|
Biodiversity Data Journal :
Research Article
|
|
Corresponding author: Lynn S. Kimsey (lskimsey@ucdavis.edu)
Academic editor: Yasen Mutafchiev
Received: 17 Oct 2017 | Accepted: 20 Nov 2017 | Published: 24 Nov 2017
© 2017 Lynn Kimsey, Thomas Zavortink, Robert Kimsey, Steven Heydon
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Kimsey LS, Zavortink TJ, Kimsey RB, Heydon SL (2017) Insect biodiversity of the Algodones Dunes of California. Biodiversity Data Journal 5: e21715. https://doi.org/10.3897/BDJ.5.e21715
|
|
Abstract
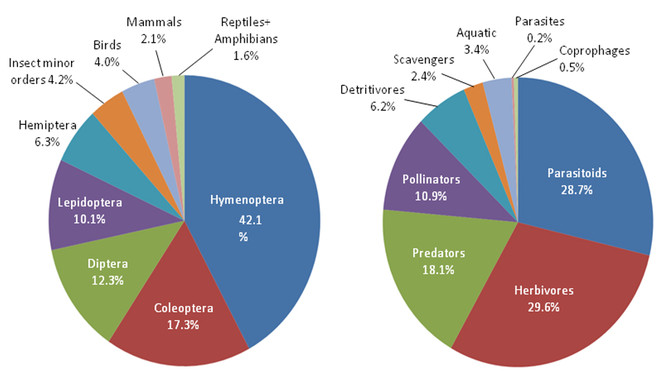
Over a nine year period beginning in 2007 we surveyed the insects of the Algodones Dunes, Imperial Count, California, as part of a study undertaken for the U.S. Bureau of Land Management. In a series of 22 collecting trips ranging in duration from 2 to 8 days we thus far have accumulated records of 1,840 species, 21 orders and 244 families from the dunes. Hymenoptera constituted the most diverse order, comprising about 45% of all the species recovered. Insect diversity and abundance peaked during the hottest part of the year between the months of May and September. Life history traits of the insects sampled included herbivores (29.6%), parasitoids (28.7%), predators (18.1%), pollen/nectar feeders (10.9%), detritivores (6.2%) and scavengers (2.4%). Seventy-nine or 4% of the insect species collected in the dunes have been solely recorded from there, and 3% of the species almost certainly derive from adjacent aquatic habitats or agricultural ecosystems, as their life histories could not be completed in Algodones Dunes habitat. The insect fauna of the Algodones Dunes is unexpectedly rich and diverse.
Keywords
Sonora, Mexico, Gran Desierto
Introduction
The overall invertebrate biodiversity of any region in North America remains very poorly studied, and the proportional representation of life history traits of the most abundant group, insects, seems largely unknown. The Great Smoky Mountains National Park All Taxa Biological Inventory (
The Algodones or Imperial Dunes constitute a major portion of the Imperial Sand Dunes Recreational Area (ISDRA), an extensive geographic feature in the southeastern corner of California. During the Pleistocene these dunes formed as part of a more extensive erg that extended from the southern end of the Salton Sea south through the Gran Desierto el Altar in Sonora, Mexico, to the Gulf of California. Periodic flooding and silt deposition by the Colorado River over thousands of years likely created the dunes (
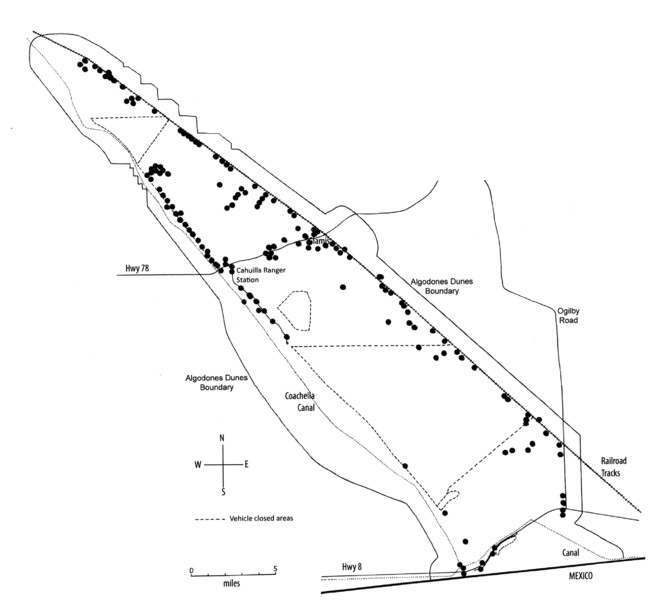
No natural surface water exists in these dunes, except for temporary pools caused by heavy rainfall run-off from the Chocolate and Cargo Muchacho Mountains. A diversity of manmade water resources on or adjacent to the dunes include wildlife “guzzlers” together with water delivery canals that run along the southern and western margins. Within the dunes the U.S. Bureau of Land Management maintains three such guzzlers, small in-ground water tanks for wildlife, primarily deer. The Coachella Canal runs along the western margin of the dunes, roughly 11 km west of Glamis. The All-American Canal transects the southern end of the dunes just above the Mexican border. The Union Pacific railroad runs the length of the eastern edge of the dunes. Where the tracks pass closest to the dunes Union Pacific drip irrigates rows of tamarisk trees as a windbreak to keep sand off the tracks (Fig.
Humans have lived in or near the dunes for thousands of years and their influence on the biota of the dunes, minimal at first, has become more pronounced through time. Prehistoric Native American trails crossed the dunes along trade routes, which are followed today by sections of California Highway 78. The compacted soil of these trails is still visible in certain places paralleling the highway where it runs through a gap in the mountains east of the dunes. Native Americans also engaged in seasonal foraging on the dunes for food resources such as the elongate roots of sand food [Pholisma sonorae (Torr. ex Gray): (Boraginaceae)]. The mineral resources of the nearby mountains attracted European settlers to the area as early as the 1780’s creating towns and settlements. The largest of these towns was Tumco, which at its peak in the early 1900’s, was inhabited by over 3,000 people. The mine there closed permanently in 1942 and the site is now abandoned. The Algodones Dunes are now bordered on the south by the All-American Canal, and on the west by the Coachella Canal. These were constructed in the mid–30’s to provide irrigation to the Imperial Valley. During World War II, the dunes were a training ground for desert warfare troops and for air to ground combat, and spent ordinance can still be encountered anywhere on the dunes. The dunes were first opened to vehicle traffic in 1916 when a road made of wooden boards was constructed to facilitate transportation between San Diego and Yuma. A short section of this “plank road” is preserved near the Grays Well Road exit on Interstate 8. A paved road replaced the plank road 10 years later, which was in turn replaced by Interstate 8, a modern four lane divided highway. However, the dunes proved to be an impassible barrier for the Southern Pacific Railroad (now the Union Pacific Railroad). The rail line west from Yuma, Arizona turns north toward Los Angles and runs the length of the dunes eastern edge. Drainage from the Chocolate and Cargo Muchacho Mountains is now channelized by a series of V shaped berms that funnel the runoff under the tracks through large culverts or bridges. In recent decades, the Algodones Dunes has become one of the premiere sites for off road vehicle recreation in California. Crowds on winter holiday weekends may approach 150,000 people. The traffic from these vehicles has largely denuded the open sand habitats south of Highway 78. This traffic largely disappears as the weather warms and the peak season for insect activity approaches. The latest human modification of the dunes is the construction of a border wall along the California–Mexican border. The border wall is a 15-foot high metal fence with an undeveloped sand road for the use of U.S. Immigration running along the wall on the United States side.
Many of these human alterations of the dunes environment have probably had little effect on the dunes insect fauna; others have caused significant alterations. The two developments that probably caused the most significant alterations to the insect fauna are the agricultural development of the Imperial Valley and the building of the agricultural canals. Spring collections are dominated by aphids and their parasitoids during what must be an annual migration. Aquatic insects can be collected at nearly any time of the year at light traps or Malaise traps.
The eastern and western edges of the dunes substantially differ geologically and biologically. The drier western side is dominated by open dune habitats (Fig.
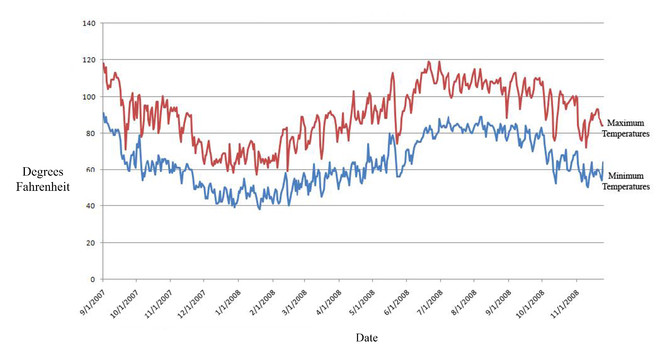
To live on the dunes, plants and animals must survive extreme temperatures, little rainfall and shifting substrates, comprising some of the harshest conditions in North America. Rainfall averages 76 mm annually, half falling during the winter, half in the summer monsoon. Prevailing winds shift seasonally, originating from the west in the fall, winter and spring months and from the southeast in the summer. Wind speeds, particularly during the summer, can reach 95 kph (60 mph). Temperatures approximately range from a nighttime low of 4°C (39.2°F) in the winter to a daytime high of 49°C (120.2°F) in June (Fig.
The flora of the ISDRA seems depauperate relative to areas elsewhere in the Colorado Desert, but vertebrate surveys suggest a rich diversity (
Thus, we sought to enumerate insect species diversity, expand the list of potentially endemic insects, characterize the systematic diversity, and parse the frequency distribution of insect life history traits in the Algodones Dunes. Accordingly, we periodically sampled diverse locations in the dunes (Fig.
Study Site
We surveyed the insects of the Imperial Sand Dunes Recreation Area from September 2007 until March 2016, making 383 collections during 22 collecting trips ranging in duration from 2 to 8 days. During the first year of the study, we repeatedly sampled five sites for five consecutive days during different seasons of the year for a seasonal faunal comparison. These sites were located in the three major vegetation types found on the dunes, psammophytic scrub, creosote bush scrub and microphyll woodland. Starting in the second year of the study and continuing into the third year, we sampled eight sites (four pairs) to compare faunal diversity between non-disturbed and badly disturbed habitats. This study has yet to be published. The four pairs were distributed approximately 0.5-2.4 km (0.03-1.5 mi) apart along Highway 78 from the Cahuilla Ranger Station on the west to Glamis on the east. One member of each pair was located approximately 0.3-1.6 km (0.2-1.0 mi) north of Highway 78 in the undisturbed North Algodones Dunes Wilderness and the other member a comparable distance south of Highway 78 in disturbed habitat heavily impacted by off-highway vehicles (Fig.
Methods
We employed a variety of collecting techniques to sample insect diversity. Simple aerial net collecting, sand sifting through 0.3 cm (0.125 in) mesh screen, and hand-capture by team members supplemented Malaise, black light, pitfall, yellow bowl, blue vane and McPhail traps. Standard Malaise traps continuously collected flying and walking insects into catcher heads filled with propylene glycol or 95% ethanol or a combination of both (Fig.
To sample fauna associated with each season, we attempted five-day-long sampling excursions to the dunes during the first year of the study, scheduling each in a different season insofar as was possible. During these excursions we set one Malaise trap, ten yellow bowls and ten pitfall traps, and ran a black light trap for one or two nights in each of the five season-comparison sites. Moderate to full gale force winds and sand storms sometimes blew equipment down or away, obliterating samples. As resources and opportunity permitted during these excursions, we also set out these devices in secondary non-permanent sites as well.
To sample fauna in disturbed and undisturbed habitats in the disturbance comparison locations along Highway 78, we set out eight black light traps simultaneously on one night, and eight Malaise traps continuously for five days in June, August and September 2009 and March 2010.
In later years of the study, sampling was primarily by hand netting and Malaise and black light traps.
Taxonomic coverage. We have developed a checklist of the insects of the Algodones Dunes, which is available on the Bohart Museum of Entomology website - http://bohart.ucdavis.edu/research.html. A more detailed database to manage all our insect specimen data is under construction but is not yet available on-line. Numerous experts in the systematic community assisted us with the identification of certain insect groups, particularly scientists at the California Department of Food & Agriculture, Plant Pest Diagnostics Branch; the University of California Davis; and the U.S. National Museum (see acknowledgments). This process of species identification remains on-going, and we have not identified some taxonomic groups where expertise remains absent, for example muscoid and nematoceran fly families, Trichoptera and Psocodea. Thus, the checklist and database remain incomplete for the taxa where identification remains on-going. Specimens are deposited in the Bohart Museum of Entomology, University of California, Davis.
Temperature, rainfall and wind speed/direction were taken from the California Department of Water Resources Cahuilla (http://cdec.water.ca.gov/cgi-progs/queryF?CAU) and Buttercup (http://cdec.water.ca.gov/cgi-progs/queryF?BUT) weather stations, located at the middle and southern end of the dunes respectively.
Results
Our nine-year survey of the insect fauna yielded five major observations. 1) The order Hymenoptera constituted the most diverse order comprising more than 45% of the insect species sampled and 42% of the total animal species recorded on the Algodones Dunes. 2) At least 79 species (roughly 4%) have thus far only been recorded from the dunes. 3) Insect species diversity and abundance peaked during the hottest part of the year between the months of May and September. 4) The insect fauna seems dominated by parasitoids and herbivores. 5) Large numbers of aquatic and agricultural pest insects that cannot survive on the dunes disperse there, likely from the Coachella and All-American Canals or from agricultural lands to the west.
Species Composition. The insect fauna of the Algodones Dunes is unexpectedly large, with 1,840 species in 21 orders and 244 families identified thus far (Table
Overview of the insects collected on the Algodones Dunes, Imperial County, California between 2007 and 2016.
|
Order |
Total number of families |
Total number of species |
Number of species known only from the dunes |
Total number of exotic species* |
Total aquatic species |
|
Blattodea |
2 |
3 |
0 |
1 |
0 |
|
Coleoptera |
54 |
343 |
17 |
9 |
15 |
|
Dermaptera |
2 |
2 |
0 |
2 |
0 |
|
Diptera |
50 |
244 |
7 |
4 |
20 |
|
Embiidina |
1 |
1 |
0 |
0 |
0 |
|
Ephemeroptera |
1 |
5 |
0 |
0 |
5 |
|
Hemiptera |
32 |
131 |
0 |
8 |
6 |
|
Hymenoptera |
45 |
837 |
52 |
2 |
0 |
|
Isoptera |
2 |
3 |
0 |
0 |
0 |
|
Lepidoptera |
28 |
200 |
0 |
6 |
2 |
|
Mantodea |
1 |
2 |
0 |
0 |
0 |
|
Microcoryphia |
1 |
1 |
0 |
0 |
0 |
|
Neuroptera |
5 |
31 |
0 |
0 |
1 |
|
Odonata |
6 |
10 |
0 |
0 |
10 |
|
Orthoptera |
4 |
11 |
1 |
1 |
0 |
|
Phasmida |
1 |
1 |
0 |
0 |
0 |
|
Psocodea |
1 |
5 |
0 |
0 |
0 |
|
Strepsiptera |
1 |
1 |
0 |
0 |
0 |
|
Thysanoptera |
2 |
2 |
0 |
1 |
0 |
|
Trichoptera |
3 |
5 |
0 |
0 |
5 |
|
Zygentoma |
2 |
2 |
0 |
0 |
0 |
|
Total |
244 |
1840 |
77 |
34 |
64 |
*Insects exotic to North America.
Comparison of species recorded from the Algodones Dunes in the literature versus those collected in the current study, and those found in both. Literature records are from: 1
| Taxon |
Number of published species |
Number of survey species |
Difference |
Number of species in both |
% of species in both |
|
Hymenoptera |
|||||
|
Aporinellus (Pompilidae)2 |
6 |
4 |
2 |
2 |
25 |
|
Arachnospila (Pompilidae)2 |
7 |
2 |
5 |
1 |
13 |
|
Episyron (Pompilidae)2 |
3 |
1 |
2 |
1 |
33 |
|
Pompilus (Pompilidae)2 |
3 |
2 |
1 |
1 |
25 |
|
Coleoptera |
|||||
|
Asbolus (Tenebrionidae)3 |
4 |
2 |
2 |
2 |
50 |
|
Chilometopon (Tenebrionidae)3 |
5 |
3 |
2 |
3 |
60 |
|
Cryptoglossa (Tenebrionidae)1 |
2 |
1 |
1 |
1 |
50 |
|
Cymatodora (Cleridae)1 |
3 |
1 |
2 |
1 |
33 |
|
Diplotaxis (Scarabaeidae)1 |
6 |
0 |
6 |
0 |
0 |
|
Eustattus (Tenebrionidae)3 |
5 |
3 |
2 |
3 |
60 |
|
Glaresis (Glaresidae)1 |
3 |
0 |
3 |
0 |
0 |
|
Horistonotus (Elateridae)3 |
0 |
2 |
2 |
0 |
0 |
|
Tricorynus (Anobiidae)1 |
3 |
0 |
3 |
0 |
0 |
With the largest number of species, 42.1%, the order Hymenoptera dominates this habitat, followed by Coleoptera 17.3%, Diptera 12.3%, Lepidoptera (10.1%) and Hemiptera 6.3% (Fig.
Potentially Endemic Species. We compiled a list of 79 species of insects recorded only from the dunes including those new to science and a number of described species (Table
Insect species only recorded from the Algodones Dunes as of the current study. (*Taxa newly recognized during this study.)
| Coleoptera | ||
| 1 | Acmaeroderoides stramineus Nelson | Buprestidae |
| 2 | Agrilus harenus Nelson | Buprestidae |
| 3 | Lepismadora algodones Velten | Buprestidae |
| 4 | Prasinalia imperialis (Barr) | Buprestidae |
| 5 | Hyperaspidius algodones Gordon | Coccinellidae |
| 6 | Trigonoscuta rothi rothi Pierce | Curculionidae |
| 7 | Trigonoscuta rothi algodones Pierce | Curculionidae |
| 8 | Trigonoscuta rothi imperialis Pierce | Curculionidae |
| 9 | Trigonoscuta rothi punctata Pierce | Curculionidae |
| 10 | Horistonotus n. sp. 1* | Elateridae |
| 11 | Horistonotus n. sp. 2* | Elateridae |
| 12 | Anomala carlsoni Hardy | Scarabaeidae |
| 13 | Anomala hardyorum Potts | Scarabaeidae |
| 14 | Cyclocephala wandae Hardy | Scarabaeidae |
| 15 | Edrotes arens La Rivers | Tenebrionidae |
| 16 | Eusattus dilatatus LeConte | Tenebrionidae |
| 17 | Nocibiotes crassipes (Casey) | Tenebrionidae |
| 18 | Tonibius sulcatus (Casey) | Tenebrionidae |
| Diptera | ||
| 19 | Apiocera warner Cazier | Apioceridae |
| 20 | Efferia macroxipha Forbes | Asilidae |
| 21 | Elachiptera n. sp.* | Chloropidae |
| 22 | Trixoscelis n. sp.* | Heleomyzidae |
| 23 | Blaesoxipha (Acanthodotheca) n. sp.* | Sarcophagidae |
| 24 | Eumacronychia n. sp. 1* | Sarcophagidae |
| 25 | Eumacronychia n. sp. 2* | Sarcophagidae |
| Hymenoptera | ||
| 26 | Perdita algodones Timberlake | Andrenidae |
| 27 | Perdita glamis Timberlake | Andrenidae |
| 28 | Psilochalcis n. sp. 1 | Chalcididae |
| 29 | Psilochalcis n. sp. 2 | Chalcididae |
| 30 | Psilochalcis n. sp. 3 | Chalcididae |
| 31 | Psilochalcis n. sp. 4 | Chalcididae |
| 32 | Microbembex elegans Griswold | Crabronidae |
| 33 | Plenoculus n. sp. 1* | Crabronidae |
| 34 | Plenoculus n. sp. 2* | Crabronidae |
| 35 | Plenoculus n. sp. 3* | Crabronidae |
| 36 | Plenoculus n. sp. 4* | Crabronidae |
| 37 | Plenoculus n. sp. 5* | Crabronidae |
| 38 | Solierella n. sp.* | Crabronidae |
| 39 | Stictiella villegasi Bohart | Crabronidae |
| 40 | Banacuniculus dis Buffington | Eucoilidae |
| 41 | Ganaspidium n. sp.* | Eucoilidae |
| 42 | Tenuipetiolus n. sp.* | Eurytomidae |
| 43 | Dasymutilla imperialis Manley & Pitts | Mutillidae |
| 44 | Dasymutilla nocturna Mickel | Mutillidae |
| 45 | Sphaerophthalma ecarinata Schuster | Mutillidae |
| 46 | Sphaerophthalma django Pitts & Wilson* | Mutillidae |
| 47 | Ageniella arenicola Wasbauer & Kimsey* | Pompilidae |
| 48 | Ageniella pernia Wasbauer & Kimsey* | Pompilidae |
| 49 | Acordulecera algodones Smith | Pergidae |
| 50 | Acordulecera kimseyi Smith | Pergidae |
| 51 | Caenocrepis n. sp.* | Pteromalidae |
| 52 | Catalaccus n. sp. 1* | Pteromalidae |
| 53 | Catalaccus n. sp. 2* | Pteromalidae |
| 54 | Chlorocytus n. sp.* | Pteromalidae |
| 55 | Epipteromalus n. sp. 1* | Pteromalidae |
| 56 | Epipteromalus n. sp. 2* | Pteromalidae |
| 57 | Epipteromalus n. sp. 3* | Pteromalidae |
| 58 | Gastrancistrus n. sp. 1* | Pteromalidae |
| 59 | Gastrancistrus n. sp. 2* | Pteromalidae |
| 60 | Gastrancistrus n. sp. 3* | Pteromalidae |
| 61 | Gastrancistrus n. sp. 4* | Pteromalidae |
| 62 | Gastrancistrus n. sp. 5* | Pteromalidae |
| 63 | Gastrancistrus n. sp. 6* | Pteromalidae |
| 64 | Halticoptera n. sp.* | Pteromalidae |
| 65 | Heteroschema n. sp.* | Pteromalidae |
| 66 | Lyrcus n. sp. 1* | Pteromalidae |
| 67 | Lyrcus n. sp. 2* | Pteromalidae |
| 68 | Lyrcus n. sp. 3* | Pteromalidae |
| 69 | Lyrcus n. sp. 4* | Pteromalidae |
| 70 | Lyrcus n. sp. 5* | Pteromalidae |
| 71 | Pachyneuron n. sp.* | Pteromalidae |
| 72 | Pteromalus sp. 1* | Pteromalidae |
| 73 | Pteromalus sp. 2* | Pteromalidae |
| 74 | Pteromalus sp. 3* | Pteromalidae |
| 75 | Pteromalus sp. 4* | Pteromalidae |
| 76 | Sedomaya glamisensis Kimsey & Wasbauer | Tiphiidae |
| 77 | Pseuderimerus n. sp.* | Torymidae |
| 78 | Euparagia unidentata Carpenter & Kimsey* | Vespidae |
| Orthoptera | ||
| 79 | Macrobaenetes algodonensis Tinkham | Raphidophoridae |
Seasonality. Adult insect diversity and abundance peaked during the hottest part of the year, between the months of May and September. Daytime temperatures during this period ranged from 32-49°C (90-120.2°F), with the highest temperatures usually occurring in June (Fig.
Species of insects native to the dunes clearly predominated in summer months, when exotic species infrequently occurred. However, during the cool winter months bean aphid (Aphis fabae Scopoli) and pea aphid (Acyrthosiphon pisum Mordvilko) (Aphididae) and a variety of pest noctuid moths including cutworms and army worms (Noctuidae) exemplified the exotic species that dominated the insect fauna on the dunes. These likely dispersed from adjacent agricultural land to the west. Winged adult bean and pea aphids but no wingless offspring, together with adults and nymphs of Acyrthosiphon kondoi Shinji, fed on the endangered Peirson’s Milk Vetch in March. Thus it seems unlikely that bean or pea aphids reproduce on this plant.
Scavengers and Fungivores. The native insect fauna contains a large number of scavengers and fungivores. These insects do not constitute the most species-rich taxa, but dominate in numbers of individuals. Predominant scavengers on the dunes include the Algodones sandtreader, Macrobaenetes algodonensis Tinkham (Rhaphidophoridae), two species of Microbembix elegans and argyropleura Bohart (Crabronidae), and a diversity of tenebrionid beetle species (Coleoptera). Fungivores, particularly larvae of fungivorous beetles, for example Anthicidae, seem to take advantage of a perennially moist subsurface stratum where mycorrhizal and decomposing fungi grow. A single, one-night black light trap sample in June 2010, when temperatures were high and water availability was low, contained approximately 22,000 individuals of the anthicid beetle, Anthicus cervinus LaFerté-Sénectère (Anthicidae).
Dunes exotics
Numerous exotic crop or horticultural pest insects that are unlikely to survive for long on the dunes, constituting about 2% of species, land on the dunes, most likely after dispersion from adjacent western agricultural lands. These include the aphid species that feed primarily on alfalfa, and Aphis nerii Boyer de Fonscolombe that feeds on milkweed (Asclepias).
Interestingly, aquatic insects, constituting over 3% of the species collected, must originate from one of the canals, guzzlers or railroad irrigation puddles, or indirectly from the Colorado River to the east and southeast. Aquatic insects often disperse into adjacent desert regions where they cannot survive, but provide food resources to local predators and scavengers (
We also occasionally observed exotic species appearing only once on the dunes and then disappearing. In 2007 we recorded large numbers of two species of earwig, Euborellia cincticollis (Gerstaecker) (Anisolabidae) and Labidura riparia (Pallas) (Labiduridae) at several localities. In the same year the house cricket, Acheta domesticus L. (Gryllidae) commonly occurred and additionally was observed in the nearby community of El Centro. We did not subsequently collect these species on the dunes.
A component of the insect fauna that cannot derive from the dunes and yet are not chance immigrants from local agricultural or aquatic habitats include known migrating species, such as painted lady butterflies, Vanessa cardui (L.) (Nymphalidae), and two dragonfly species, Sympetrum corruptum (Hagen) (Libellulidae) and Aeshna junius Say (Aeshnidae).
Long range aerial plankton would also appear to land/fall on the dunes. For example, we recorded two specimens of Amphidecius schickae Heydon (Pteromalidae) from the dunes; the only known hosts of this species, Neuroderus sp. (Cynipidae), feed on oaks, yet the nearest oaks to the dunes are approximately 100 km to the west. One specimen of the Aristolochia pipevine specialist Battus philenor (L.) (Papilionidae) was also found on the open dunes nearly 100 km from the closest known source of native Aristolochia in western Arizona.
Conclusions
Species in the order Hymenoptera clearly dominate this sand dune habitat. Even if expanded sampling techniques added Coleoptera species, the number of species would have to double to approach the number of Hymenoptera. Such numbers make sense; more than half of Hymenoptera collected were parasitoids of other insects or non-insect arthropods. Additionally, given the large number of fungivorous insects in our samples fungi must be a major yet cryptic presence in these dunes.
Acknowledgements
The study was supported by a U.S. Bureau of Land Management under an Assistance Agreement, BAA033001/BAA06044 issued under the umbrella Cooperative Agreement for the Californian Cooperative Ecosystems Unit (CESU). The project was in large part an extended collaborative effort. The staff of the El Centro Office of the Bureau of Land Management greatly supported us, particularly Daniel Steward, Andrew Truette and Erin Dreyfuss, as well as U.S. Border Patrol, and MCI and Union Pacific Railroad employees. A large number of undergraduate and graduate students assisted with collecting and specimen preparation. Species identifications were provided by the staff of the Bohart Museum and by the following specialists: Rolf Albuu, Charles Bellamy, Robert Beiriger, Matt Buffington, James M. Carpenter, Michael Caterino, Andy Cline, Theodore Cohen, Eric Fisher, Ray Gagné, Steve Gaimari, Rosser Garrison, Michael Gates, Ray Gill, Martin Hauser, Michael Haverty, Peter Kerr, Wayne Mathis, John Oswald, Thomas Pape, James Pitts, David Smith, Jeff Smith, Norman Smith, Catherine Tauber, Robbin Thorp, Marius Wasbauer, Gillian Watson, Kevin Williams and Robert Zuparko. The systematists at the California Department of Food & Agriculture, Plant Pest Diagnostics Branch were particularly helpful.
References
- The coleopterous fauna of selected California sand dunes.Report for the Bureau of Land Management Contract CA-960-1285-1225-DEOO, March 15, 1979.1‑147.
- Petition to list 16 endemic insect species from the Algodones Sand Dunes, Imperial County, California as federally endangered or threatened under the federal endangered species act. http://www.biologicaldiversity.org/swcbd/PROGRAMS/deserts/algodones/petition_17.pdf
- Final environmental impact statement for the Imperial Sand Dunes Recreation Area Management Plan and proposed amendment to the California Desert Conservation Plan, 1980.US Department of Interior, Bureau of Land Management report, May 2003.1‑471.
- Breeding bird densities, species composition, and bird species diversity of the Algodones Dunes.Western Birds9:9‑20.
- Breeding bird densities, species composition, and bird species diversity of the Algodones Dunes.Ecology67(3):629‑638. URL: http://onlinelibrary.wiley.com/doi/10.2307/1937686/full
- Rarity and diversity in forest ant assemblages of Great Smoky Mountains National Park.Southeastern Naturalist(special issue)215‑228. https://doi.org/10.1656/1528-7092(2007)6[215:RADIFA]2.0.CO;2
- Speculations on the origin of the Algodones Dunes, California.Bulletin of the Geological Society of America80:1039‑1044. https://doi.org/10.1130/0016-7606(1967)78[1039:SOTOOT]2.0.CO;2
- Source of sand dunes of southeastern California and northwestern Sonora, Mexico.Bulletin of the Geological Society of America80:531‑534. https://doi.org/10.1130/0016-7606(1969)80[531:SOSDOS]2.0.CO;2
- Biodiversity hotspots for conservation priorities.Nature403:853‑858. https://doi.org/10.1038/35002501
- Craneflies (Diptera: Tipuloidea) of Great Smoky Mountains National Park.Zootaxa1013:1‑18. [InEnglish]. https://doi.org/10.11646/zootaxa.1013.1.1
- The all taxa biological inventory of the Great Smoky Mountains national park.Florida Entomologist84(4):556‑564. https://doi.org/10.2307/3496388
- Unscented color traps for non-Apis bees (Hymenoptera: Apiformes).Journal of the Kansas Entomological Society78:4373‑4380.
- The Pompilidae of the Algodones Dunes, California, with description of new species.Pan-Pacific Entomologist86:2‑9. https://doi.org/10.3956/2009-30.1