|
Biodiversity Data Journal :
Methods
|
|
Corresponding author: Maria Salnitska (m.salnitska@gmail.com)
Academic editor: Stylianos Chatzimanolis
Received: 07 Oct 2022 | Accepted: 03 Dec 2022 | Published: 19 Dec 2022
© 2022 Maria Salnitska, Alexey Solodovnikov, Igor Orlov
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Salnitska M, Solodovnikov A, Orlov I (2022) Sampling and curation of rove beetles (Insecta, Coleoptera, Staphylinidae) for comprehensive and DNA-grade collections to enhance biodiversity exploration in Northern Eurasia. Biodiversity Data Journal 10: e96080. https://doi.org/10.3897/BDJ.10.e96080
|
|
Abstract
Staphylinidae beetles form a major portion of terrestrial biodiversity globally and, in particular, in Northern Eurasia, a large area with a historically better known north temperate, subarctic and arctic biota. However, even here, rove beetles remain amongst the so-called “dark taxa” with a high fraction of taxonomically unknown lineage diversity. The propagation of DNA-based technologies in systematic entomology in recent decades has brought new opportunities for biodiversity exploration, true also for Staphylinidae. Simultaneously, new methods have revealed limitations of specimens sampled and curated by traditional practices, as existing legacy collections, whether institutional or private, unfortunately do not always qualify as a source of DNA-grade material. In addition, both legacy and newly-collected DNA-grade material of Staphylinidae remain highly biased towards Central Europe, a region with a traditionally well-developed scientific infrastructure and long-established culture for the maintenance of entomological collections. To increase the degree of biodiversity knowledge for our target organismal group across the globe, efficient sampling of DNA-grade material and, in particular, the development of comprehensive local collections in under-studied regions is highly desirable. To facilitate that, here we provide a practical guide for collecting and curation of Staphylinidae with a focus on capacity building for DNA-grade collections in Siberia and elsewhere in Northern Eurasia.
Keywords
fauna, systematics, phylogenomics, barcoding, species, expeditions, tissues, vouchers, collection management
Introduction
With more than 64 000 described species, Staphylinidae (Insecta, Coleoptera), the rove beetles, is the second largest family amongst the Animal Kingdom after weevils (
The great diversity and abundance of rove beetles (Figs
Diversity of Staphylinidae, some subfamilies in Northern Eurasia. A Aleochara laevigata Gyllenhal, 1810 (Aleocharinae) 0.5 mm; B Gyrophaena affinis Mannerheim, 1830 (Aleocharinae) 0.5 mm; C Cypha seminulum (Erichson, 1839) (Aleocharinae) 0.5 mm; D Bolitochara obliqua Erichson, 1837 (Aleocharinae) 0.5 mm; E Euaesthetus bipunctatus (Ljungh, 1804) (Euaesthetinae) 0.5 mm; F Habrocerus capillaricornis (Gravenhorst, 1806) (Habrocerinae) 0.5 mm; G Micropeplus fulvus Erichson, 1840 (Micropeplinae) 0.5 mm; H Lordithon lunulatus (Linnaeus, 1760) (Mycetoporinae) 1 mm; I Eusphalerum tenenbaumi (Bernhauer, 1932) (Omaliinae) 0.5 mm; J Omalium rivulare (Paykull, 1789) (Omaliinae) 0.5 mm; K Bledius spectabilis Kraatz, 1857 (Oxytelinae) 1 mm; L Oxytelus laqueatus (Marsham, 1802) (Oxytelinae) 0.5 mm; M Paederus riparius (Linnaeus, 1758) (Paederinae) 1 mm; N Lathrobium elongatum (Linnaeus, 1767) (Paederinae) 1 mm; O Siagonium quadricorne Kirby, 1815 (Piestinae) 1 mm.
Diversity of Staphylinidae, some subfamilies in Northern Eurasia. A Megarthrus denticollis (Beck, 1817) (Proteininae) 0.5 mm; B Claviger longicornis Müller, 1818 (Pselaphinae) 0.5 mm; C Scaphidium quadrimaculatum Olivier, 1790 (Scaphidiinae) 1 mm (image by Anders Illum); D Euconnus hirticollis (Illiger, 1798) (Scydmaeninae) 0.5 mm; E Staphylinus erythropterus Linnaeus, 1758 (Staphylininae) 1 mm; F Gauropterus fulgidus (Fabricius, 1787) (Staphylininae) 1 mm; G Quedius levicollis (Brüllé, 1832) (Staphylininae) 1 mm; H Philonthus decorus (Gravenhorst, 1802) (Staphylininae) 1 mm; I Stenus comma Le Conte, 1863 (Steninae) 1 mm; J Sepedophilus testaceus (Fabricius, 1793) (Tachyporinae) 0.5 mm; K Tachinus bipustulatus (Fabricius, 1793) (Tachyporinae) 1 mm.
Scientific collections, institutional and private, general and specific, have always played a vital role in the process of the study of Staphylinidae. Originating as small noble collections in Europe and growing in the era of geographic discoveries in major European urban centres, currently, entomological collections constitute a vital part of public scientific infrastructure globally. Large sections of Staphylinidae form a part of any notable entomological collection today, not to mention the various specialised institutional or private collections of so-called amateur taxonomists. Despite some diversity of such collections, all of them are essentially assemblages of dry pinned specimens in entomological drawers filed in a cabinet. Seldom, they are complemented by more specialised types, for example, larval collections in 70% ethyl alcohol or bulk samples of unprocessed material kept in 70% ethyl alcohol, formaldehyde or dry on cotton layers awaiting sorting and mounting on insect pins.
The rise of molecular methods for the study of biodiversity during the last few decades has changed some approaches to the study of insects and, naturally, added new demands to the preservation and storage of material in collections in order to be DNA-grade (
Molecular methods have revealed that classic collections of pinned Staphylinidae specimens are not always suitable for obtaining molecular data due to their collecting and storage conditions. Advances of phylogenetic and phylogeographic studies, dependent on a representative taxon sample, have also revealed how patchy and geographically biased available material is. In particular, Russia and adjacent countries, although covering major areas of Northern Eurasia, have a much thinner network of collections in contrast to the geographically smaller central and northern European countries. Even in well-sampled areas, legacy material, even if DNA-grade, may not be useful for detailed phylogeographic studies as often it comes with poorly-georeferenced locality data.
As an attempt to mitigate this bias, our paper provides guidelines on how to modernise existing collections or develop new ones to accelerate systematic studies of Staphylinidae in the target region using not only classic but and mainly so, modern molecular-based methods. It combines a succinct review of the currently available main literature and institutional resources which form the basis for building new collections, general recommendations and specific, practical tips for collecting, sorting and preserving Staphylinidae material, especially DNA-grade.
Literature and other sources of published taxonomic information
The strategy of collecting material for a collection largely depends on the research tasks and projects, i.e. the short- and long-term goals. For example, whether one targets a comprehensive faunistic study of a particular region for Staphylinidae overall or searches for a certain taxon within limited or broad geographic areas for revisionary or phylogenetic work, will impact one's sampling approach. While Staphylinidae of Europe are better sampled, other territories of Northern Eurasia are unevenly studied, poorly or even barely touched by exploration. Sampling approaches for faunistic, taxonomic, phylogenetic, biogeographic or ecological studies will differ. The most valuable sources of information for planning any sampling effort or collection management are published specialised catalogues and comprehensive regional checklists, as well as taxonomic revisions and monographs. The amount of such literature is enormous and here we highlight a few of the most inclusive sources. The only recent printed World Catalogue of Staphylinidae (
While the checklists and catalogues are the baseline resources, details must be sought in the main body of taxonomic literature, both legacy and recent. There is no single resource that would aggregate detailed information for all species of Staphylinidae for the entirety of Northern Eurasia. One of the most recent detailed resources is a volume covering several subfamilies of Staphylinidae for several European countries within the well-known German series on the beetles of Central Europe (
It is worth mentioning that the community of researchers studying Staphylinidae has accumulated all literature on the taxonomy and ecology of this group in one digital collection of PDF files, available for copying on a portable disc. The most updated PDF collection can be freely obtained from colleagues, for example, at the annual international meetings on biology and systematics of Staphylinidae, which have been held in May every year in Europe for more than 20 years. These meetings are an important venue to obtain information on all aspects of collecting Staphylinidae. Single references or any other bits of information can be obtained at any time by posting questions to the specialised listserver (https://sympa.uio.no/nhm.uio.no/info/staphlist) where the majority of contemporary colleagues with expertise on Staphylinidae are subscribed and can instantly provide help.
Taxonomic information on Staphylinidae, often supplemented by the high-resolution beetle images, is also available through a growing number of web resources, such as GBIF (https://www.gbif.org/), BeetleBase (http://beetlebase.com/), Danish Beetle Bank (https://danbiller.dk/), SCAN (https://scan-bugs.org/portal/), BioMap (https://baza.biomap.pl) and many others. However, coverage for Staphylinidae and the credibility of these online resources vary, often necessitating checking the primary resources from where these data came to these aggregators.
Main Staphylinidae collections of the world relevant for Northern Eurasia
The most comprehensive collections of Staphylinidae with world coverage and a strong focus on Europe or Eurasia are concentrated in Europe, especially in the United Kingdom, countries of central Europe and Italy. Large legacy collections at the national natural history museums of London, Paris, Berlin, Vienna, Brussels, Geneva, Prague, Budapest and other European capitals, form the most crucial data banks for the taxonomic knowledge on Staphylinidae in Northern Eurasia. Collecting and collections of Staphylinidae are going strong in Japan, with the focus on the fauna of Japan itself. In addition, private collections of the prominent taxonomists working on Staphylinidae, mainly in Europe, most of which are bound by agreements to be integrated into institutional collections, are spectacular. Some institutional collections, not necessarily the largest, are bound to the active research programmes on Staphylinidae systematics or at least they are curated by the staff members with taxonomic expertise in this group (e.g. in Vienna and Geneva, Oslo, Wroclaw, Hamburg or Copenhagen-based natural history museums). A few institutions are particularly important for Staphylinidae systematics because of the large amount of type material. These are natural history museums in London (types of D. Sharp, M. Cameron, W. Kirby, T. Marsham and many others) and Vienna (types of O. Scheerpeltz, A. Horion, E. Eppelsheim, F. Wagner and others), the Royal Belgian Institute of Natural Science in Brussels (types of C. Fauvel, G. Fagel, A. Schuster and others), the Field Museum of Natural History in Chicago (USA) and the National Museum of Nature and Science (Japan). The Field Museum, in particular, is a depository for M. Bernhauer’s collection, one of the largest and most important legacy collections for Staphylinidae systematics. The National Museum of Nature and Science in Japan recently accessed the large collection of A. Smetana (
The Zoological Institute of the Russian Academy of Science, which has one of the largest zoological collections with a geographic focus on large areas poorly represented in other European collections, also has a notable collection of Staphylinidae. However, without specialised curatorial staff, this collection is difficult to navigate and a very large portion of it consists of poorly-georeferenced legacy material. Acceleration of taxonomic research on Staphylinidae in recent decades in China has prompted growth of respective collections there, especially in Beijing and Shanghai. Given that Russia and China occupy large areas of Northern Eurasia, the capacity of the Staphylinidae collections in those countries is still insufficient to support and promote the study of this mega-diverse taxon there. For example, in Russia, scientific institutions in Siberia or the Far East have collections that are an order of magnitude smaller compared to the collection in St. Petersburg, located in European Russia. Legacy material is predominating over recent accessions in the main institutional collection of Staphylinidae in Ukraine, Romania, Bulgaria and some other biodiversity-rich countries at the southern periphery of Northern Eurasia. Finally, large and biodiversity-rich areas of the Caucasus or Middle Asia do not have notable local collections of Staphylinidae at all. To promote the development of more collections in relevant local institutions, especially with a focus on desirable DNA-grade material, the following sections contain relevant guidelines.
Collecting Staphylinidae
Target habitats and microhabitats
Northern Eurasia comprises diverse landscapes (or biomes or habitats) from extensive tundras in the north to deserts in the south. Any habitat is a network of different microhabitats populated by certain species of Staphylinidae so that each collecting locality is represented by a microhabitat mosaic with different species communities. Thus, when the purpose of collecting is to study the entire fauna, it is necessary to cover all possible microhabitats within a locality by various collecting methods. If the target is to collect a certain taxon, it is necessary to focus sampling in its specific microhabitat and use the most efficient method. Overall, as staphylinids are mostly predators or saprophages in leaf litter and other humid decaying organic matters (
The most diverse community of species of Staphylinidae in Northern Eurasia is confined to the forests. For example, some of the largest genera, such as Stenus (Fig.
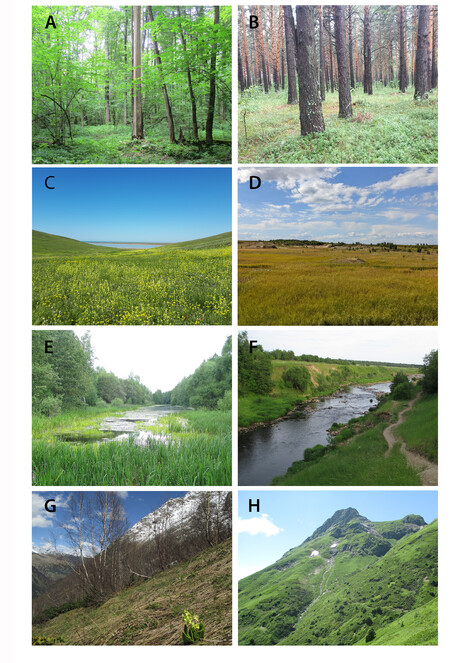
Main zonal and extra-zonal habitats for collecting Staphylinidae in Northern Eurasia. A broad-leaved forest (European Russia); B coniferous forest (European Russia); C steppe (European Russia); D tundra (West Siberia, Russia); E lake (West Siberia, Russia); F river; G, H subalpine zone in the mountains (Caucasus, Russia).
Regardless of the biome, rove beetles prefer humid microhabitats and are always abundant around water bodies such as rivers, lakes, bogs, estuaries or sea shores (E and F). There are many species and genera that are specialised to such wet habitats, for example, some Stenus (Fig.
In addition, regardless of the biome, the mountainous landscapes (Fig.
Humid and fungusy thick leaf litter of mature broad-leaved forests is the most productive microhabitat (or substrate) for collecting rove beetles in Northern Eurasia. Patches of leaf litter with nearby piles of dead wood, growth of mosses or tree trunks with flowing sap are especially promising. Leaf litter of coniferous forests or drier forests or subalpine shrubs harbours less diverse species communities, but still is quite productive. Grassy leaf litter of the meadows, steppes, alpine grasslands or swamp tussocks is harder to process, but it is also an important microhabitat for Staphylinidae. Hydrophilous Staphylinidae are also often confined to the assemblages of wet leaf and other ground-based litter at the shores or splash-zones of various stagnant or running water bodies (Fig.
Despite the highly diverse species community associated with the water-edge habitats, there are no aquatic forms amongst rove beetles and, interestingly, only a few groups have evolved into specialised forms for life in the deeper layers of soil or in caves and other subterranean cavities. For example, MSS (Milieu Souterrain Superficiel according to
Certain Staphylinidae species can be found in the nests and burrows of mammals and birds, where they mainly occur in the debris of the animal latrines. Some species, from time-to-time or exclusively, are found in the nests of social insects (Fig.
Staphylinidae are regularly found in decaying wood or bark (Fig.
Many Staphylinidae, sometimes entire lineages, are more or less specialised to living inside the fruiting bodies of various types of fungi (Fig.
A significant number of Staphylinidae species are distinctly coprophilous and can be found in faeces, mainly of vertebrate animals. For example, moist cattle dung (Fig.
Hand-collecting and trapping methods
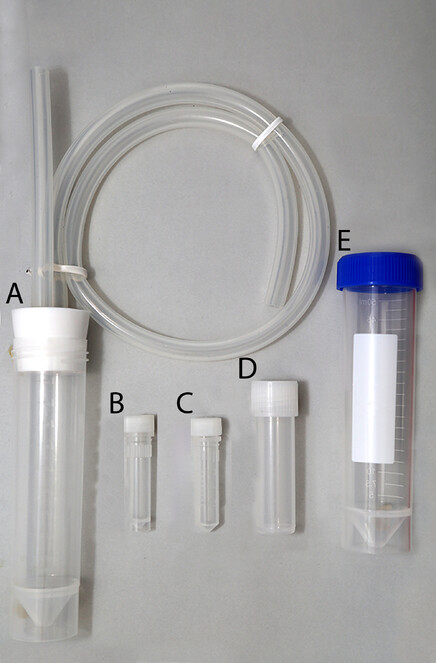
Most Staphylinidae in the temperate or colder conditions of Northern Eurasia can be picked up by hand-collecting directly from the habitat they live in, as they rarely attempt to fly away quickly and none of Staphylinidae in that region is poisonous or otherwise hazardous to humans. Only a few large species from the subtribe Staphylinina may attempt to bite with their mandibles, which are, however, not strong enough to pose a significant threat. Having a collecting plastic tray and an aspirator or pooter (Fig.
The Winkler eclector is the most universal as it is very portable and does not require electricity as a source of heat. It represents a long bag made from breathable white cloth internally sewed to two square rings, fastened on a top (Fig.
The classical Berlese funnel eclector consists of a plastic or metallic cone (funnel) often with a mesh ring inside on which to place the sifted material. A cup with the preservation liquid is attached underneath the funnel, while the funnel is covered by a lid with an installed light bulb on its inner side. The funnel can be fixed on the ground in different ways, for example, by specially designed racks or simple plastic bucket depending on the funnel size. One of the best, most efficient and portable Berlese funnels we are aware of, are those used by Margaret Thayer and Alfred Newton (Fig.
Using a combination of several Winkler eclectors and Berlese funnels, one can efficiently process large amounts of sifted leaf litter and other debris in various conditions. During expeditions, the sifted material from various localities can be accumulated in breathable labelled canvas litter bags, which can be kept for several hours or even days until they are transported to suitable conditions for extraction.
Unfortunately, not all kinds of debris are easy or possible to sift. For example, very wet moss or flood debris may be too sticky to be sifted. Additionally, liquified rotten mushrooms or dung or very fine-grained soil are difficult or pointless to sift. In those cases, piles of moss or similar substrates can be placed in the Berlese funnels directly. When this is not possible, for example, with soil or dung, a step of flotation may be added. For that, a target substrate is placed into a wide container with warm water to let Staphylinidae or other target animals float to the surface together with light organic debris. The latter material is collected by a small net, soaked on paper and afterwards placed for extraction of beetles into the eclectors. Using such flotation for large amounts of soil (so-called soil washing) was the only method that allowed us to discover diversity of the endogean subfamily Leptotyphlinae. Although the endogean fauna is mainly poor in Northern Eurasia due to the wiping out effect of Quaternary glaciation, many areas here are worth exploring by this method, described in detail in
Naturally, hand-collecting of rove beetles that occur in specialised, hidden habitats should be assisted by additional techniques and equipment. For example, caving skills and equipment are needed to explore caves for rove beetles or knowledge of ant and other animal biology and often digging equipment, are required to explore ant hills or underground animal nests. Occasionally a pyrethrum-based rapid killer insecticide (Raid or similar) may be used to spray fungusy or mossy logs to knock them out from crevices to a large piece of white cloth or plastic spread underneath (often referred to as small scale fogging). Interestingly, such fogging is often and efficiently used in tropical and south temperate conditions, while in north temperate zones, especially in Northern Eurasia, this method is not popular and, in our experience, not as efficient.
In addition to hand collecting aided by various extraction methods, there are a number of trap types used for collecting Staphylinidae. Traps bring non-stop long-term possibilities to sample rove beetles in the variety of habitats, but they demand time for installation and monitoring. Additionally, they non-selectively collect rove beetles along with any other similarly behaving invertebrates (and sometimes vertebrates, such as rodents and amphibians), so they produce mixed bulk samples that may demand labour-intensive subsequent sorting. Most trap types are the most effective when installed for longer periods of several days or weeks, which brings certain demands for the properties of preserving liquid used to kill and preserve collected organisms (for details see below).
Pitfall traps (Fig.
Unlike pitfalls, the flight intercept or window traps (
The somewhat similar Malaise traps (
While the window and Malaise traps are static, a vehicle-mounted net (car-net) is a useful tool to actively sample flying Staphylinidae and other insects (Fig.
Finally, many night-flying insects, including some Staphylinidae, can be attracted to light traps (Fig.
As a concluding remark here, we stress that one should always stay open to new data about the biology of target groups, technological development and new inventions facilitating efficient collecting. For example, we plan to extensively try a powerful electrical vacuum cleaner to collect Staphylinidae from ground-based debris in Northern Eurasian grasslands, a method now becoming popular and productive in Central Europe.
Preserving liquids for field collecting and bulk sample storage
Traditionally, rove beetle specimens collected in the field are killed and then preserved to be pinned (or point- or card-mounted on the insect pin). The main goal of pinning and drying a specimen on the pin is to preserve the exoskeleton intact for morphological examination. Most of the internal tissues decay and dry to some extent with such a curation technique. This is the main way to keep Staphylinidae in entomological collections, as with other Coleoptera, for decades to centuries. Due to the time lag between collecting and pin-mounting steps, there are a number of ways to keep specimens after they are killed and before they are mounted. One of the widely used methods is killing the material with and keeping it in 70% ethyl alcohol or in 70% alcohol with a slight addition of acetic acid. This solution keeps the specimens soft enough to be easily mounted after decades of storage. Another common way is to kill specimens with ethyl acetate and either keeping them in the same killing vials with sawdust or filter paper in the freezer before mounting or spreading them dry on layers of cotton. The latter method is especially widespread in Russia.
New requirements for material to be DNA-grade changed this practice. Now the purpose of the mainstream preservation is not only to keep a beetle body “intact” due to well-preserved sclerites of the exoskeleton connected by half-decayed, half-dried muscles and membranes, but to preserve its DNA for as long as possible.
Strategies for preservation of DNA and maintaining specimen qualities for easy mounting on insect pins do not always align (
Killing by and preserving in 96% or even more highly concentrated ethyl alcohol is obviously a proven and simple method of inhibiting the initial post-mortem DNA degradation. It is important to understand that, to properly preserve a sample in 100% alcohol, it is necessary to dehydrate it by exchanging ethanol in the vial several times before storage. To facilitate that, the volume of alcohol should significantly exceed the volume of tissue, i.e. one should not fill the vial with specimens for more than ¼-⅓ of its volume. Specimens preserved that way may be mounted on insect pins afterwards or often, they are kept in absolute alcohol indefinitely, ideally at low (-20° – -80°C) temperatures. Some material meant for long-term DNA preservation for years and decades, if not centuries, is kept at ultralow temperatures in specialised cryo-tanks with liquid nitrogen (
As mentioned above, another challenge for DNA-grade preservation is posed by the long-term window and pitfall traps, where it is not possible to use high percentage alcohol as a preservation medium due to its high rate of evaporation. When these traps are not checked regularly, more viscous media like 100% (or as concentrated as possible) propylene glycol is used to preserve DNA-grade material. Moreover, unlike concentrated alcohol, propylene glycol does not make the specimens stiff and, as far as known (
Collecting events and field labelling
Regardless of the collecting technique, all samples from the same collecting event must be securely labelled in the field, to avoid any confusion or loss of information. A collecting event is an arbitrary concept, but usually this is a sample from the same trap collected during a certain period or a hand-collected sample from a microhabitat within a given locality taken on a certain date. Specimens hand-collected at the same collecting event are usually placed together in sizeable plastic vials (50 ml, for example, Falcon tubes) (Fig.
Basic entomological tools for mounting and dissecting specimens. A glue; B pinning block; C pipettes; D forceps; E soft forceps; F insect pins (No. 3); G genitalia vial; H minuten-based dissection tools; I mounting boards; J, N glass slides; K porcelain cup; L small Petri dishes; M scissors; O brushes; P alcohol-proof ink pens.
Short-term storage and transportation of samples from the field to laboratory conditions
For longer multi-day or week field trips, storage of samples in Falcon collecting vials or larger plastic containers is not practical, as they take significant space and have to be filled with alcohol up to the lid to avoid damage to specimens from shaking. Therefore, transferring them into securely sealed compact Whirl packs with a smaller amount of alcohol in a large plastic container that is kept away from day light and heat as much as possible is more practical. For air transportation, alcohol can be poured out from these bags completely for a few hours, to significantly reduce weight of the samples and comply with the safety regulations for luggage. Back in the laboratory, samples are transferred from the Whirl packs to collection cryo-vials or glass jars with fresh alcohol and printed finalised labels (for details, see below). Samples can be stored at room temperature for up to two weeks; for longer storage they should be deposited in a freezer at -20°C or colder.
Labelling: principles and label content
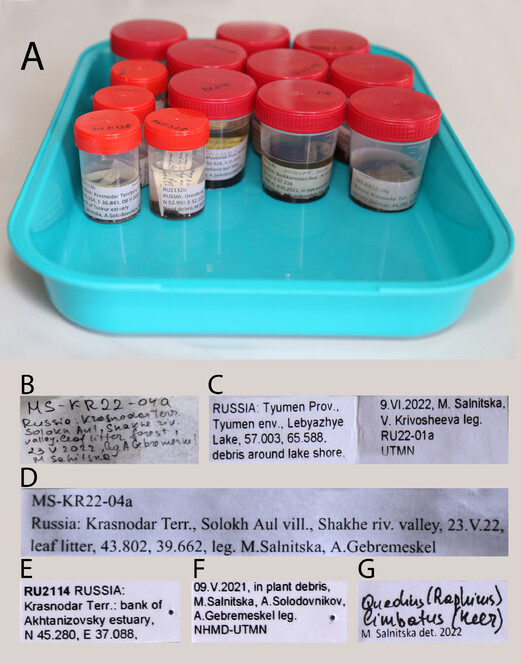
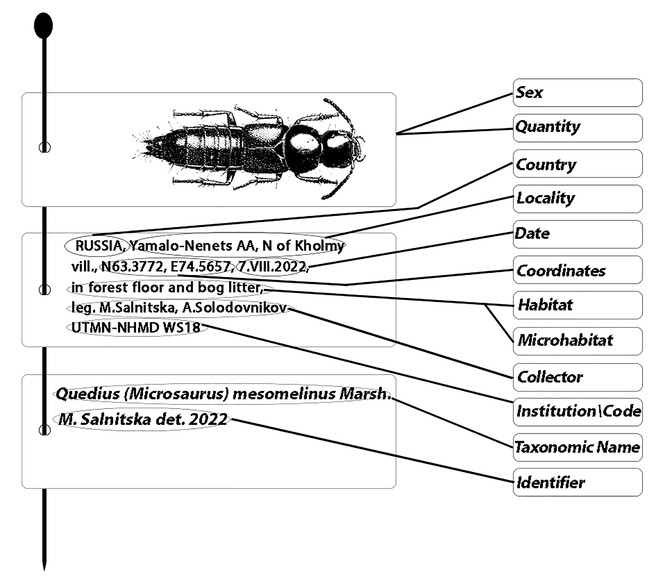
Proper labelling is the most crucial condition for scientific material. The label must briefly, but unambiguously record the place, date and other conditions of a collecting event by having such information as country, province, locality, coordinates, habitat, microhabitat, date and collectors (Fig.
Examples of bulk sample and specimen labels. A temporary storage of bulk samples with the finalised geographic labels; B primary field hand-written labels; C finalised geographic labels for long term storage in 2 ml vials; D finalised geographic labels for temporary storage; E, F geographic labels for pinned specimens; G identification label.
Dry and wet collections, curation process from bulk samples to individual DNA-grade specimens
In the countries of Northern Eurasia with a temperate climate, collections of Staphylinidae that serve as a source of DNA-grade material mostly comprise the following main types of material: (1) pinned adult specimens traditionally kept in drawers placed in cabinets at room or slightly cooler temperature, (2) wet material in 70% ethyl alcohol or other preservation liquids kept at room or slightly cooler temperature (bulk samples or individual adults or larvae awaiting to be sorted or mounted on pins) and 3) wet material in 96% ethyl alcohol permanently kept in freezers (usually bulk samples or individually sorted and identified adult or larval beetles). Dry pinned specimens may be DNA-grade to varying degrees, depending on the method of their collecting and preservation and their age (
Storage of wet bulk samples
If the purpose is to keep mixed bulk samples in 96% alcohol under low temperatures long-term, the main standard to follow is proper labelling, using alcohol-proof thin (80 g/m2) paper (Southworth Paper, Hammermill, TerraSlate, BledProof and others) and ink (Canon, Xerox and others) and well-sealed quality jars or vials (Spectrum, Bormioli, IKEA and others).
Morphosorting of wet bulk samples
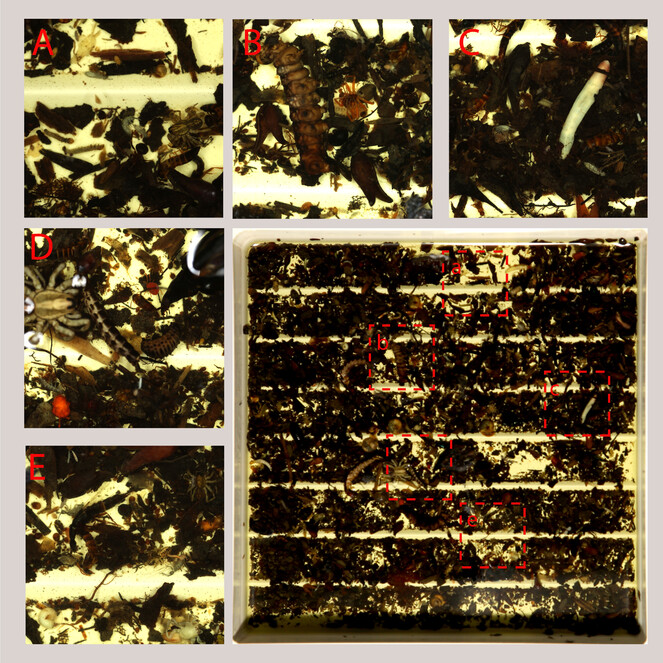
Morphosorting into certain taxonomic groups is the initial step of curating bulk samples for further, more detailed investigations. Morphosorting can be conducted at various taxonomic levels (for example, sort invertebrates into main classes, sort insects into orders, orders into families and so on) with the immediate aim to select target taxa (for example, family Staphylinidae) from the bulk sample (Fig.
Sorting sifted leaf litter bulk sample, example of main groups of arthropods (and other invertebrates) to sort. A rove beetle, spider, springtail, ants; B spiders, larvae, ants; C rove beetles, springtails, annelids, aphids; D rove beetles, larvae, mites; E rove beetles, springtails, molluscs.
To efficiently perform morphosorting of a wet bulk sample, one must spread it (or its subset) on the bottom of a Petri dish or white sorting tray (Fig.
Storage of wet individual samples for DNA grade cryo-collection
Individual wet samples in 96% alcohol after morphosorting are kept in properly labelled 2-20 ml cryo-vials (SSI, Eppendorf, Thermo FC, Corning, Accumax and others) (Fig.
Example of infrastructure for a cryo-collection. A, B liquid nitrogen generator and tanks for storing samples under ultra-low (up to -80ºC) temperatures; C standing freezers for sample storage under low (-20ºC or lower) temperatures; D arrangement of jars and cryo-boxes with samples on shelves of a standing -20ºC freezer.
Mounting on insect pins
Traditionally, mounting large rove beetles on insect pins directly or, for smaller beetles, via gluing them to the card or point (Fig.
Mounting on insect pins with dissection of genitalia or other structures
Identification of a species of Staphylinidae often requires examination of the genitalia or terminalia. For that, these structures need to be dissected and prepared differently from the specimen. Occasionally, mouthparts or other structures are dissected too for closer examination. In all cases, it is best when dissected parts are mounted on the same pin with their respective specimen, usually in a special container (e.g. genitalia vial) (Fig.
Organisation, storage and curation of specimens in dry collections
Despite some differences in collection organisation between institutions, the best and most accessible practice is to keep pinned Staphylinidae beetles in cardboard unit trays of two sizes (small and large) packed in wooden drawers (Fig.
Databasing and digitalisation of collections
Modern advances in biological research that require processing information from publications, specimen meta-data, specimen images, DNA sequences and other large and diverse data are impossible without databasing and managing digital archives. Databases create a unified source of data for a certain project, geographic area or taxon. Databases are crucial for the digitisation of biological collections, a priority for the development of scientific infrastructure in recent decades (
Acknowledgements
We sincerely thank peer reviewers Fangshuo Hu, Adam Brunke and Michael Caterino for their many useful comments and suggestions that improved the manuscript. Michael Caterino is specially acknowledged for his generous offer to provide linguistic proof-reading of the paper, which we gladly accepted. Margaret Thayer, Alfred Newton (Chicago, USA), Oleg Semionenkov (Smolensk, Russia), Roman Dudko (Novosibirsk, Russia) and Fedor Konstantinov (Saint-Petersburg, Russia) kindly shared the photos used in this paper. This study was supported by the Ministry of Science and Higher Education of the Russian Federation within the framework of the Federal Scientific and Technical Program for the Development of Genetic Technologies for 2019-2027 (agreement №075-15-2021-1345, unique identifier RF----193021X0012).
Funding program
The Federal Scientific and Technical Program
Grant title
Development of Genetic Technologies for 2019-2027 (agreement №075-15-2021-1345, unique identifier RF----193021X0012).
Hosting institution
The Institute of Environmental and Agricultural Biology (X-BIO), University of Tyumen, Tyumen, Russian Federation
Ethics and security
Ethics and security standards not violated.
Conflicts of interest
All authors declare that they have no conflicts of interest.
References
- Can Townes type Malaise traps be improved? Some recent developments.Entomologische Berichten69:129‑135.
- Checklist of the Staphylinidae (Coleoptera) in Korea.Journal of Asia-Pacific Biodiversity10(3):279‑336. https://doi.org/10.1016/j.japb.2017.06.006
- The effect of silica desiccation under different storage conditions on filter-immobilized environmental DNA.BMC Research Notes14(1):1‑6. https://doi.org/10.1186/s13104-021-05530-x
- DNA Extraction from Museum Specimens of Parasitic Hymenoptera.PLoS ONE7(10):45549. https://doi.org/10.1371/journal.pone.0045549
- Endogean beetles (Coleoptera) of Madagascar: deep soil sampling and illustrated overview.Zootaxa4963(2):317‑334. https://doi.org/10.11646/zootaxa.4963.2.4
- Staphylinidae (Insecta: Coleoptera) in Latin America: synopsis, annotated catalog, diversity and distribution.Zootaxa4621(1):1‑406. https://doi.org/10.11646/ZOOTAXA.4621.1.1
- Freude-Harde-Lohse-Klausnitzer - Die Käfer Mitteleuropas. Band 4. Staphylinidae I.Zweite neubearbeitete Auflage.Spektrum Akademischer Verlag,Heidelberg,560pp.
- Comparative efficiency of ultra violet black light lamp and mercury vapour lamp as a light source in light trap against major insect pest of Kharif crops.Journal of Entomology and Zoology Studies7(1):532‑537.
- Morphospecies as a substitute for Coleoptera species identification, and the value of experience in improving accuracy.Journal of the Royal Society of New Zealand33(2):583‑590. https://doi.org/10.1080/03014223.2003.9517746
- Biology of rove beetles (Staphylinidae).Springer International Publishinghttps://doi.org/10.1007/978-3-319-70257-5_1
- No specimen left behind: industrial scale digitization of natural history collections.ZooKeys209:133‑146. https://doi.org/10.3897/zookeys.209.3178
- Evaluation of window flight traps for effectiveness at monitoring dead wood‐associated beetles: the effect of ethanol lure under contrasting environmental conditions.Agricultural and Forest Entomology11(2):143‑152. https://doi.org/10.1111/j.1461-9563.2008.00400.x
- Are subcortical rove beetles truly Holarctic? An integrative taxonomic revision of north temperate Quedionuchus (Coleoptera: Staphylinidae: Staphylininae.Organisms Diversity & Evolution20(1):77‑116. https://doi.org/10.1007/s13127-019-00422-2
- Integrative taxonomy of Nearctic and Palaearctic Aleocharinae: new species, synonymies, and records (Coleoptera, Staphylinidae.ZooKeys1041:27‑99. https://doi.org/10.3897/zookeys.1041.64460
- The limits of Quediini at last (Staphylinidae: Staphylininae): a rove beetle mega‐radiation resolved by comprehensive sampling and anchored phylogenomics.Systematic Entomology46(2):396‑421. https://doi.org/10.1111/syen.12468
- Integrated phylogenomics and fossil data illuminate the evolution of beetles.BioRxivhttps://doi.org/10.1101/2021.09.22.461358
- Phylogeny of the hyper‐diverse rove beetle subtribe Philonthina with implications for classification of the tribe Staphylinini (Coleoptera: Staphylinidae.Cladistics34(1):1‑40. https://doi.org/10.1111/cla.12188
- Window flight traps for insects.The Canadian Entomologist87(1):46‑47. https://doi.org/10.4039/Ent8746-1
- Silica gel: an ideal material for field preservation of leaf samples for DNA studies.Taxon40(2):215‑220. https://doi.org/10.2307/1222975
- Coléoptères Staphylinidae de la région Paléarctique occidentale. I. Généralités. Sous-familles: Xantholininae et Leptotyphlinae.Nouvelle Revue d’Entomologie Supplément2(2, i–ix):1‑651.
- Coléoptères staphylinides de la région paléarctique occidentale II. Sous famille Staphylininae, Tribus Philonthini et Staphylinini.Nouvelle Revue d’Entomologie. Supplément4(4):1‑593.
- Coléoptères staphylinides de la région paléarctique occidentale III. Sous famille Staphylininae, Tribu Quediini. Sous famille Paederinae, Tribu Pinophilini.Nouvelle Revue d’Entomologie Supplément8(4):1‑364.
- A review of adhesives for entomotaxy.PeerJhttps://doi.org/10.7287/peerj.preprints.27184v1
- Molecular phylogeny of the Athetini-Lomechusini-Ecitocharini clade of aleocharine rove beetles (Insecta).Zoologica Scripta41(6):617‑636. https://doi.org/10.1016/j.ympev.2010.05.023
- Coastal Staphylinidae (Coleoptera): A worldwide checklist, biogeography and natural history.ZooKeys107:1‑98. https://doi.org/10.3897/zookeys.107.1651
- Empirical evaluation of preservation methods for faecal DNA.Molecular Ecology7(10):1423‑1428. https://doi.org/10.1046/j.1365-294x.1998.00449.x
- Die Kafer Mitteleuropas. Band 1.Heidelberg, Spektrum Akademischer Verlag,214pp.
- Die Käfer Mitteleuropas, Band 5, Staphylinidae II (Hypocyphtinae und Aleocharinae). Pselaphidae Goecke & Evers.Krefeld,381pp.
- Insect morphology in the age of phylogenomics: innovative techniques and its future role in systematics.Entomological Science17(1):1‑24. https://doi.org/10.1111/ens.12053
- The subterranean environment. Hypogean life, concepts and collecting techniques.WBA Handbooks 3,Verona,132pp.
- DNA extraction from dry museum beetles without conferring external morphological damage.PLOS One2(3):e272. https://doi.org/10.1371/journal.pone.0000272
- Effects of preservation and storage of microcrustaceans in RNAlater on RNA and DNA degradation.Limnology and Oceanography: Methods3(2):143‑148. https://doi.org/10.4319/lom.2005.3.143
- The Global Ant Biodiversity Informatics (GABI) database: synthesizing data on the geographic distribution of ant species (Hymenoptera: Formicidae).Myrmecological News/Osterreichische Gesellschaft fur Entomofaunistik24:83‑89.
- Phylogeny of the family Staphylinidae based on molecular data: A review biology of rove beetles (Staphylinidae). In:Biology of rove beetles (Staphylinidae).Springerhttps://doi.org/10.1007/978-3-319-70257-5_2
- Rove beetles (Coleoptera: Staphylinidae) on agrolandscape herbaceous vegetation in the Leningrad Region.Russian Entomological Journal28(4):373‑376. https://doi.org/10.15298/rusentj.28.4.05
- Digitization and the future of natural history collections.BioScience70(3):243‑251. https://doi.org/10.1093/biosci/biz163
- Catalog of the Staphylinidae (Insecta: Coleoptera). 1758 to the end of the second Millennium. I-VII (Parts 1-7).Bulletin of the American Museum of Natural History265:1‑4218. https://doi.org/10.1206/0003-0090.265.1.1
- Why not use window traps for collecting Coleoptera and other flying insects?Entomologische Berichten40(9):131‑132.
- Einsatz eines Autokeschers im Ziegelrodaer Forst-Ergebnisse und Bemerkungen zur Methode.Hercynia-Ökologie und Umwelt in Mitteleuropa50(1):31‑94.
- Sur l’existence d’un milieu souterrain superficiel en zone non calcaire.Comptes rendus de l'Académie des Sciences, Paris290:49‑52.
- The interactive digital key to rove beetles (Coleoptera: Staphylinidae) of Denmark.danbiller.dk/keyx/Staphylinidae
- Car catching: An interesting method for collecting beetles and other insects.Entomologiske Tidsskrift99:115‑118.
- The past and the present through phylogenetic analysis: the rove beetle tribe Othiini now and 99 Ma.Systematic Entomology44(1):1‑18. https://doi.org/10.1111/syen.12305
- How old can we go? Evaluating the age limit for effective DNA recovery from historical insect specimens.Systematic Entomology45(3):505‑515. https://doi.org/10.1111/syen.12411
- Catalogue of Chinese Coleoptera.3, Staphylinidae.Science Press,Beijing,xix + 720pp.
- Coleoptera: Staphylinidae: Scaphidiinae.In:World Catalogue of Insects 16.EJ Brill,Leiden, Boston,xvi, 418pp.
- Scaphisomatini of Arizona (Coleoptera, Staphylinidae, Scaphidiinae) collected by V-flight intercept traps.Revue suisse de Zoologie128(1):173‑185. https://doi.org/10.35929/RSZ.0043
- A new insect trap.Entomologische Tidskrift58:148‑160.
- Ecology and sampling techniques of an understudied subterranean habitat: the Milieu Souterrain Superficiel (MSS).The Science of Nature103(11):1‑24. https://doi.org/10.1007/s00114-016-1413-9
- Factors affecting DNA preservation from museum‐collected lepidopteran specimens.Entomologia Experimentalis et Applicata120(3):239‑244. https://doi.org/10.1111/j.1570-7458.2006.00451.x
- Insect collections and DNA analyses: how to manage collections?Museum Management and Curatorship23(2):193‑199. https://doi.org/10.1080/09647770802012375
- The effect of ethanol concentration on the morphological and molecular preservation of insects for biodiversity studies.PeerJ9:e10799. https://doi.org/10.7717/peerj.10799
- Propylene glycol and non-destructive DNA extractions enable preservation and isolation of insect and hosted bacterial DNA.Agriculture11(1):77. https://doi.org/10.3390/agriculture11010077
- When data management meets project management.Biodiversity Information Science and Standards3:e37224. https://doi.org/10.3897/biss.3.37224
- Collecting in collections: a PCR strategy and primer set for DNA barcoding of decades‐old dried museum specimens.Molecular Ecology Resources15(5):1102‑1111. https://doi.org/10.1111/1755-0998.12380
- An upgrade pinning block: a mechanical practical aid for fast labelling of the insect specimens.Biodiversity Data Journal5:e20648. https://doi.org/10.3897/BDJ.5.e20648
- DNA preservation: a test of commonly used preservatives for insects.Invertebrate Systematics27(1):81‑86. https://doi.org/10.1071/IS12067
- Trap responses of flying insects. The influence of trap design on capture efficiency.Academic Press, Harcourt Brace Jovanovich,New York,93pp. https://doi.org/10.1016/B978-0-12-509755-0.50009-8
- First large‐scale quantification study of DNA preservation in insects from natural history collections using genome‐wide sequencing.Methods in Ecology and Evolutionhttps://doi.org/10.1111/2041-210X.13945
- A hands-on overview of tissue preservation methods for molecular genetic analyses.Organisms Diversity & Evolution10(1):91‑105. https://doi.org/10.1007/s13127-010-0012-4
- Museum specimens: An overlooked and valuable material for conservation genetics.Ecological Research36(1):13‑23. https://doi.org/10.1111/1440-1703.12181
- Propylene glycol: a promising preservative for insects, comparable to ethanol, from trapping to DNA analysis.Entomologia Experimentalis et Applicata168(2):158‑165. https://doi.org/10.1111/eea.12876
- World catalog of the genera of Pselaphidae (Coleoptera).Fieldiana Zoology93.
- World catalog of the genera of Scydmaenidae.Koleopterologische Rundschau68:137‑165.
- StaphBase: Staphyliniformia world catalog database (version Aug 2022).The Catalogue of Life, 2022URL: www.catalogueoflife.org
- Subterranean pitfall traps: is it worth including them in your ant sampling protocol?Psyche2012https://doi.org/10.1155/2012/870794
- Staphylinids: quick guide.Current Biology27:43‑56.
- Collecting small beetles with large-area "window" traps.The Coleopterists Bulletin34:237‑239.
- The value of digitising natural history collections.Research Ideas and Outcomes7:e78844. https://doi.org/10.3897/rio.7.e78844
- Obtaining, Storing and Archiving Specimens and Tissue Samples for Use in Molecular Studies.In:Techniques in Molecular Systematics and Evolution. Methods and Tools in Biosciences and Medicine.Birkhäuser, Basel.. https://doi.org/10.1007/978-3-0348-8125-8_11
- Combining pitfall traps and soil samples to collect Collembola for site scale biodiversity assessments.Applied Soil Ecology45(3):293‑297. https://doi.org/10.1016/j.apsoil.2010.05.005
- Field preservation of Coleoptera for molecular genetic analyses.Environmental Entomology24(3):716‑719. https://doi.org/10.1093/ee/24.3.716
- Coleoptera collected with a car net in Finland.Notulae Entomologicae62:69‑72.
- Rove beetles of the genus Quedius (Coleoptera, Staphylinidae) of Russia: a key to species and annotated catalogue.ZooKeys847:1‑100. https://doi.org/10.3897/zookeys.847.34049
- DNA barcode sheds light on species boundaries in the common morphologically variable rove beetle Quedius umbrinus-complex that puzzled taxonomists for more than a century (Coleoptera, Staphylinidae).Systematics and Biodiversity19(7):859‑874. https://doi.org/10.1080/14772000.2021.1943559
- Collecting and preserving insects and mites.Techniques and Tools1‑52.
- Hypogaeic pitfall traps: methodological advances and remarks to improve the sampling of a hidden ant fauna.Insectes Sociaux57(3):261‑266. https://doi.org/10.1007/s00040-010-0078-1
- Phylogenetic placement of the austral rove beetle genus Hyperomma triggers changes in classification of Paederinae (Coleoptera: Staphylinidae).Zoologica Scripta46(3):336‑347. https://doi.org/10.1111/zsc.12209
- Integrating specimen databases and revisionary systematics.ZooKeys209:255‑267. https://doi.org/10.3897/zookeys.209.3288
- Staphylinidae, 304–1134.In:Catalogue of Palaearctic Coleoptera.Volume 2. Hydrophiloidea–Staphylinoidea, Revised and updated edition.Vol. 3.Brill,Leiden, Boston,304-1134pp.
- A catalog of the Nicrophorinae (Coleoptera: Silphidae) of the world.Zootaxa65(1):1‑304. https://doi.org/10.11646/zootaxa.65.1.1
- Sampling of soil mesofauna and macrofauna with pitfall trap.Revista Verde de Agroecologia e Desenvolvimento Sustentável8(5):108‑115.
- A review of terrestrial and canopy Malaise traps.Annals of the Entomological Society of America114(1):27‑47. https://doi.org/10.1093/aesa/saaa044
- Taxonomic review of the ‘quediine’ subtribes of Staphylinini (Coleoptera: Staphylinidae: Staphylininae) of mainland China.Nakladatelstvi Jan Farkač,Prague:434 pp.
- Species delimitation in the Aleochara fucicola species complex (Coleoptera: Staphylinidae: Aleocharinae) and its phylogenetic relationships.Zoologica Scripta43(6):629‑640. https://doi.org/10.1111/zsc.12077
- Flight activity of insects sampled with a truck trap. I. Flight activity of Staphylinidae (Coleoptera).Konfyu56:410‑416.
- Preservation of DNA for data storage.Russian Chemical Reviews90(2):280. https://doi.org/10.1070/RCR4994
- High‐performance digitization of natural history collections: automated imaging lines for herbarium and insect specimens.Taxon63(6):1307‑1313. https://doi.org/10.12705/636.13
- Staphylinidae Latreille, 1802. Handbook of Zoology. Coleoptera, Beetles. In:Handbook of Zoology. Coleoptera, Beetles.2nd,Volume 1: Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim).Walter de Gruyter,GmbH Berlin/Boston, Massachusetts,394–442.pp.
- Morphoecological features and phylogeny of the Staphylinidae with a catalog of the fauna of the USSR.NaukaMoscow:191 pp. [InRussian].
- Methods for dissecting dry insects and insects preserved in fixative solutions or by refrigeration.Bulletin of the World Health Organization47(2):239‑244.
- Olfactory response of rove beetle, Paederus fuscipes (Curtis) to flower volatiles.Journal of Pharmacognosy and Phytochemistry8(1):2258‑2260.
- The effects of preservatives and temperatures on arachnid DNA.Invertebrate Systematics19(2):99‑104. https://doi.org/10.1071/IS04039
- The value of RTUs and parataxonomy versus taxonomic species.New Zealand Entomologist27(1):3‑9. https://doi.org/10.1080/00779962.2004.9722118
- Application of propylene glycol in DNA-based studies of invertebrates.Metabarcoding and Metagenomics5:57278. https://doi.org/10.3897/mbmg.5.57278
- DESS: a versatile solution for preserving morphology and extractable DNA of nematodes.Nematology8(3):367‑376. https://doi.org/10.1163/156854106778493448
- Arthropod diversity and abundance along the Kihansi Gorge (Kihansi River) in the southern Udzungwa Mountains, Tanzania.Journal of East African Natural History87:233‑240. https://doi.org/10.2982/0012-8317(1998)87[233:ADAAAT]2.0.CO;2
- Multilocus phylogeny defines a new classification of Staphylininae (Coleoptera, Staphylinidae), a rove beetle group with high lineage diversity.Systematic Entomology45(1):114‑127. https://doi.org/10.1111/syen.12382