|
Biodiversity Data Journal :
Taxonomic paper
|
Review of the genus Namadytes Hesse, 1969 (Insecta: Diptera: Mydidae: Syllegomydinae)
|
Corresponding author:
Academic editor: Pierfilippo Cerretti
Received: 13 Feb 2014 | Accepted: 05 Mar 2014 | Published: 10 Mar 2014
© 2014 Torsten Dikow, Stephanie Leon
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Dikow T, Leon S (2014) Review of the genus Namadytes Hesse, 1969 (Insecta: Diptera: Mydidae: Syllegomydinae). Biodiversity Data Journal 2: e1071. https://doi.org/10.3897/BDJ.2.e1071
|
|
Abstract
The Mydidae genus Namadytes Hesse, 1969 is reviewed. It is known from five species, primarily occurring in Namibia. The study of newly available material from both Namibia and South Africa deposited in several natural history collections results in the recognition of three species and new synonymy of two, i.e., Namadytes pallidus Hesse, 1972 is a new junior synonym of Namadytes maculiventris (Hesse, 1969) and Namadytes prozeskyi Hesse, 1969: 282 is a new junior synonym of Namadytes vansoni Hesse, 1969: 280. All three species are re-described and comments on sexual dimorphism and intraspecific variation are made, a dichotomous key for their identification is presented, and illustrations and photographs are provided to support the descriptions and facilitate future identification. Distribution, occurrence in biodiversity hotspots sensu Conservation International, and seasonal incidence with associated weather and climatic data are discussed for all species. A morphological structure ventral to the halter and posterior to the metathoracic spiracle, the infra-halter sclerite, is here newly termed.
Keywords
Diptera, Mydidae, Syllegomydinae, Namadytes, Afrotropical Region, taxonomy
Introduction
The southern African Mydidae fauna is the most diverse world-wide both in terms of species numbers and generic diversity. The seminal work by
Taxonomic history
At the start of this review, Namadytes Hesse, 1969 is known from five species with an interesting taxonomic history.
Hesse (1969) described the genus Namadytes (p. 278) based on two female specimens and representing two distinct species, i.e., Namadytes vansoni Hesse, 1969: 280 from Seeheim, Namibia and Namadytes prozeskyi Hesse, 1969: 282 from Arechadamab, Namibia. On page 284, Hesse describes the genus Namamydas Hesse, 1969 based on a single male specimen, identified as Namamydas maculiventris Hesse, 1969, collected by himself and his colleagues from the South African Museum (now Iziko South African Museum) at Vioolsdrift on the South African bank of the Orange River, which represents the border with Namibia. Hesse comments on the unique arrangement of the male aedeagal prongs being fused medially in this species.Hesse (1972) established the synonymy of Namadytes and Namamydas based on the collection of female and male specimens at the Excelsior farm No. 127, Namibia, of a new species, Namadytes cimbebasiensis Hesse, 1972. He writes (p. 139), "The discovery of two additional species of Namadytes from South West Africa, described below, and of which one is represented by both sexes, proves without doubt that the male sex of Namadytes (unknown at the time of description) is identical generically with the male described by me as Namamydas. The latter genus thus falls away as a synonym of Namadytes."Bowden (1980) cataloged all five valid Namadytes species.
Goals of this review
As can be seen from the above information, Namadytes and its five species were represented by nine specimens prior to this study. Two species, i.e., N. prozeskyi and N. vansoni, were known from females only, while N. maculiventris and N. pallidus were known only from males, and N. cimbebasiensis from both sexes.
This review is based on an additional 61 specimens from numerous natural history collections accumulated over the past 35 years representing all previously known species. Such an increase in specimen number, their geographic occurrence expanding the range of the genus considerably, and substantial morphological variation suggested that a few new species might be represented among the material. However, this not the case and to the contrary the number of valid species is reduced to three by synonymy.
Materials and methods
Morphological terminology and abbreviations for setae follows
The female genitalia and male terminalia are first excised and macerated in 10% potassium hydroxide (KOH) at 55 °C followed by neutralization in acetic acid (CH3COOH) and rinsing in distilled water (H2O). They are temporarily stored in 75% ethanol (C2H5OH) for examination and illustration and eventually sealed in polyethylene vials containing 100% glycerine (C3H8O3) and attached to the specimen's pin. Morphological features were examined using a Zeiss SteREO Discovery.V12 stereo microscope. Illustrations were observed with a camera lucida, drawn, inked, and scanned. The setation on terminalia is not shown. Wing length is measured from the tegula to the distal tip of the wing. Whole habitus photographs of pinned specimens were taken using a Visionary Digital Passport II system (base and StackShot only), an Olympus E-30 digital SLR, a 50 mm macro lens (equivalent to 100 mm focal length in 35 mm photography), and a 25 mm extension tube. The specimens were illuminated by a Falcon FLDM-i200 LED dome-light for even and soft light. Adobe DNG-format images were stacked using HeliconFocus software. Photographs of particular features were taken on a Zeiss SteREO Discovery.V12 stereo microscope and an attached Olympus PEN E-PL5 digital camera. All specimen photographs have been deposited in Morphbank:: Biological Imaging. These images can be automatically harvested by the Encyclopedia of Life (EOL) and are available under the respective species page.
The following data on species occurrences are given (where available): country, state/province, county, locality, geographic co-ordinates (formatted in both decimal and degrees minutes seconds latitude/longitude), elevation (in meters), date of collection (format: yyyy-mm-dd), habitat information, sampling protocol (if other than hand netting), collector, catalog number (a unique specimen number and any other identifying number), depository (institution and collection code), number of specimens and sex, life stage, name of person who identified the specimen, and any other previous identifications (note that for synonymized species the holotype still retains its status as a primary type specimen and therefore the particular material examined list will include two (or more) holotypes; see the entry under 'previousIdentifications' for the original identification by the author). Each specimen is listed with a unique specimen number (either an institutional catalog number or an AAM-XXXXXX number used by the senior author) that will allow the re-investigation as well as provide a unique Life Science Identifier (LSID). The occurrence of all species is illustrated in distribution maps plotted with SimpleMappr (
Institutions providing specimens
Institutions providing specimens are listed below, together with the abbreviations used in the text when citing depositories (institutionCode), a link to the record in the Global Registry of Biodiversity Repositories (GRBio), and the people who kindly assisted: AMGS – Albany Museum, Grahamstown, Eastern Cape, South Africa (A. Kirk-Spriggs, S. Gess); BMNH – The Natural History Museum, London, UK (E. McAlister); CSCA – California State Collection of Arthropods, Sacramento, California, USA (M. Hauser); INHS – Illinois Natural History Survey, Urbana-Champaign, Illinois, USA (D. Webb, M. Irwin); NMNW – National Museum of Namibia, Windhoek, Khomas, Namibia (A. Kirk-Spriggs); NMSA – KwaZulu-Natal Museum, Pietermaritzburg, KwaZulu-Natal, South Africa (B. Muller); SAMC – Iziko South African Museum, Cape Town, Western Cape, South Africa (M. Cochrane); SANC – South African National Collection of Insects, Pretoria, Gauteng, South Africa (R. Urban); TMSA – Ditsong National Museum of Natural History (formerly Transvaal Museum), Pretoria, Gauteng, South Africa (M. Krüger); USNM – National Museum of Natural History, Smithsonian Institution, Washington, DC, USA.
Data resources
- DRYAD: natural-language species descriptions from Lucid Builder in SDD format (also available as Suppl. material
1 ). - GBIF: specimen occurrence data – 5e6acf4c-e913-45fd-8466-5c0b92c322dd.
- Morphbank:: Biological Imaging: high-resolution photographs – 835218.
- SimpleMappr: distribution map – 2368 (as in figure 1) and in Google Earth as a KML file.
- Lucid Builder: illustrated, multi-entry, matrix-based identification key – asilodflies.si.edu and IdentifyLife.
- Lucid Phoenix: illustrated, dichotomous identification key – asilodflies.si.edu.
Taxon treatments
Namadytes
Nomenclature
Namadytes Hesse, 1969: 278.
Namamydas Hesse, 1969: 284 junior synonym sensu
Type species
Description
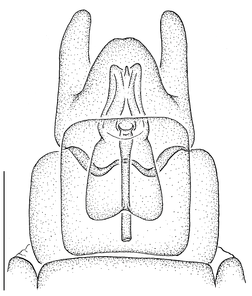
Female genitalia (Fig.
Male terminalia (e.g., Fig.
Diagnosis
The genus (Fig.
Distribution
Taxon discussion
Males are unique in the arrangement of their medially fused aedeagal prongs and the yellow to light brown abdominal coloration. Females in contrast are more generalized and similar to other female Mydidae occurring in southern Africa. However, the antero-median V-shaped indentation on the antepronotum and the presence of white setae on the infra-halter sclerite are relatively easy to observe and distinguish the females from other Mydidae. There is considerable sexual dimorphism and the setation, for example on the anatergites, is always easier to observe in males. Intra-specific variation in the abdominal coloration, especially in females, is likewise substantial, which probably led Hesse to describe a species twice.
Namadytes cimbebasiensis
Nomenclature
Namadytes cimbebasiensis Hesse, 1972: 143.
-
scientificName: Namadytes cimbebasiensis Hesse, 1972; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:cimbebasiensis; scientificNameAuthorship:Hesse, 1972; country:Namibia; stateProvince:Hardap; county:Maltahöhe; locality:Excelsior No. 127; verbatimCoordinates:25°24'00''S 016°12'00''E; decimalLatitude:-25.4; decimalLongitude:16.2; eventDate:1969-05-07; sex:1 male; lifeStage:Adult; catalogNumber:SAM-DIP-A007146; recordedBy:H. Brown; otherCatalogNumbers:AAM-000450; identifiedBy:A. Hesse; dateIdentified:1972; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes cimbebasiensis Hesse, 1972; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:cimbebasiensis; scientificNameAuthorship:Hesse, 1972; country:Namibia; stateProvince:Hardap; county:Maltahöhe; locality:Excelsior No. 127; verbatimCoordinates:25°24'00''S 016°12'00''E; decimalLatitude:-25.4; decimalLongitude:16.2; eventDate:1969-05-07; sex:1 female; lifeStage:Adult; catalogNumber:SAM-DIP-A007146; recordedBy:H. Brown; otherCatalogNumbers:AAM-000451; identifiedBy:A. Hesse; dateIdentified:1972; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes cimbebasiensis Hesse, 1972; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:cimbebasiensis; scientificNameAuthorship:Hesse, 1972; country:Namibia; stateProvince:Hardap; county:Maltahöhe; locality:Excelsior No. 127; verbatimCoordinates:25°24'00''S 016°12'00''E; decimalLatitude:-25.4; decimalLongitude:16.2; eventDate:1969-05-07; sex:1 male; lifeStage:Adult; catalogNumber:SAM-DIP-A007146; recordedBy:H. Brown; otherCatalogNumbers:AAM-000452; identifiedBy:A. Hesse; dateIdentified:1972; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes cimbebasiensis Hesse, 1972; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:cimbebasiensis; scientificNameAuthorship:Hesse, 1972; country:Namibia; stateProvince:Hardap; county:Maltahöhe; locality:Excelsior No. 127; verbatimCoordinates:25°24'00''S 016°12'00''E; decimalLatitude:-25.4; decimalLongitude:16.2; eventDate:1969-05-07; sex:1 male; lifeStage:Adult; catalogNumber:SAM-DIP-A007146; recordedBy:H. Brown; otherCatalogNumbers:AAM-000453; identifiedBy:A. Hesse; dateIdentified:1972; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes cimbebasiensis Hesse, 1972; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:cimbebasiensis; scientificNameAuthorship:Hesse, 1972; country:Namibia; stateProvince:Karas; county:Keetmanshoop; locality:Swartbaas West No. 276; verbatimCoordinates:27°00'16''S 019°41'08''E; decimalLatitude:-27.00444; decimalLongitude:19.68556; eventDate:1972-04-19–1972-04-22; sex:1 female; lifeStage:Adult; catalogNumber:NMNW-H7809; otherCatalogNumbers:AAM-003014; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMNW; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes cimbebasiensis Hesse, 1972; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:cimbebasiensis; scientificNameAuthorship:Hesse, 1972; country:Namibia; stateProvince:Karas; county:Keetmanshoop; locality:Swartbaas West No. 276; verbatimCoordinates:27°00'16''S 019°41'08''E; decimalLatitude:-27.00444; decimalLongitude:19.68556; eventDate:1972-04-19–1972-04-22; sex:1 male; lifeStage:Adult; catalogNumber:NMNW-H7808; otherCatalogNumbers:AAM-003013; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMNW; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes cimbebasiensis Hesse, 1972; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:cimbebasiensis; scientificNameAuthorship:Hesse, 1972; country:South Africa; stateProvince:Northern Cape; locality:Van Zylsrus, 90 km W; verbatimCoordinates:27°04'47''S 021°17'08''E; decimalLatitude:-27.07972; decimalLongitude:21.28556; eventDate:1990-03-26; sex:1 female; lifeStage:Adult; catalogNumber:AAM-003000; recordedBy:M. Schwarz; otherCatalogNumbers:AAM-003000; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:CSCA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes cimbebasiensis Hesse, 1972; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:cimbebasiensis; scientificNameAuthorship:Hesse, 1972; country:South Africa; stateProvince:Northern Cape; locality:58 km N on R360 Upington-Kgalagadi; verbatimCoordinates:27°59'22''S 020°59'51''E; decimalLatitude:-27.98944; decimalLongitude:20.9975; eventDate:2000-04-06; sex:1 male; lifeStage:Adult; catalogNumber:AAM-003021; recordedBy:F. and S. Gess; otherCatalogNumbers:AAM-003021; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:AMGS; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes cimbebasiensis Hesse, 1972; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:cimbebasiensis; scientificNameAuthorship:Hesse, 1972; country:South Africa; stateProvince:Northern Cape; locality:Kgalagadi Transfontier Park, Kalahari Tented Camp; verbatimElevation:947 m; verbatimCoordinates:25°47'08''S 020°01'01''E; decimalLatitude:-25.78556; decimalLongitude:20.01694; eventDate:2012-04-11; habitat:dune scrub; sex:1 male; lifeStage:Adult; catalogNumber:NMSA-DIP-66615; recordedBy:J. Londt; identifiedBy:T. Dikow; dateIdentified:2013; institutionCode:NMSA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes cimbebasiensis Hesse, 1972; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:cimbebasiensis; scientificNameAuthorship:Hesse, 1972; country:South Africa; stateProvince:Northern Cape; locality:Kgalagadi Transfontier Park, Twee Rivieren; verbatimElevation:864 m; verbatimCoordinates:26°28'27''S 020°36'46''E; decimalLatitude:-26.47417; decimalLongitude:20.61278; eventDate:2012-04-08–2012-04-14; habitat:Acacia savanna; sex:1 male; lifeStage:Adult; catalogNumber:NMSA-DIP-67240; recordedBy:J. and A. Londt; identifiedBy:T. Dikow; dateIdentified:2013; institutionCode:NMSA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
Description
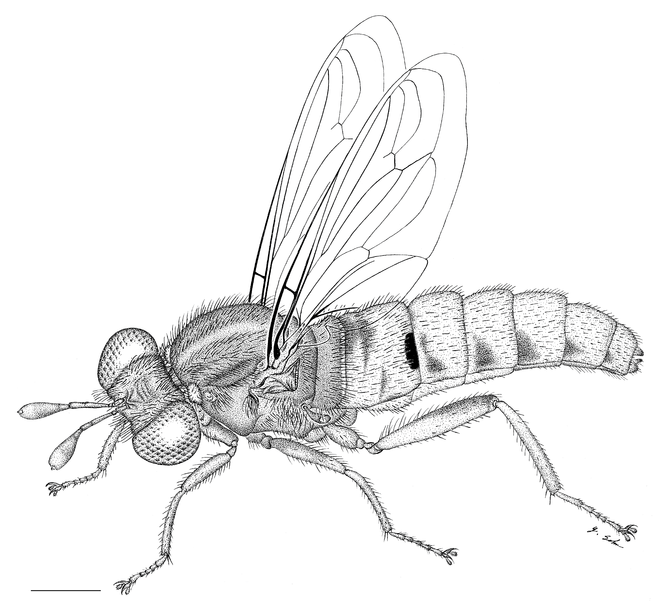
Male: Fig.
Head: brown, in general grey pubescent; width distinctly greater than thorax, interocular distance on vertex larger than at ventral eye margin, vertex between compound eyes ± horizontally straight, medially only slightly below dorsal eye margin, parafacial area about as wide as ½ the width of central facial gibbosity; facial gibbosity distinct, well-developed and discernible in lateral view; mystax yellow, covering entire facial gibbosity; frons not elevated, predominantly apubescent; vertex entirely grey pubescent; postgena lightly grey pubescent; setation: vertex yellow, frons white or yellow, ocp setae white, pocl macrosetae absent; ocellar triangle apubescent; proboscis light brown, very short, vestigial, knob-like; labellum small, as wide as prementum, as long as prementum, unsclerotized laterally; maxillary palpus cylindrical, yellow, minute.
Antenna: brown, scape and pedicel white setose dorsally and ventrally; postpedicel cylindrical in proximal ½, symmetrically bulbous in distal ½, ≥ 4.0 times as long as combined length of scape and pedicel, asetose; apical seta-like sensory element situated apically in cavity on postpedicel.
Thorax: brown, lightly grey pubescent; scutum uniformly black or uniformly brown, surface entirely smooth, lightly grey pubescent, broad sublateral stripes apubescent, scutal setation comprised of scattered long white to yellow setae; dc setae pre- and postsuturally white or yellow, acr setae absent, lateral scutal setae white or yellow, npl setae 0, spal setae 0, pal setae 0; antepronotum dorso-medially with V-shaped indentation; postpronotal lobe light brown, grey pubescent; proepisternum, lateral postpronotum, and postpronotal lobe long white setose; scutellum grey pubescent, asetose, apical scutellar setae absent; mesopostnotum, anatergite, and katatergite partly grey pubescent, mesopostnotum apubescent, mesopostnotum asetose, anatergite asetose, katatergite long white setose; katatergite ± flat; anterior anepisternum white setose, supero-posterior anepisternum long white setose; posterior anepimeron asetose, katepimeron asetose; metanepisternum grey pubescent, asetose, metepimeron ± flat, yellow, grey pubescent, long white setose; infra-halter sclerite asetose or white setose.
Leg: light brown, setation yellow; pro, mes, and met coxa lightly white pubescent, short yellow setose; met trochanter setose medially; femur brown or light brown, met femur ± cylindrical only slightly wider than pro and mes femur, in distal ½ macrosetose, 1 antero-ventral and 1 postero-ventral row of macrosetae, postero-ventrally regular setose only; pro, mes, and met tibia straight, met tibia cylindrical, ventral keel absent, latero-posteriorly regular setose only; pro and mes tarsomere 1 longer than tarsomere 2, but less than combined length of tarsomeres 2–3, met tarsomere 1 as long as combined length of tarsomeres 2–4; pulvillus well-developed, as long as well-developed claw, and as wide as base of claw; empodium absent.
Wing: length = 6.6–8.1 mm; hyaline throughout, veins light brown, microtrichia absent; cells r1, r4, r5, m3, + cup closed; C terminates at junction with R1; R4 terminates in R1; R5 terminates in R1; stump vein (R3) at base of R4 present, short not reaching R2; R4 and R5 widest apart medially; r-m distinct, R4+5 and M1 apart, connected by crossvein; M1 straight at r-m (not curving anteriorly), M1 (or M1+M2) terminates in R1; CuA1 and CuA2 split proximally to m-cu (cell m3 narrow proximally); M3+CuA1 do not terminate together in C; A1 undulating, cell a1 wide, A1 and wing margin further apart proximally than distally; alula well-developed; halter light yellow.
Abdomen: yellow to brown; setation comprised of dense white setae, surface entirely smooth; T1 brown, T2 brown anteriorly and postero-medially, otherwise yellow, T3–7 brown and yellow posteriorly; T1 and anterior ½ of T2 long white setose, remaining T short white setose; T predominantly apubescent; S1–7 light brown; S1 asetose, S2–7 sparsely white setose; S predominantly apubescent; T2–4 parallel-sided and not constricted waist-like; bullae on T2 black, transversely elongate, surface entirely smooth, T2 surface anterior to bullae smooth.
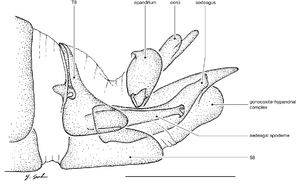
♂ terminalia: Fig.
Female: Fig.
Head: brown, facial gibbosity light brown; mystax white or yellow, sparsely covering entire facial gibbosity; setation: vertex white or yellow, pocl macrosetae light brown; maxillary palpus light brown.
Thorax: light brown, predominantly grey pubescent; scutum uniformly brown, predominantly brown pubescent, narrow sublateral stripes (wider anteriorly) and lateral and posterior margins grey pubescent, scutal setation comprised of scattered short yellow setae; proepisternum, lateral postpronotum, and postpronotal lobe short white setose; mesopostnotum, anatergite, and katatergite lightly grey pubescent, katatergite short white setose; anterior anepisternum white to yellow setose, supero-posterior anepisternum short white to yellow setose; metepimeron light brown; infra-halter sclerite asetose.
Leg: femur brown; pulvillus reduced, half length of well-developed claw.
Wing: length = 9.6–12.7 mm.
Abdomen: setation comprised of sparsely scattered short yellow setae; T1–7 brown, T2–6 light brown medially; T1–7 sparsely yellow setose; S1–7 brown; S1–7 sparsely short yellow setose; bullae on T2 oval.
♀ genitalia (Fig.
Diagnosis
This rather small species (wing length in males 6.6–8.1 mm and in females 9.6–12.7 mm) is distinguished from congeners by the entirely grey pubescent vertex, the short postpedicel (only about 4 times as long as combined length of scape and pedicel), the grey pubescent scutellum, and the few white setae on the infra-halter sclerite, which are absent in some females.
Distribution
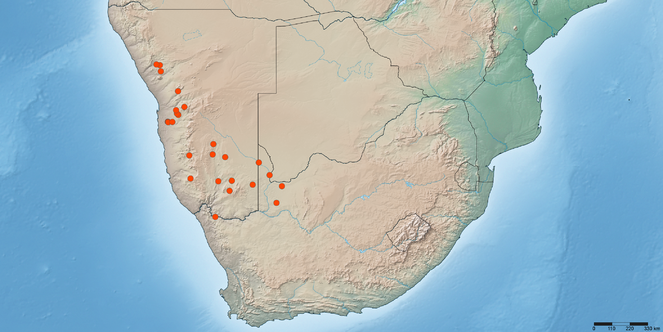
Namibia (Hardap, Karas) and South Africa (Northern Cape) (Fig.
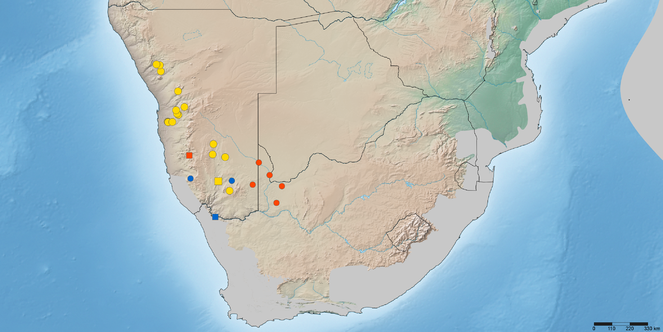
Map of southern Africa with elevational relief and biodiversity hotspots (in grey) showing distribution of Namadytes cimbebasiensis (red), N. maculiventris (blue), and N. vansoni (yellow). Map data available in Google Earth KML file and also through GBIF (data-set #5e6acf4c-e913-45fd-8466-5c0b92c322dd).
Biology
N. cimbebasiensis has recently been collected on dune scrub and in Acacia savanna in the Kgalagadi Transfrontier Park of the Kalahari Desert by J.G.H. Londt.
Taxon discussion
N. cimbebasiensis is very distinct and the smallest species of Namadytes. All specimens, originating from only five collecting events, are either greasy or are not in the best condition so that in particular the pubescence patterns might differ in freshly mounted material.
Type locality
Namibia: Hardab: Excelsior No. 127 (25°24'00''S, 016°12'00''E) (Fig.
Biodiversity hotspot
Not known to occur in any of the southern African biodiversity hotspots (Cape Floristic Region, Maputaland-Pondoland-Albany, or Succulent Karoo) (Fig.
Namadytes maculiventris
Nomenclature
Namamydas maculiventris Hesse, 1969: 285.
Namadytes maculiventris (Hesse, 1969) new combination sensu
Namadytes pallidus Hesse, 1972: 146 syn. nov. (ZooBank LSID).
-
scientificName: Namadytes maculiventris Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:maculiventris; scientificNameAuthorship:Hesse, 1969; country:South Africa; stateProvince:Northern Cape; locality:Vioolsdrift; verbatimCoordinates:28°46'10''S 017°37'37''E; decimalLatitude:-28.76944; decimalLongitude:17.62694; eventDate:1935-03-00; sex:1 male; lifeStage:Adult; catalogNumber:SAM-DIP-A007147; recordedBy:SAM Museum Staff; previousIdentifications:Namamydas maculiventris by A. Hesse in 1969; identifiedBy:A. Hesse; dateIdentified:1972; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes maculiventris Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:maculiventris; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Karas; locality:Keetmanshoop, 48 km SE; verbatimCoordinates:26°46'47''S 018°32'15''E; decimalLatitude:-26.77972; decimalLongitude:18.5375; eventDate:1968-10-30; sex:1 male; lifeStage:Adult; catalogNumber:SAM-DIP-A007148; recordedBy:J. Rozen E. Martinez; otherCatalogNumbers:AAM-000454; previousIdentifications:Namadytes pallidus by A. Hesse in 1972; identifiedBy:T. Dikow S. Leon; dateIdentified:2013; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes maculiventris Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:maculiventris; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Karas; locality:Keetmanshoop, 48 km SE; verbatimCoordinates:26°46'47''S 018°32'15''E; decimalLatitude:-26.77972; decimalLongitude:18.5375; eventDate:1968-10-30; sex:1 male; lifeStage:Adult; catalogNumber:SAM-DIP-A007148; recordedBy:J. Rozen E. Martinez; otherCatalogNumbers:AAM-000455; previousIdentifications:Namadytes pallidus by A. Hesse in 1972; identifiedBy:T. Dikow S. Leon; dateIdentified:2013; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes maculiventris Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:maculiventris; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Karas; locality:Aus; verbatimCoordinates:26°40'00''S 016°16'00''E; decimalLatitude:-26.66667; decimalLongitude:16.26667; eventDate:1990-02-11; sex:1 male; lifeStage:Adult; catalogNumber:USNMENT00779997; recordedBy:M. Schwarz; identifiedBy:T. Dikow S. Leon; dateIdentified:2013; institutionCode:USNM; collectionCode:Entomology; basisOfRecord:PreservedSpecimen
Description
Male: Fig.
Head: brown, facial gibbosity light brown, in general densely grey pubescent; width distinctly greater than thorax, interocular distance on vertex larger than at ventral eye margin, vertex between compound eyes ± horizontally straight, medially only slightly below dorsal eye margin, parafacial area about as wide as ½ the width of central facial gibbosity; facial gibbosity distinct, well-developed and discernible in lateral view; mystax white, densely covering entire facial gibbosity; frons not elevated, predominantly apubescent; vertex entirely white pubescent; postgena white pubescent; setation: vertex white, frons white, ocp setae white, pocl macrosetae white; ocellar triangle apubescent; proboscis yellow, very short, vestigial, knob-like; labellum small, as wide as prementum, as long as prementum, unsclerotized laterally; maxillary palpus cylindrical, yellow, minute.
Antenna: brown, scape and pedicel white setose dorsally and ventrally; postpedicel cylindrical in proximal ½, symmetrically bulbous in distal ½, ≥ 7.0 times as long as combined length of scape and pedicel, asetose; apical seta-like sensory element situated apically in cavity on postpedicel.
Thorax: brown, lightly grey pubescent; scutum predominantly black, anteriorly and laterally yellow to light brown, surface entirely smooth, lightly grey pubescent, scutal setation comprised of long white setae with distinct rows of long dorsocentral setae and dense lateral scutal setae; dc setae pre- and postsuturally white, acr setae absent, lateral scutal setae white, npl setae 0, spal setae 0, pal setae 0; antepronotum dorso-medially with V-shaped indentation; postpronotal lobe yellow, white pubescent; proepisternum, lateral postpronotum, and postpronotal lobe long white setose; scutellum apubescent, asetose, apical scutellar setae absent; mesopostnotum, anatergite, and katatergite grey pubescent, mesopostnotum asetose, anatergite asetose, katatergite long white setose; katatergite ± flat; anterior anepisternum white setose, supero-posterior anepisternum long white setose; posterior anepimeron asetose, katepimeron asetose; metanepisternum grey pubescent, asetose, metepimeron ± flat, yellow, grey pubescent, long white setose; infra-halter sclerite white setose.
Leg: yellow to light brown, setation predominantly white; pro, mes, and met coxa lightly white pubescent, long white setose; met trochanter setose medially; femur yellow to light brown, met femur ± cylindrical only slightly wider than pro and mes femur, in distal ½ macrosetose, 1 antero-ventral and 1 postero-ventral row of macrosetae, postero-ventrally long white, erect setose with setae arranged in distinct row; pro, mes, and met tibia straight, met tibia cylindrical, ventral keel absent, latero-posteriorly long white, erect setose with setae arranged in distinct row; pro and mes tarsomere 1 longer than tarsomere 2, but less than combined length of tarsomeres 2–3, met tarsomere 1 as long as combined length of tarsomeres 2–4; pulvillus well-developed, as long as well-developed claw, and as wide as base of claw; empodium absent.
Wing: length = 9.6–12.2 mm; hyaline throughout, veins light brown, microtrichia absent; cells r1, r4, r5, m3, + cup closed; C terminates at junction with R1; R4 terminates in R1; R5 terminates in R1; stump vein (R3) at base of R4 present, short not reaching R2; R4 and R5 widest apart medially; r-m distinct, R4+5 and M1 apart, connected by crossvein; M1 straight at r-m (not curving anteriorly), M1 (or M1+M2) terminates in R1; CuA1 and CuA2 split proximally to m-cu (cell m3 narrow proximally); M3+CuA1 do not terminate together in C; A1 undulating, cell a1 wide, A1 and wing margin further apart proximally than distally; alula well-developed; halter light yellow.
Abdomen: yellow to brown; setation comprised of dense white setae, surface entirely smooth; T1–2 anteriorly yellow otherwise brown, T3 antero-medially brown otherwise yellow, T4–7 yellow to light brown; T1 and anterior ½ of T2 long white setose, remaining T short white setose; T predominantly apubescent; S1–7 yellow; S1–7 short white setose; S predominantly apubescent; T2–4 parallel-sided and not constricted waist-like; bullae on T2 black, transversely elongate, surface entirely smooth, T2 surface anterior to bullae smooth.
♂ terminalia: Fig.
Female: unknown.
Diagnosis
This large species (wing length in males 9.6–12.2 mm, females unknown) is distinguished from congeners by the entirely white pubescent vertex and postgena, by the long postpedicel (about 7 times as long as combined length of scape and pedicel), the long white scutal setation, the yellow postpronotal lobes, the densely grey pubescent mesopostnotum, anatergite, and katatergite, the yellow to light brown coloration of the legs, and the long, erect white setae dorsally on the metathoracic femur.
Distribution
Namibia (Karas) and South Africa (Northern Cape) (Fig.
Taxon discussion
Type locality
South Africa: Northern Cape: Vioolsdrift (28°46'10''S, 17°37'37''E) (Fig.
Biodiversity hotspot
Not known to occur in any of the southern African biodiversity hotspots (Cape Floristic Region, Maputaland-Pondoland-Albany, or Succulent Karoo) (Fig.
Namadytes vansoni
Nomenclature
Namadytes vansoni Hesse, 1969: 280.
Namadytes prozeskyi Hesse, 1969: 282 syn. nov. (ZooBank LSID)
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Karas; locality:Seeheim; verbatimCoordinates:26°48'53''S 017°47'57''E; decimalLatitude:-26.81472; decimalLongitude:17.79917; eventDate:1933-05-00; sex:1 female; lifeStage:Adult; catalogNumber:TMSA-Dip34; recordedBy:G. van Son; otherCatalogNumbers:AAM-000456; identifiedBy:A. Hesse; dateIdentified:1969; institutionCode:TMSA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Arechadamab, Game Reserve No. 3 (= Namib Naukluft Park); verbatimCoordinates:23°10'00''S 015°36'00''E; decimalLatitude:-23.16667; decimalLongitude:15.6; eventDate:1959-10-11; sex:1 female; lifeStage:Adult; catalogNumber:TMSA-Dip35; recordedBy:O. Prozesky; otherCatalogNumbers:AAM-000457; previousIdentifications:Namadytes prozeskyi by A. Hesse in 1969; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:TMSA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Duineveld No. 529, SW Khorixas; verbatimCoordinates:20°47'00''S 014°38'00''E; decimalLatitude:-20.78333; decimalLongitude:14.63333; eventDate:1978-05-14; sex:1 male; lifeStage:Adult; catalogNumber:SAM-DIP-A012469; recordedBy:V. Whitehead; otherCatalogNumbers:AAM-003049; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Rooiberg No. 517, W Khorixas; verbatimCoordinates:20°27'00''S 014°35'00''E; decimalLatitude:-20.45; decimalLongitude:14.58333; eventDate:1978-05-12; sex:1 male; lifeStage:Adult; catalogNumber:SAM-DIP-A012479; recordedBy:V. Whitehead; otherCatalogNumbers:AAM-003037; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Kuiseb Namib; verbatimCoordinates:23°32'33''S 015°01'18''E; decimalLatitude:-23.5425; decimalLongitude:15.02167; eventDate:1959-05-04; sex:1 female; lifeStage:Adult; catalogNumber:AAM-000672; recordedBy:H. Brown; otherCatalogNumbers:AAM-000672; previousIdentifications:Namadytes prozeskyi by J. Bowden in; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:BMNH; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Namib-Naukluft Park, Namib Desert Research Station, Kuiseb River; verbatimElevation:420 m; verbatimCoordinates:23°33'45''S 015°02'38''E; decimalLatitude:-23.5625; decimalLongitude:15.04389; samplingProtocol:Malaise trap; eventDate:1997-03-14–1997-03-26; habitat:riparian vegetation; sex:1 male; lifeStage:Adult; catalogNumber:INHS-503368; recordedBy:I. Kapofi M. Irwin; previousIdentifications:Namadytes maculiventris by B. Kondratieff in 2000; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:INHS; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Namib-Naukluft Park, Namib Desert Research Station, Kuiseb River; verbatimElevation:420 m; verbatimCoordinates:23°33'45''S 015°02'38''E; decimalLatitude:-23.5625; decimalLongitude:15.04389; samplingProtocol:Malaise trap; eventDate:1997-03-05–1997-03-14; habitat:riparian vegetation; sex:1 male; lifeStage:Adult; catalogNumber:INHS-503373; recordedBy:I. Kapofi M. Irwin; previousIdentifications:Namadytes maculiventris by B. Kondratieff in 2000; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:INHS; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Gobabeb, Kuiseb River; verbatimCoordinates:23°33'37''S 015°02'26''E; decimalLatitude:-23.56028; decimalLongitude:15.04056; eventDate:1979-06-10; sex:1 female; lifeStage:Adult; catalogNumber:AAM-002824; recordedBy:R. Wharton; otherCatalogNumbers:AAM-002824; previousIdentifications:Namadytes prozeskyi by R. Wharton in 1979; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMSA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Gobabeb, Kuiseb River; verbatimCoordinates:23°33'37''S 015°02'26''E; decimalLatitude:-23.56028; decimalLongitude:15.04056; eventDate:1979-06-12; sex:1 male; lifeStage:Adult; catalogNumber:AAM-002825; recordedBy:R. Wharton; otherCatalogNumbers:AAM-002825; previousIdentifications:Namadytes prozeskyi by R. Wharton in 1979; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMSA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Gobabeb, Kuiseb River; verbatimCoordinates:23°33'37''S 015°02'26''E; decimalLatitude:-23.56028; decimalLongitude:15.04056; eventDate:1979-06-09; sex:1 female; lifeStage:Adult; catalogNumber:AAM-002827; recordedBy:R. Wharton; otherCatalogNumbers:AAM-002827; previousIdentifications:Namadytes prozeskyi by R. Wharton in 1979; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMSA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Gobabeb; verbatimCoordinates:23°33'37''S 015°02'26''E; decimalLatitude:-23.56028; decimalLongitude:15.04056; eventDate:1979-05-11; sex:1 male; lifeStage:Adult; catalogNumber:AAM-002828; recordedBy:R. Wharton; otherCatalogNumbers:AAM-002828; previousIdentifications:Namadytes prozeskyi by R. Wharton in 1979; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMSA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Namib-Naukluft Park, Namib Desert Research Station, Kuiseb River; verbatimElevation:420 m; verbatimCoordinates:23°33'45''S 015°02'38''E; decimalLatitude:-23.5625; decimalLongitude:15.04389; samplingProtocol:Malaise trap; eventDate:1997-03-14–1997-03-26; habitat:riparian vegetation; sex:1 male; lifeStage:Adult; catalogNumber:INHS-503356; recordedBy:I. Kapofi M. Irwin; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:INHS; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Namib-Naukluft Park, Namib Desert Research Station, Kuiseb River; verbatimElevation:420 m; verbatimCoordinates:23°33'45''S 015°02'38''E; decimalLatitude:-23.5625; decimalLongitude:15.04389; samplingProtocol:Malaise trap; eventDate:1997-03-26–1997-04-02; habitat:riparian vegetation; sex:1 male; lifeStage:Adult; catalogNumber:INHS-503365; recordedBy:I. Kapofi M. Irwin; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:INHS; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Namib-Naukluft Park, Namib Desert Research Station, Kuiseb River; verbatimElevation:420 m; verbatimCoordinates:23°33'45''S 015°02'38''E; decimalLatitude:-23.5625; decimalLongitude:15.04389; samplingProtocol:Malaise trap; eventDate:1997-04-21–1997-04-28; habitat:riparian vegetation; sex:1 female; lifeStage:Adult; catalogNumber:INHS-503360; recordedBy:I. Kapofi M. Irwin; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:INHS; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Namib-Naukluft Park, Namib Desert Research Station, Kuiseb River; verbatimElevation:420 m; verbatimCoordinates:23°33'45''S 015°02'38''E; decimalLatitude:-23.5625; decimalLongitude:15.04389; samplingProtocol:Malaise trap; eventDate:1997-04-09–1997-04-21; habitat:riparian vegetation; sex:1 male; lifeStage:Adult; catalogNumber:INHS-503359; recordedBy:I. Kapofi M. Irwin; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:INHS; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Namib-Naukluft Park, Namib Desert Research Station, Kuiseb River; verbatimElevation:420 m; verbatimCoordinates:23°33'45''S 015°02'38''E; decimalLatitude:-23.5625; decimalLongitude:15.04389; samplingProtocol:Malaise trap; eventDate:1997-02-26–1997-03-05; habitat:riparian vegetation; sex:1 male; lifeStage:Adult; catalogNumber:INHS-503364; recordedBy:I. Kapofi M. Irwin; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:INHS; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Ganab, Game Reserve No. 3 (= Namib Naukluft Park); verbatimCoordinates:23°06'10''S 015°31'45''E; decimalLatitude:-23.10278; decimalLongitude:15.52917; eventDate:1967-04-21; sex:1 female; lifeStage:Adult; catalogNumber:AAM-002985; recordedBy:J. Potgieter; otherCatalogNumbers:AAM-002985; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMSA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Windhoek, 158 km W; verbatimCoordinates:22°44'21''S 015°55'57''E; decimalLatitude:-22.73917; decimalLongitude:15.9325; eventDate:1983-04-22; habitat:thornveld in dry river bed; sex:1 male; lifeStage:Adult; catalogNumber:AAM-002986; recordedBy:J. Londt B. Stuckenberg; otherCatalogNumbers:AAM-002986; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMSA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Swakopmund, 110 km E; verbatimCoordinates:22°55'01''S 015°28'12''E; decimalLatitude:-22.91694; decimalLongitude:15.47; eventDate:1983-04-22; habitat:barren gravel plain; sex:1 female; lifeStage:Adult; catalogNumber:AAM-002989; recordedBy:B. Stuckenberg J. Londt; otherCatalogNumbers:AAM-002989; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMSA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Namib-Naukluft Park, Namib Desert Research Station, Kuiseb River; verbatimElevation:420 m; verbatimCoordinates:23°33'45''S 015°02'38''E; decimalLatitude:-23.5625; decimalLongitude:15.04389; samplingProtocol:Malaise trap; eventDate:1997-03-05–1997-03-14; habitat:riparian vegetation; sex:1 male; lifeStage:Adult; catalogNumber:AAM-002997; recordedBy:I. Kapofi M. Irwin; otherCatalogNumbers:AAM-002997; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:CSCA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Namib-Naukluft Park, Namib Desert Research Station, Kuiseb River; verbatimElevation:420 m; verbatimCoordinates:23°33'45''S 015°02'38''E; decimalLatitude:-23.5625; decimalLongitude:15.04389; samplingProtocol:Malaise trap; eventDate:1997-04-21–1997-04-28; habitat:riparian vegetation; sex:1 female; lifeStage:Adult; catalogNumber:AAM-002999; recordedBy:I. Kapofi M. Irwin; otherCatalogNumbers:AAM-002999; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:CSCA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Outjo, Bethanis No. 514; verbatimCoordinates:20°24'00''S 014°24'00''E; decimalLatitude:-20.4; decimalLongitude:14.4; eventDate:1973-05-08–1973-05-10; sex:1 female; lifeStage:Adult; catalogNumber:NMNW-H12725; otherCatalogNumbers:AAM-003007; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMNW; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Karas; county:Keetmanshoop; locality:Rotegab No. 95; verbatimCoordinates:27°20'00''S 018°25'00''E; decimalLatitude:-27.33333; decimalLongitude:18.41667; eventDate:1972-04-27; sex:1 female; lifeStage:Adult; catalogNumber:NMNW-H8279; otherCatalogNumbers:AAM-003009; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMNW; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Karas; county:Namaland; locality:Mukorob No. 14; verbatimCoordinates:25°29'00''S 018°10'00''E; decimalLatitude:-25.48333; decimalLongitude:18.16667; eventDate:1974-04-12–1974-04-14; sex:1 male; lifeStage:Adult; catalogNumber:NMNW-H18282; otherCatalogNumbers:AAM-003011; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMNW; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Damaraland, Duineveld No. 529; verbatimCoordinates:20°47'00''S 014°38'00''E; decimalLatitude:-20.78333; decimalLongitude:14.63333; eventDate:1978-05-14–1978-05-16; sex:1 female; lifeStage:Adult; catalogNumber:NMNW-H36196; recordedBy:M.-L. Penrith S. Louw; otherCatalogNumbers:AAM-003015; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMNW; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Karas; locality:Gibeon, 41 km SW on 1089; verbatimCoordinates:25°20'00''S 017°29'00''E; decimalLatitude:-25.33333; decimalLongitude:17.48333; eventDate:1999-03-24; sex:1 male; lifeStage:Adult; catalogNumber:AAM-003019; recordedBy:F. and S. Gess; otherCatalogNumbers:AAM-003019; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:AMGS; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Usakos, Phillips Caves; verbatimCoordinates:21°52'16''S 015°35'18''E; decimalLatitude:-21.87111; decimalLongitude:15.58833; eventDate:2002-04-23; sex:1 male; lifeStage:Adult; catalogNumber:AAM-003020; recordedBy:F. and S. Gess; otherCatalogNumbers:AAM-003020; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:AMGS; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Namib-Naukluft Park, Kuiseb River near Gobabeb; verbatimCoordinates:23°34'00''S 015°03'00''E; decimalLatitude:-23.56667; decimalLongitude:15.05; samplingProtocol:Malaise trap; eventDate:1983-02-18–1983-03-20; sex:1 male; lifeStage:Adult; catalogNumber:AAM-003058; recordedBy:National Collection Kuiseb Survey; otherCatalogNumbers:AAM-003058; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SANC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Mariental, 52 km W; verbatimCoordinates:24°46'35''S 017°31'13''E; decimalLatitude:-24.77639; decimalLongitude:17.52028; eventDate:1983-03-27; sex:1 male; lifeStage:Adult; catalogNumber:AAM-003059; recordedBy:C. Eardley; otherCatalogNumbers:AAM-003059; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SANC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Hope Mine, 48 km N, Kuiseb River; verbatimCoordinates:23°33'56''S 015°16'16''E; decimalLatitude:-23.56556; decimalLongitude:15.27111; eventDate:1959-05-11; sex:1 female; lifeStage:Adult; catalogNumber:SAM-DIP-A007149; recordedBy:H. Brown; otherCatalogNumbers:AAM-002909; previousIdentifications:Namadytes prozeskyi by A. Hesse in 1969; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Outjo, Bethanis No. 514; verbatimCoordinates:20°24'00''S 014°24'00''E; decimalLatitude:-20.4; decimalLongitude:14.4; eventDate:1973-05-08–1973-05-10; sex:1 female; lifeStage:Adult; catalogNumber:NMNW-H12726; otherCatalogNumbers:AAM-003008; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMNW; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Outjo, Bethanis No. 514; verbatimCoordinates:20°24'00''S 014°24'00''E; decimalLatitude:-20.4; decimalLongitude:14.4; eventDate:1973-05-08–1973-05-10; sex:1 male; lifeStage:Adult; catalogNumber:NMNW-H12727; otherCatalogNumbers:AAM-003006; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMNW; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Namib-Naukluft Park, Namib Desert Research Station, Kuiseb River; verbatimElevation:420 m; verbatimCoordinates:23°33'45''S 015°02'38''E; decimalLatitude:-23.5625; decimalLongitude:15.04389; samplingProtocol:Malaise trap; eventDate:1997-03-14–1997-03-26; habitat:riparian vegetation; sex:1 male; lifeStage:Adult; catalogNumber:INHS-503370; recordedBy:I. Kapofi M. Irwin; previousIdentifications:Namadytes maculiventris by B. Kondratieff in 2000; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:INHS; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Namib-Naukluft Park, Namib Desert Research Station, Kuiseb River; verbatimElevation:420 m; verbatimCoordinates:23°33'45''S 015°02'38''E; decimalLatitude:-23.5625; decimalLongitude:15.04389; samplingProtocol:Malaise trap; eventDate:1997-03-14–1997-03-26; habitat:riparian vegetation; sex:1 male; lifeStage:Adult; catalogNumber:INHS-503371; recordedBy:I. Kapofi M. Irwin; previousIdentifications:Namadytes maculiventris by B. Kondratieff in 2000; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:INHS; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Namib-Naukluft Park, Namib Desert Research Station, Kuiseb River; verbatimElevation:420 m; verbatimCoordinates:23°33'45''S 015°02'38''E; decimalLatitude:-23.5625; decimalLongitude:15.04389; samplingProtocol:Malaise trap; eventDate:1997-03-14–1997-03-26; habitat:riparian vegetation; sex:1 male; lifeStage:Adult; catalogNumber:INHS-503369; recordedBy:I. Kapofi M. Irwin; previousIdentifications:Namadytes maculiventris by B. Kondratieff in 2000; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:INHS; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Namib-Naukluft Park, Namib Desert Research Station, Kuiseb River; verbatimElevation:420 m; verbatimCoordinates:23°33'45''S 015°02'38''E; decimalLatitude:-23.5625; decimalLongitude:15.04389; samplingProtocol:Malaise trap; eventDate:1997-03-05–1997-03-14; habitat:riparian vegetation; sex:1 male; lifeStage:Adult; catalogNumber:INHS-503372; recordedBy:I. Kapofi M. Irwin; previousIdentifications:Namadytes maculiventris by B. Kondratieff in 2000; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:INHS; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Namib-Naukluft Park, Namib Desert Research Station, Kuiseb River; verbatimElevation:420 m; verbatimCoordinates:23°33'45''S 015°02'38''E; decimalLatitude:-23.5625; decimalLongitude:15.04389; samplingProtocol:Malaise trap; eventDate:1997-03-05–1997-03-14; habitat:riparian vegetation; sex:1 male; lifeStage:Adult; catalogNumber:AAM-002998; recordedBy:I. Kapofi M. Irwin; otherCatalogNumbers:AAM-002998; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:CSCA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Duineveld No. 529, SW Khorixas; verbatimCoordinates:20°47'00''S 014°38'00''E; decimalLatitude:-20.78333; decimalLongitude:14.63333; eventDate:1978-05-14; sex:1 male; lifeStage:Adult; catalogNumber:SAM-DIP-A012469; recordedBy:V. Whitehead; otherCatalogNumbers:AAM-003043; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Duineveld No. 529, SW Khorixas; verbatimCoordinates:20°47'00''S 014°38'00''E; decimalLatitude:-20.78333; decimalLongitude:14.63333; eventDate:1978-05-14; sex:1 male; lifeStage:Adult; catalogNumber:SAM-DIP-A012467; recordedBy:V. Whitehead; otherCatalogNumbers:AAM-003046; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Duineveld No. 529, SW Khorixas; verbatimCoordinates:20°47'00''S 014°38'00''E; decimalLatitude:-20.78333; decimalLongitude:14.63333; eventDate:1978-05-14; sex:1 male; lifeStage:Adult; catalogNumber:SAM-DIP-A012469; recordedBy:V. Whitehead; otherCatalogNumbers:AAM-003048; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Duineveld No. 529, SW Khorixas; verbatimCoordinates:20°47'00''S 014°38'00''E; decimalLatitude:-20.78333; decimalLongitude:14.63333; eventDate:1978-05-14; sex:1 male; lifeStage:Adult; catalogNumber:SAM-DIP-A012469; recordedBy:V. Whitehead; otherCatalogNumbers:AAM-003047; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Duineveld No. 529, SW Khorixas; verbatimCoordinates:20°47'00''S 014°38'00''E; decimalLatitude:-20.78333; decimalLongitude:14.63333; eventDate:1978-05-14; sex:1 female; lifeStage:Adult; catalogNumber:SAM-DIP-A012470; recordedBy:V. Whitehead; otherCatalogNumbers:AAM-003050; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Duineveld No. 529, SW Khorixas; verbatimCoordinates:20°47'00''S 014°38'00''E; decimalLatitude:-20.78333; decimalLongitude:14.63333; eventDate:1978-05-14; sex:1 female; lifeStage:Adult; catalogNumber:SAM-DIP-A012468; recordedBy:V. Whitehead; otherCatalogNumbers:AAM-003041; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Duineveld No. 529, SW Khorixas; verbatimCoordinates:20°47'00''S 014°38'00''E; decimalLatitude:-20.78333; decimalLongitude:14.63333; eventDate:1978-05-14; sex:1 female; lifeStage:Adult; catalogNumber:SAM-DIP-A012470; recordedBy:V. Whitehead; otherCatalogNumbers:AAM-003042; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Duineveld No. 529, SW Khorixas; verbatimCoordinates:20°47'00''S 014°38'00''E; decimalLatitude:-20.78333; decimalLongitude:14.63333; eventDate:1978-05-14; sex:1 female; lifeStage:Adult; catalogNumber:SAM-DIP-A012470; recordedBy:V. Whitehead; otherCatalogNumbers:AAM-003044; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Duineveld No. 529, SW Khorixas; verbatimCoordinates:20°47'00''S 014°38'00''E; decimalLatitude:-20.78333; decimalLongitude:14.63333; eventDate:1978-05-14; sex:1 female; lifeStage:Adult; catalogNumber:SAM-DIP-A012470; recordedBy:V. Whitehead; otherCatalogNumbers:AAM-003051; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Duineveld No. 529, SW Khorixas; verbatimCoordinates:20°47'00''S 014°38'00''E; decimalLatitude:-20.78333; decimalLongitude:14.63333; eventDate:1978-05-14; sex:1 female; lifeStage:Adult; catalogNumber:SAM-DIP-A012468; recordedBy:V. Whitehead; otherCatalogNumbers:AAM-003045; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Rooiberg No. 517, W Khorixas; verbatimCoordinates:20°27'00''S 014°35'00''E; decimalLatitude:-20.45; decimalLongitude:14.58333; eventDate:1978-05-14; sex:1 male; lifeStage:Adult; catalogNumber:SAM-DIP-A012474; recordedBy:V. Whitehead; otherCatalogNumbers:AAM-003040; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Rooiberg No. 517, W Khorixas; verbatimCoordinates:20°27'00''S 014°35'00''E; decimalLatitude:-20.45; decimalLongitude:14.58333; eventDate:1978-05-14; sex:1 male; lifeStage:Adult; catalogNumber:SAM-DIP-A012479; recordedBy:V. Whitehead; otherCatalogNumbers:AAM-003038; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Kunene; locality:Rooiberg No. 517, W Khorixas; verbatimCoordinates:20°27'00''S 014°35'00''E; decimalLatitude:-20.45; decimalLongitude:14.58333; eventDate:1978-05-14; sex:1 female; lifeStage:Adult; catalogNumber:SAM-DIP-A012473; recordedBy:V. Whitehead; otherCatalogNumbers:AAM-003039; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:SAMC; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Karas; county:Namaland; locality:Mukorob No. 14; verbatimCoordinates:25°29'00''S 018°10'00''E; decimalLatitude:-25.48333; decimalLongitude:18.16667; eventDate:1974-04-12–1974-04-14; sex:1 female; lifeStage:Adult; catalogNumber:NMNW-H18283; otherCatalogNumbers:AAM-003012; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMNW; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Karas; county:Namaland; locality:Mukorob No. 14; verbatimCoordinates:25°29'00''S 018°10'00''E; decimalLatitude:-25.48333; decimalLongitude:18.16667; eventDate:1974-04-12–1974-04-14; sex:1 male; lifeStage:Adult; catalogNumber:NMNW-H18282; otherCatalogNumbers:AAM-003010; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMNW; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Windhoek, 158 km W; verbatimCoordinates:22°44'21''S 015°55'57''E; decimalLatitude:-22.73917; decimalLongitude:15.9325; eventDate:1983-04-22; habitat:thornveld in dry river bed; sex:1 male; lifeStage:Adult; catalogNumber:AAM-002987; recordedBy:J. Londt B. Stuckenberg; otherCatalogNumbers:AAM-002987; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMSA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Windhoek, 158 km W; verbatimCoordinates:22°44'21''S 015°55'57''E; decimalLatitude:-22.73917; decimalLongitude:15.9325; eventDate:1983-04-22; habitat:thornveld in dry river bed; sex:1 male; lifeStage:Adult; catalogNumber:AAM-002988; recordedBy:J. Londt B. Stuckenberg; otherCatalogNumbers:AAM-002988; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMSA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
-
scientificName: Namadytes vansoni Hesse, 1969; scientificNameID: ; family:Mydidae; genus:Namadytes; specificEpithet:vansoni; scientificNameAuthorship:Hesse, 1969; country:Namibia; stateProvince:Erongo; locality:Gobabeb, Kuiseb River; verbatimCoordinates:23°33'37''S 015°02'26''E; decimalLatitude:-23.56028; decimalLongitude:15.04056; eventDate:1979-12-06; sex:1 male; lifeStage:Adult; catalogNumber:AAM-002826; recordedBy:R. Wharton; otherCatalogNumbers:AAM-002826; previousIdentifications:Namadytes prozeskyi by R. Wharton in 1979; identifiedBy:T. Dikow S. Leon; dateIdentified:2012; institutionCode:NMSA; collectionCode:Insects; basisOfRecord:PreservedSpecimen
Description
Male: Fig.
Head: brown, in general lightly silver pubescent; width distinctly greater than thorax, interocular distance on vertex larger than at ventral eye margin, vertex between compound eyes ± horizontally straight, medially only slightly below dorsal eye margin, parafacial area about as wide as ½ the width of central facial gibbosity; facial gibbosity distinct, well-developed and discernible in lateral view; mystax white, densely covering entire facial gibbosity; frons not elevated, predominantly apubescent; vertex predominantly apubescent, only lateral margin grey pubescent; postgena lightly grey pubescent; setation: vertex white, frons white, ocp setae white, pocl macrosetae absent; ocellar triangle apubescent; proboscis brown, short, about ½ length of oral cavity; labellum small, as wide as prementum, as long as prementum, unsclerotized laterally; maxillary palpus cylindrical, light brown, minute.
Antenna: brown, scape and pedicel white setose dorsally and ventrally; postpedicel cylindrical in proximal ½, symmetrically bulbous in distal ½, ≥ 7.0 times as long as combined length of scape and pedicel, asetose; apical seta-like sensory element situated apically in cavity on postpedicel.
Thorax: brown, lightly grey pubescent; scutum uniformly brown, surface entirely smooth, apubescent, scutal setation comprised of long white setae with distinct rows of long dorsocentral setae and dense lateral scutal setae; dc setae pre- and postsuturally white, acr setae absent, lateral scutal setae white, npl setae 0, spal setae 0, pal setae 0; antepronotum dorso-medially with V-shaped indentation; postpronotal lobe light brown, grey pubescent; proepisternum, lateral postpronotum, and postpronotal lobe long white setose; scutellum apubescent, asetose, apical scutellar setae absent; mesopostnotum, anatergite, and katatergite lightly grey pubescent, mesopostnotum asetose, anatergite long white setose, katatergite long white setose; katatergite ± flat; anterior anepisternum white setose, supero-posterior anepisternum long white setose; posterior anepimeron long white setose, katepimeron long white setose; metanepisternum grey pubescent, asetose, metepimeron ± flat, yellow, grey pubescent, long white setose; infra-halter sclerite white setose.
Leg: light brown to brown, setation predominantly white; pro, mes, and met coxa lightly white pubescent, long white setose; met trochanter setose medially; femur light brown to brown, met femur evenly clubbed in distal 3/4, in distal ½ macrosetose, 1 antero-ventral and 1 postero-ventral row of macrosetae, postero-ventrally long white, erect setose proximally with setae arranged in distinct row; pro, mes, and met tibia straight, met tibia cylindrical, ventral keel absent, latero-posteriorly long white, erect setose with setae arranged in distinct row; pro and mes tarsomere 1 longer than tarsomere 2, but less than combined length of tarsomeres 2–3, met tarsomere 1 as long as combined length of tarsomeres 2–4; pulvillus well-developed, as long as well-developed claw, and as wide as base of claw; empodium absent.
Wing: length = 7.1–8.9 mm; hyaline throughout, veins brown, microtrichia absent; cells r1, r4, r5, m3, + cup closed except r5 open; C terminates at junction with M1 (or M1+M2); R4 terminates in R1; R5 terminates in R1; stump vein (R3) at base of R4 present, short not reaching R2; R4 and R5 widest apart medially; r-m distinct, R4+5 and M1 apart, connected by crossvein; M1 straight at r-m (not curving anteriorly), M1 (or M1+M2) terminates in C; CuA1 and CuA2 split proximally to m-cu (cell m3 narrow proximally); M3+CuA1 do not terminate together in C; A1 undulating, cell a1 wide, A1 and wing margin further apart proximally than distally; alula well-developed; halter light brown.
Abdomen: yellow to brown; setation comprised of dense white setae, surface entirely smooth; T1 brown, T2 predominantly yellow with brown medially and antero-laterally, T3–7 yellow with brown antero-laterally; T1 and anterior ½ of T2 long white setose, remaining T short white setose; T predominantly apubescent; S1–7 light brown; S1–7 short white setose; S predominantly apubescent; T2–4 parallel-sided and not constricted waist-like; bullae on T2 black, transversely elongate, surface entirely smooth, T2 surface anterior to bullae smooth.
♂ terminalia: Fig.
Female: Fig.
Head: mystax white, covering entire facial gibbosity, sparse; pocl macrosetae white.
Antenna: postpedicel ≥ 5.0–≥ 6.0 times as long as combined length of scape and pedicel.
Thorax: scutum predominantly brown pubescent, narrow sublateral stripes (wider anteriorly) and lateral and posterior margins grey pubescent, scutal setation comprised of scattered short white setae; scutellum grey pubescent proximally, apubescent distally; supero-posterior anepisternum short white setose; posterior anepimeron short white setose, katepimeron short white setose; metepimeron light brown or yellow, grey pubescent, short white setose.
Leg: setation yellow; met femur ± cylindrical only slightly wider than pro and mes femur, postero-ventrally regular setose only; met tibia latero-posteriorly regular setose only; pulvillus reduced, half length of well-developed claw.
Wing: length = 10.9–14.2 mm; hyaline throughout, slightly brown stained along veins.
Abdomen: setation comprised of sparsely scattered short yellow setae; T1–5 brown with yellow posterior margin, T6 brown (sometimes yellow posteriorly), T7 brown; T1–7 sparsely yellow setose; S1–7 brown; S1–7 sparsely short yellow setose.
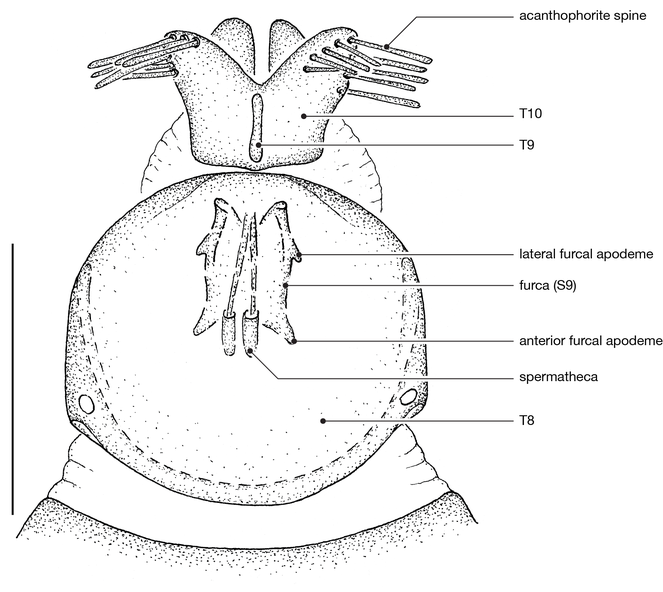
♀ genitalia: 8–9 acanthophorite spines per plate.
Diagnosis
This large species (wing length in males 7.1–8.9 mm and in females 10.9–14.2 mm) is distinguished from congeners by the wing venation in that cell r5 is open and therefore M1 terminates in C (and not in R1), the predominantly apubescent vertex, the short proboscis that is only about half the length of the oral cavity, the long white setose anatergite, and the setose katepimeron.
Distribution
Namibia (Erongo, Karas, Kunene) (Fig.
Biology
Females of this species (as Namadytes prozeskyi syn. nov.) were observed by
Females usually oviposited in shallow depressions, such as hoof prints and in particular on the lip of these prints, in the sandy Kuiseb river bed and followed a Mydidae-characteristic oviposition sequence of sand-ovipositing species (for details see
N. vansoni has been collected in riparian vegetation along a dry river bed, in thornveld in a dry river bed, and on barren gravel plains.
Taxon discussion
This species exhibits substantial intra-specific variation (Figs
Type locality
Namibia: Karas: Seeheim (26°48'53''S, 017°47'57''E) (Fig.
Biodiversity hotspot
Not known to occur in any of the southern African biodiversity hotspots (Cape Floristic Region, Maputaland-Pondoland-Albany, or Succulent Karoo) (Fig.
Identification keys
|
Key to Namadytes species There is considerable sexual dimorphism and the setation, for example on the anatergites, is always easier to observe in males. Intraspecific variation in the abdominal coloration, especially in females, is likewise substantial. |
||
| 1 | Anatergite setose; cell r5 open (M1+2 terminating into C); proboscis about ½ length of oral cavity; katepimeron setose | vansoni |
| – | Anatergite asetose; cell r5 closed (M1+2 terminating into R1); proboscis short, less than ½ length of oral cavity; katepimeron asetose | 2 |
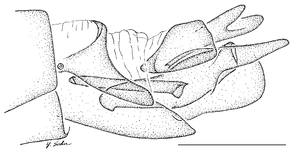
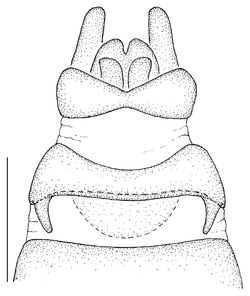
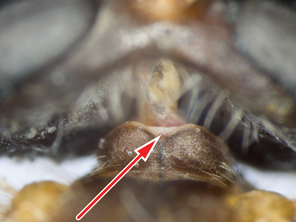
| 2 | Infra-halter sclerite (ventral to halter base and posterior to metathoracic spiracle) with only very few white setae (Fig. |
cimbebasiensis |
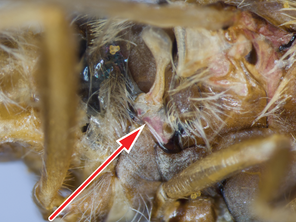
| – | Infra-halter sclerite long white setose (Fig. |
maculiventris |
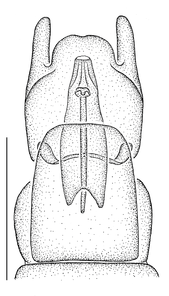
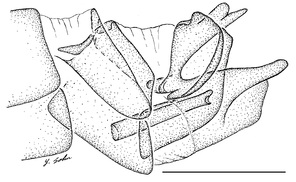
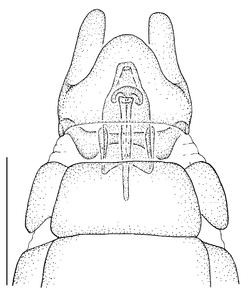
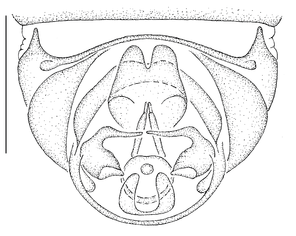
Antepronotum and infra-halter sclerite of Namadytes species.
b: Sparsely setose infra-halter sclerite of Namadytes cimbebasiensis in lateral view (♂ AAM-003021, #835222).
c: Densely setose infra-halter sclerite of Namadytes vansoni in lateral view (♂ AAM-002899, #835290)
Discussion
Morphological characteristics
Namadytes species exhibit two remarkable morphological characteristics unknown in any other Mydidae genus.
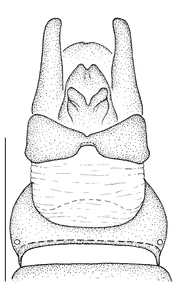
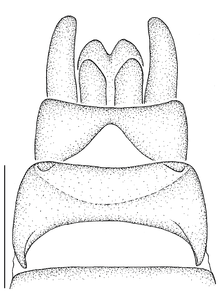
- The antepronotum is anteriorly not entire as in all Mydidae, but has a V-shaped indentation medially that is easily visible (Fig.
12 a ). - A small sclerite ventral to the halter and posterior to the metathoracic spiracle, here termed the infra-halter sclerite (Fig.
12 b ,c ), is unique within Mydidae. This sclerite is usually long, densely white setose, but only sparsely white setose N. cimbebasiensis males and sometimes even asetose in females of this species.
Biology
The knowlegde of the biology of Namadytes is very scarce as there is hardly any information on habitat preferences or flight behavior available on collecting labels. However,
Although not directly observed by Wharton, he suggests that mating with teneral or very young females might occur in Namadytes as well and is an important adaptation for short-lived species in desert environments (
Seasonal incidence
Namadytes has primarily been collected during February through June, during the Southern Hemisphere late summer to early winter, as well as in October (Southern Hemisphere spring) (Table
Month |
N. cimbebasiensis |
N. maculiventris |
N. vansoni |
January |
– |
– |
– |
February |
– |
√ |
√ |
March |
√ |
√ |
√ |
April |
√ |
– |
√ |
May |
√ |
– |
√ |
June |
– |
– |
√ |
July |
– |
– |
– |
August |
– |
– |
– |
September |
– |
– |
– |
October |
– |
√ |
√ |
November |
– |
– |
– |
December |
– |
– |
– |
Namadytes has not been sampled during the hottest months of the year, i.e., December–January, but has been collected in the cooler spring and late summer to early winter months. However, when one takes a closer look at the temperature and rainfall patterns at specific localities, taken from World Weather Online (
Namadytes species and the temperature and occurrence at selected localities. Temperature data from WorldWeatherOnline. Note that selected localities are places for which temperature data are available, for example, N. cimbebasiensis has not been collected at Upington, but north, north-east, and north-west of it.
Month |
N. cimbebasiensis |
N. maculiventris |
N. vansoni |
|||||||||||||
Maltahöhe |
Upington |
Aus |
Keetmanshoop |
Vioolsdrift |
Gobabeb |
Keetmanshoop |
Khorixas |
|||||||||
°C |
coll. |
°C |
coll. |
°C |
coll. |
°C |
coll. |
°C |
coll. |
°C |
coll. |
°C |
coll. |
°C |
coll. |
|
January |
34 |
– |
37 |
– |
24 |
– |
36 |
– |
20 |
– |
30 |
– |
36 |
– |
31 |
– |
February |
33 |
– |
37 |
– |
24 |
✓ |
35 |
– |
20 |
– |
30 |
✓ |
35 |
– |
27 |
– |
March |
31 |
– |
34 |
✓ |
24 |
– |
34 |
– |
19 |
✓ |
32 |
✓ |
34 |
– |
24 |
– |
April |
28 |
– |
30 |
✓ |
23 |
– |
30 |
– |
19 |
– |
31 |
✓ |
30 |
✓ |
24 |
✓ |
May |
25 |
✓ |
26 |
– |
22 |
– |
26 |
– |
20 |
– |
29 |
– |
26 |
✓ |
28 |
✓ |
June |
21 |
– |
23 |
– |
22 |
– |
23 |
– |
19 |
– |
27 |
✓ |
23 |
– |
26 |
– |
July |
22 |
– |
23 |
– |
21 |
– |
23 |
– |
18 |
– |
26 |
– |
23 |
– |
26 |
– |
August |
24 |
– |
25 |
– |
19 |
– |
25 |
– |
17 |
– |
24 |
– |
25 |
– |
25 |
– |
September |
27 |
– |
29 |
– |
20 |
– |
29 |
– |
17 |
– |
26 |
– |
29 |
– |
29 |
– |
October |
30 |
– |
32 |
– |
21 |
– |
32 |
✓ |
18 |
– |
28 |
– |
32 |
– |
30 |
– |
November |
32 |
– |
34 |
– |
22 |
– |
34 |
– |
18 |
– |
30 |
– |
34 |
– |
32 |
– |
December |
33 |
– |
37 |
– |
23 |
– |
36 |
– |
19 |
– |
29 |
– |
36 |
– |
30 |
– |
These data provide a glimpse at the temperature tolerance within species as, for example, N. maculiventris occurs at Vioolsdrift with an average high temperature of 19 °C (March) and near Keetmanshoop with an average high temperature of 32 °C (October).
Biodiversity hotspots
The biodiversity hotspots sensu Conservation International (
Acknowledgements
We would like to thank the museum curators who made specimens available through loans and for their hospitality when visiting the collections. We thank Fritz Geller-Grimm (Frankfurt a.M., Germany) for donating the sole recently collected Namadytes maculiventris specimen to the USNM. This project is funded by a U.S. National Science Foundation REVSYS Grant (DEB 0919333; PI T. Dikow, Co-PI David Yeates). Any opinions, findings, and conclusions or recommendations expressed in this manuscript are those of the authors and do not necessarily reflect the views of the National Science Foundation. Partial funding is also provided by the John D. and Catherine T. MacArthur Foundation funding of the Biodiversity Synthesis Group of the Encyclopedia of Life. We thank Y. Sohn (NMNH, Smithsonian Institution) for skillfully illustrating the specimen and male and female terminalia. We thank the peer reviewers for suggestions that enhanced the manuscript.
Author contributions
TD conceptualized the project and gathered material from numerous collections. SL worked on this review during her NSF-funded summer internship at the Biodiversity Synthesis Center at the Field Museum of Natural History, Chicago, IL, USA where she was trained and supervised by TD in 2012.
References
- Bowden J (1980) 27. Family Mydidae. In: Crosskey RW (Ed.) Catalogue of the Diptera of the Afrotropical Region.British Museum (Natural History),London,325-333pp. [InEnglish]. [ISBN0-565-00821-8].
- Cumming JM, Wood DM (2009) Adult morphology and terminology. In: Brown BV, Borkent A, Cumming JM, Wood DM, Woodley NE, Zumbado MA (Eds) Manual of Central American Diptera.1.NRC Press,Ottawa,9-50pp. [InEnglish]. [ISBN978-0-660-19833-0].
- Phylogeny of Asilidae inferred from morphological characters of imagines (Insecta: Diptera: Brachycera: Asiloidea).Bulletin of the American Museum of Natural History319:1‑175. [InEnglish]. URL: http://hdl.handle.net/2246/5949
- New species and new records of Mydidae from the Afrotropical and Oriental regions (Insecta, Diptera, Asiloidea).ZooKeys64:33‑75. [InEnglish]. https://doi.org/10.3897/zookeys.64.464
- Review of Namibimydas Hesse, 1972 and Nothomydas Hesse, 1969 (Diptera: Mydidae: Syllegomydinae: Halterorchini) with the description of new species.African Invertebrates53(1):79‑111. [InEnglish]. URL: http://africaninvertebrates.org/ojs/index.php/AI/article/view/25
- Dikow T, Meier R, Vaidya G, Londt JH (2009) Biodiversity Research Based On Taxonomic Revisions — A Tale Of Unrealized Opportunities. In: Pape T, Bickel D, Meier R (Eds) Diptera Diversity: Status, Challenges and Tools.Brill Academic Publishers,Leiden,323-345pp. https://doi.org/10.1163/ej.9789004148970.i-459.55
- The Mydaidae (Diptera) of Southern Africa.Annals of the South African Museum54:1‑388. [InEnglish]. URL: http://www.biodiversitylibrary.org/item/126406
- New Mydaidae (Diptera) from the Namib Desert and South-Western Africa.Annals of the South African Museum60(3):109‑171. [InEnglish]. URL: http://www.biodiversitylibrary.org/pdf4/015634000127256.pdf
- McAlpine JF (1981) Morphology and terminology–adults. In: McAlpine JF, Peterson BV, Shewell GE, Teskey HJ, Vockeroth JR, Wood DM (Eds) Manual of Nearctic Diptera.1.Research Branch, Agriculture Canada,Ottawa,9-63pp. [InEnglish]. URL: http://www.esc-sec.ca/aafcmono.php [ISBN0-660-10731-7].
- Biodiversity hotspots for conservation priorities.Nature403(6772):853‑858. [InEnglish]. https://doi.org/10.1038/35002501
- The Torre-Bueno Glossary of Entomology.The New York Entomological Society,New York,840pp.
- Zoobank: Developing a nomenclatural tool for unifying 250 years of biological information.Zootaxa1950:39‑50. [InEnglish]. URL: http://www.mapress.com/zootaxa/2008/f/zt01950p050.pdf
- SimpleMappr, an online tool to produce publication-quality point maps. URL: http://www.simplemappr.net
- Antennal evolution in the Brachycera (Diptera), with a reassessment of terminology relating to the flagellum.Studia dipterologica6(1):33‑48. [InEnglish].
- Observations on the behaviour, phenology and habitat preferences of mydas flies in the central Namib Desert (Diptera: Mydidae).Annals of the Transvaal Museum33(9):145‑151. [InEnglish]. URL: http://content.sabinet.co.za/u?/00411752,165
- World Weather Online. http://www.worldweatheronline.com. Accessed on: 2013-10-06.
- Conservation International Biodiversity Hotspots 2013. http://www.conservation.org/where/priority_areas/hotspots/Pages/hotspots_main.aspx. Accessed on: 2013-10-06.
Supplementary materials
The XML file includes the natural-language species descriptions in SDD (Structure of Descriptive Data) format.
Average temperature Aus
Average rainfall Aus
Average temperature Gobabeb
Average rainfall Gobabeb
Average temperature Keetmanshoop
Average rainfall Keetmanshoop
Average temperature Khorixas
Average rainfall Khorixas
Average temperature Maltahöhe
Average rainfall Maltahöhe
Average temperature Upington
Average rainfall Upington
Average temperature Vioolsdrift
Average rainfall Vioolsdrift