Brien Collection, Tempe, AZ
- AUMNH – Auburn University Museum of Natural History, Auburn, AL
The remaining 47 records of preserved specimens were not previously available online; information for these records is available in Suppl. material
- CMNC – Canadian Museum of Nature Collection, Ottawa, Canada
- FSCA – Florida State Collection of Arthropods, Gainesville, FL
- LSAM – Louisiana State Arthropod Museum, Baton Rouge, LA
- MEM – Mississippi Entomological Museum, Mississippi State, MS
- NCSU – North Carolina State University Insect Collection, Raleigh, NC
- UGCA – University of Georgia Collection of Arthropods, Athens, GA
- VMNH – Virginia Museum of Natural History, Martinsville, VA
In addition to specimens from natural history collections, 43 observations posted on the websites BugGuide (
Literature sources citing H. squamosus occurrences from additional localities (
Botanical names used are the accepted name from
Taxon treatment
Heilipus squamosus
Nomenclature
Original combination: Pissodes squamosus LeConte, 1824: 161
Description: https://www.biodiversitylibrary.org/page/15913340
Holotype: https://mczbase.mcz.harvard.edu/guid/MCZ:Ent:5176
Synonyms:
Heilipus squamosus Boheman, 1836: 171 (not LeConte, 1824).
Description: https://www.biodiversitylibrary.org/page/4109434
Distribution
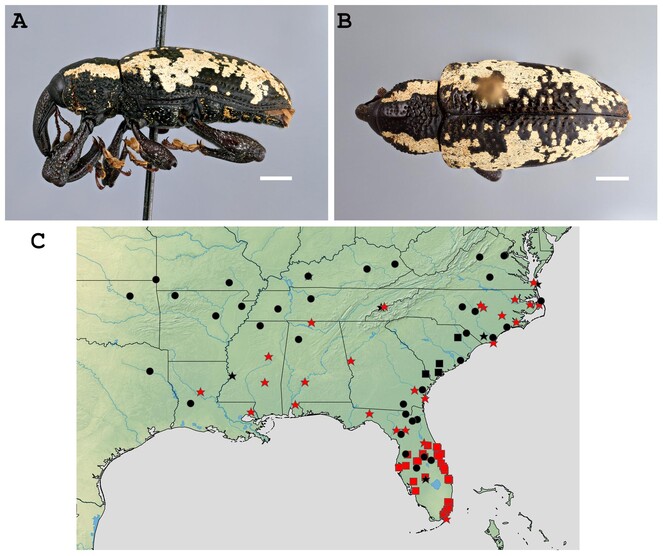
The previously-published state distribution of Heilipus squamosus is in the south-eastern United States—Georgia (
In the past 20 years, citizen science initiatives (e.g. BugGuide, iNaturalist) have yielded observations of H. squamosus from all States in its previously reported range plus seven additional States, expanding the distribution of this species to the northern and western regions of the United States:
Alabama, Arkansas (new State record), Florida, Georgia, Kansas (new State record), Kentucky (new State record), Louisiana (new State record), Missouri (new State record), Mississippi, North Carolina, Oklahoma (new State record), South Carolina, Tennessee, Texas (new State record) and Virginia (Fig.
Biology
The biology of H. squamosus is still incompletely known. Before it became a pest of non-native avocado in Florida, the published natural history information was limited to a record from “under pine bark” (
The native hosts for the weevil have been suspected to be other members of the Lauraceae, namely Lindera melissifolia (Walter) Blume (pondberry), Litsea aestivalis (L.) Fernald (pondspice), Persea borbonia (L.) Spreng. (redbay) and Sassafras albidum (Nutt.) Nees (sassafras), though no definitive rearing records are known. Of these species, sassafras is widely distributed throughout the eastern United States; the other three are restricted to coastal plains and swampy areas, with pondberry and pondspice being listed as a federally endangered and threatened species, respectively.
Adult weevils have been found on pondberry in North Carolina (
Additional records:
Discussion
Heilipus squamosus was previously known from eight States in the south-eastern United States and is herein reported from an additional seven States, expanding the distribution in the country to the west and north. The reason for the poorly-documented distribution is likely related to the weevil’s rarity, as well as a possible recent range expansion. The species has been historically reported to be rare and this is supported by only 57 adult specimens of this large and distinctive species being located in natural history collections. Removing records from Florida, where H. squamosus is known to be a pest on avocado, 33 specimens were found from only nine of the other 14 States where the species is now recorded. The rarity of the species can possibly be attributed to the rarity of the potential host plants: pondberry and pondspice. Pondberry is thought to have been uncommon even before recent habitat destruction and extant populations are known from only a few counties in each State in its range (
Another explanation for the poorly-documented distribution is that the range of H. squamosus has recently expanded. In the 21st century, citizen science initiatives like BugGuide and iNaturalist have provided an easy way for users to contribute observation data. Forty-three additional records of H. squamosus came from these sources from all 15 States from where the weevil is currently recognised, including the only known observations from five of the seven newly-recorded States (Fig.
Native Lauraceae, including the possible host plants discussed here, are threatened by the spread of laurel wilt disease (
Conclusion
- Heilipus squamosus (LeConte, 1824) is the valid name for the single species of Heilipus occurring in the United States. Much of the prior literature and current internet resources refer to this species as H. apiatus (Olivier, 1807), which is an incorrect name for this species.
- Heilipus squamosus was previously recorded from Alabama, Florida, Mississippi, North Carolina, South Carolina, Tennessee and Virginia and is newly recorded from Arkansas, Kansas, Kentucky, Louisiana, Missouri, Oklahoma and Texas.
- Possible host plants of H. squamosus include Lindera melissifolia (Walter) Blume (pondberry), Litsea aestivalis (L.) Fernald (pondspice), Persea borbonia (L.) Spreng. (redbay) and Sassafras albidum (Nutt.) Nees (sassafras), which are all species of Lauraceae, although larval associations with these plants are currently limited.
- Heilipus apiatus (Olivier, 1807) is a valid name that applies to a species known only from French Guiana.
Acknowledgements
This research was made possible, in part, by a PPA 7721-funded Cooperative Agreement (AP21PPQS&T00C035) from the United States Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS). It may not necessarily express APHIS’ views. We would like to thank the following collections personnel for looking for specimens in their collection and providing records if specimens were present: Robert Anderson (CMNC), Thomas Atkinson (University of Texas), Victoria Bayless (LSAM), Bob Blinn (NCSU), Janet Braun (University of Oklahoma), Melissa Callahan (AUMNH), Eric Chapman (University of Kentucky), Art Evans (VMNH), Rick Hoebeke (UGCA), Terence Schiefer (MEM), Kyle Schnepp (FSCA) and Kristin Simpson (University of Missouri). Additionally, thank you to iNaturalist users Thomas Shahan and John Abrams for providing more information on their observations in Oklahoma and Kentucky, respectively and Antoine Mantilleri, Hélène Perrin and Maxime Le Cesne for locating and providing images of H. apiatus syntypes at the MNHN.
References
- Molytinae Schoenherr 1823. In:American beetles - Polyphaga: Scarabaeoidea through Curculionoidea.Volume 2.CRC Press,Boca Raton,786-792pp.
- Note on the avocado weevil (Heilipus lauri Boheman).Proceedings of the Entomological Society of Washington14:181‑183. URL: https://www.biodiversitylibrary.org/item/20238#page/217/mode/1up
- Entomologisches aus dem Indianergebiet der Pampa.Stettiner Entomologische Zeitung42(1-3):36‑72. URL: https://www.biodiversitylibrary.org/item/35945#page/42/mode/1up
- An assessment of the potential impact of laurel wilt on clonal populations of Lindera melissifolia (Pondberry).Southeastern Naturalist17(4):616‑628. https://doi.org/10.1656/058.017.0409
- Rhynchophora or weevils of North Eastern America.The Nature Publishing Company, Indianapolishttps://doi.org/10.5962/bhl.title.1557
- Distribution and spread of laurel wilt disease in Georgia: 2006-08 Survey and Field Observations.Georgia Forestry Commission.
- Weevils of South Carolina (Coleoptera: Nemonychidae, Attelabidae, Brentidae, Ithyceridae, and Curculionidae).Biota of South Carolina. Vol. 6.Clemson University Public Service Publishing,Clemson, South Carolina,276pp. [ISBN9780979877742]
- The endangered pondberry (Lindera melissifolia [Walter] Blume, Lauraceae).Natural Areas Journal33(4):455‑465. https://doi.org/10.3375/043.033.0409
- Nuevos registros de especies y un hospedero vegetal del género Heilipus Germar (Curculionidae: Molytinae: Hylobiini) para Colombia.Acta Zoológica Mexicana (nueva serie)37:1‑12. https://doi.org/10.21829/azm.2021.3712334
- Ecdysis. A portal for live-data arthropod collections. https://serv.biokic.asu.edu/ecdysis/index.php. Accessed on: 2022-1-05.
- Susceptibility to laurel wilt and disease incidence in two rare plant species, pondberry and pondspice.Plant Disease95(9):1056‑1062. https://doi.org/10.1094/PDIS-11-10-0841
- GBIF Occurrence Dataset. Release date:2022-8-24. URL: https://doi.org/10.15468/e3gyvh
- GBIF Occurrence Download. Release date:2022-8-22. URL: https://doi.org/10.15468/dl.dsx827
- Catalogus Coleopterorum hucusque descriptorum synonymicus et systematicus.Vol. 8. Curculionidae.E. H. Gummi (G. Beck),Monachii,2181–2668pp. URL: https://www.biodiversitylibrary.org/item/37367#page/9/mode/1up
- Insectorum species novae aut minus cognitae, descriptionibus illustratae.Vol. 1. Coleoptera.J.C. Hendelii et filii,Halae,xxiv + 624pp. https://doi.org/10.5962/bhl.title.130964
- Introduction aux Charançons de Guyane. In:Insectes de Guyane: Beauté et Diversité.SEPANGUY, Collection Nature Guayanaise,Cayenne,Pp. 95–104pp.
- Potential effects of laurel wilt on the flora of North America.Southeastern Naturalist9(4):827‑836. https://doi.org/10.1656/058.009.0417
- Heilipus apiatus, a striking large weevil new to the Virginia fauna (Coleoptera: Curculionidae).Banisteria22:58‑59. URL: https://www.biodiversitylibrary.org/page/57144986#page/60/mode/1up
- https://www.inaturalist.org. Accessed on: 2021-8-20.
- New Coleopterous insects of North America.Annals of the Lyceum of Natural History of New York1:169‑173 + pl. XI. URL: https://www.biodiversitylibrary.org/item/54048#page/179/mode/1up
- The Rhynchophora of America North of Mexico.Proceedings of the American Philosophical Society15(96):i-xvi + 1‑455. URL: https://www.biodiversitylibrary.org/item/85593#page/8/mode/1up
- Annotated checklist of the weevils (Curculionidae sensu lato) of North America, Central America, and the West Indies (Coleoptera: Curculionidae).Memoirs of the American Entomological Institute34:1‑382.
- Annotated checklist of the weevils (Curculionidae sensu lato) of North America, Central America, and the West Indies - Supplement I.Southwestern Entomologist9(3):286‑307.
- Entomologie, ou Histoire Naturelle des Insectes, avec leurs caractères génériques et spécifiques, leur description, leur synonymie, et leur figure enluminée. Coléoptères.Vol. 5.Desray,Paris,612pp. URL: https://www.biodiversitylibrary.org/item/125992#page/11/mode/1up
- Status of Sassafras albidum (Nutt.) Nees in the presence of laurel wilt disease and throughout the eastern United States.Southeastern Naturalist16(1):37‑58. https://doi.org/10.1656/058.016.0104
- Les Hylobiini de Guyane (Coleoptera, Curculionidae). In:Contribution à l‘étude des coléoptères de Guyane. Tome I. – Supplément au Bulletin de liaison d‘ACOREP-France "Le Coléoptériste".59-72pp.
- Neue Arten der Tribus Hylobiini und Cryptorhynchini aus Französisch Guayana.Koleopterologische Rundschau87:297‑324.
- The Symbiota Collections of Arthropods Network (SCAN) serves specimen occurrence records and images from North American arthropod collections. https://scan-bugs.org/. Accessed on: 2022-1-05.
- Genera et species curculionidum, cum synonymia hujus familiae. Species novae aut hactenus minus cognitae, descriptionibus a Dom. Leonardo Gyllenhal, C.H. Boheman, et entomologis aliis illustratae.Vol. 3 (1).Roret,Paris,1-505pp. URL: https://www.biodiversitylibrary.org/item/24767#page/5/mode/1up
- Genera et species curculionidum, cum synonymia hujus familiae. Species novae aut hactenus minus cognitae, descriptionibus a Dom. Leonardo Gyllenhal, C.H. Boheman, et entomologis aliis illustratae.Vol. 7 (2).Roret,Paris,1–461pp. URL: https://www.biodiversitylibrary.org/item/24771#page/499/mode/1up
- SimpleMappr, an online tool to produce publication-quality point maps.https://www.simplemappr.net. Accessed on: 2021-11-30.
- Missouri Botanical Garden. http://www.tropicos.org. Accessed on: 2021-11-30.
- Recovery plan for pondberry (Lindera melissifolia).U.S. Fish and Wildlife Service,Atlanta, Georgia,56pp.
- BugGuide.Net: Identification, images, & information for insects, spiders & their kin for the United States & Canada. Iowa State University.https://bugguide.net/. Accessed on: 2021-11-30.
- Annotated checklist of the weevils (Curculionidae sensu lato) of South America (Coleoptera: Curculionoidea).Memoirs of the American Entomological Institute39:i–xvi + 1‑563.
- Avocado production in Florida.Cooperative Extension Work in Agriculture and Home Economics, Gainesville, Florida.Bulletin 112, 111 pp.
- Avocado production in Florida.Cooperative Extension Work in Agriculture and Home Economics, Gainesville, Florida.Bulletin 141, 110 pp.
- Heilipus squamosus Lec., a new enemy of the avocado.Proceedings of the Florida State Horticultural Society61:260‑264.
- On the distribution of Heilipus squamosos (Lec.) a pest of the avocado.The Florida Entomologist33(4):139‑141. https://doi.org/10.2307/3492736
- Updating of changes in pests, pesticides and other factors affecting subtropical insect pest control.Proceedings of the Florida State Horticultural Society84:318‑320.
- An avocado weevil (Heilipus apiatus Oliv.) (Coleoptera: Curculionidae).Florida Department of Agriculture.Contribution No. 19, Entomology Circular No. 11.
Supplementary materials
An Excel spreadsheet with three tables: 1) iNaturalist and BugGuide observations, 2) natural history collection specimens and 3) literature records.
The file includes all records (not only research grade) on iNaturalist at the time of access pertaining to the species Heilipus squamosus.