|
Biodiversity Data Journal :
Taxonomic Paper
|
|
Corresponding author: Gerlien Verhaegen (gerlienverhaegen@hotmail.com)
Academic editor: Yasen Mutafchiev
Received: 29 May 2021 | Accepted: 03 Aug 2021 | Published: 16 Aug 2021
© 2021 Gerlien Verhaegen, Emiliano Cimoli, Dhugal Lindsay
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Verhaegen G, Cimoli E, Lindsay D (2021) Life beneath the ice: jellyfish and ctenophores from the Ross Sea, Antarctica, with an image-based training set for machine learning. Biodiversity Data Journal 9: e69374. https://doi.org/10.3897/BDJ.9.e69374
|
|
Abstract
Background
Southern Ocean ecosystems are currently experiencing increased environmental changes and anthropogenic pressures, urging scientists to report on their biodiversity and biogeography. Two major taxonomically diverse and trophically important gelatinous zooplankton groups that have, however, stayed largely understudied until now are the cnidarian jellyfish and ctenophores. This data scarcity is predominantly due to many of these fragile, soft-bodied organisms being easily fragmented and/or destroyed with traditional net sampling methods. Progress in alternative survey methods including, for instance, optics-based methods is slowly starting to overcome these obstacles. As video annotation by human observers is both time-consuming and financially costly, machine-learning techniques should be developed for the analysis of in situ /in aqua image-based datasets. This requires taxonomically accurate training sets for correct species identification and the present paper is the first to provide such data.
New information
In this study, we twice conducted three week-long in situ optics-based surveys of jellyfish and ctenophores found under the ice in the McMurdo Sound, Antarctica. Our study constitutes the first optics-based survey of gelatinous zooplankton in the Ross Sea and the first study to use in situ / in aqua observations to describe taxonomic and some trophic and behavioural characteristics of gelatinous zooplankton from the Southern Ocean. Despite the small geographic and temporal scales of our study, we provided new undescribed morphological traits for all observed gelatinous zooplankton species (eight cnidarian and four ctenophore species). Three ctenophores and one leptomedusa likely represent undescribed species. Furthermore, along with the photography and videography, we prepared a Common Objects in Context (COCO) dataset, so that this study is the first to provide a taxonomist-ratified image training set for future machine-learning algorithm development concerning Southern Ocean gelatinous zooplankton species.
Keywords
Southern Ocean, gelatinous zooplankton, siphonophore, video annotation, remotely-operated vehicle (ROV), Common Objects in Context (COCO), machine learning
Introduction
Southern Ocean ecosystems have experienced increasing environmental changes over the last decades (
Gelatinous zooplankton, comprising jellyfish, ctenophores and chordate tunicates (
Recently, the drawbacks in the collection and identification of gelatinous zooplankton have slowly started to be overcome through progress in methodologies using, for instance, molecular tools (e.g. metabarcoding, environmental DNA:
Surveys of gelatinous zooplankton in the Southern Ocean flourished in the late 19th and early 20th centuries. These surveys were conducted during famous expeditions, such as the Gauss expedition 1901–1903 (that is the first German expedition to Antarctica, also known as the “Deutsche Südpolar-Expedition 1901–1903”) (e.g.
Reports employing alternative survey methods for gelatinous zooplankton in the Southern Ocean, such as genetics or optics-based surveys, are few. For instance, sequences suitable for DNA barcoding remain rare, especially at species-level taxonomic resolution, with the notable exceptions of some siphonophore species (e.g.
Materials and methods
Study location
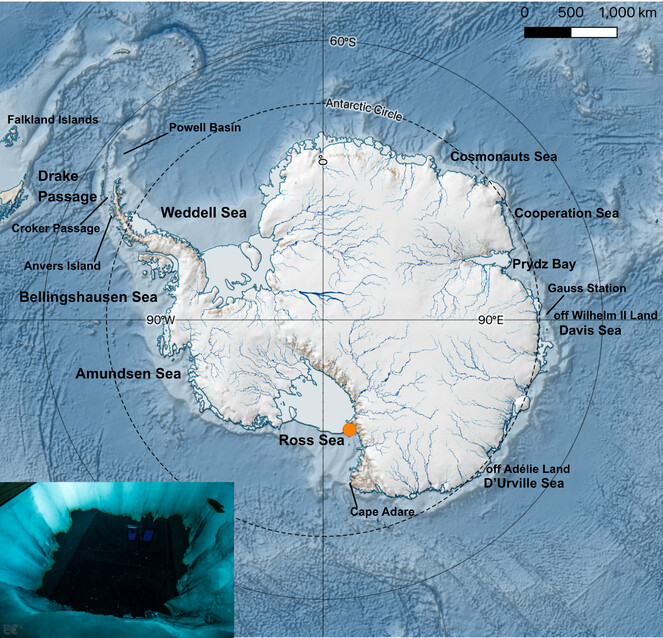
Imagery and video data of under-ice gelatinous zooplankton were acquired at Cape Evans (McMurdo Sound, Ross Sea) over two different field campaigns conducted during the period of November-December 2018 and 2019 (Antarctic summer). A field camp was established for a duration of 3 weeks for each campaign, and was located approximately 200 m from the coast on Antarctic fast-ice (77.637° S, 166.401°E) (Fig.
Photography and videography
For both campaigns, a large 2 × 1.8 m ice-hole was made through a combination of 6” Jiffy auger holes and hot-water drilling (Fig.
Underwater footage of the entire study area was conducted using two different Remotely Operated Vehicles (ROVs), equipped with a GoPro Hero 5; a Seabotix LBV-300 ROV (Teledyne Marine, California, USA) for the 2018 campaign and a BlueROV2 (Blue Robotics, California, USA) for the 2019 campaign. Additional underwater footage straight beneath the ice hole was acquired using Boxfish 360’s three large Micro Four Thirds cameras (Boxfish Research Limited, Auckland, New Zealand) deployed at different depths of the water columns using a weighted rope.
Treatment of images and videos
The raw, untreated images and videos were used to build online datasets (see "Data resources" section). The brightness and contrast of the images to build the plates (Figs. 2-19) were sometimes altered to reveal underlying morphological structures. The Common Objects in Context (COCO) dataset was generated by annotating the specimens in the images and videos using the free, open source, Computer Vision Annotation Tool (CVAT) (https://github.com/openvinotoolkit/cvat). COCO is a large-scale object detection, segmentation and captioning dataset. It is the most popular type of dataset used for training deep learning programmes.
Data resources
The occurence data reported in this paper are deposited at GBIF, the Global Biodiversity Information Facility, http://ipt.pensoft.net/resource?r=life_beneath_the_ice-jellyfish_and_ctenophores_from_the_ross_sea_antarctica&. The raw, untreated images and videos are available at http://morphobank.org/permalink/?P3993 and https://www.youtube.com/playlist?list=PL5Njywnb4yMJQa7koLOM3BhjKU7Ii2HiZ, respectively. The COCO datasets can be found on https://zenodo.org/ with the following DOI:10.5281/zenodo.5118013.
Anthoathecata
Koellikerina maasi
-
scientificName: Koellikerina maasi; kingdom:Animalia; phylum:Cnidaria; class:Hydrozoa; order:Anthoathecata; family:Bougainvilliidae; genus:Koellikerina; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2019-11-26; individualID:MCMEC2019_Koellikerina_maasi_a; lifeStage:adult; associatedMedia:"http://morphobank.org/permalink/?P3993", "https://youtu.be/QiBPf_HYrQ8", "https://youtu.be/-BonvTRljY8"; identifiedBy:Dhugal Lindsay; type:StillImage, Video; language:en; rightsHolder:Emiliano Cimoli
-
scientificName: Koellikerina maasi; kingdom:Animalia; phylum:Cnidaria; class:Hydrozoa; order:Anthoathecata; family:Bougainvilliidae; genus:Koellikerina; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:NIKON D500 camera equipped with a TAMRON SP 90mm F2.8 Di Macro VC USD F017N lens; eventDate:2018-11-27; individualID:MCMEC2018_Koellikerina_maasi_b; lifeStage:adult; associatedMedia:http://morphobank.org/permalink/?P3993; identifiedBy:Dhugal Lindsay; type:StillImage; language:en; rightsHolder:Emiliano Cimoli
-
scientificName: Koellikerina maasi; kingdom:Animalia; phylum:Cnidaria; class:Hydrozoa; order:Anthoathecata; family:Bougainvilliidae; genus:Koellikerina; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:NIKON D500 camera equipped with a TAMRON SP 90mm F2.8 Di Macro VC USD F017N lens; eventDate:2018-11-29; individualID:MCMEC2018_Koellikerina_maasi_c; lifeStage:adult; associatedMedia:http://morphobank.org/permalink/?P3993; identifiedBy:Dhugal Lindsay; type:StillImage; language:en; rightsHolder:Emiliano Cimoli
Southern Ocean, in the McMurdo Sound (
Original description after
Koellikerina maasi. A, B. Drawing from the original species description by
Additional information from specimens from the Southern Ocean: from Gauss Station (0-385 m depth) (
Additional information on specimens identified as same species from outside the Southern Ocean: from west coast of Madagascar (
Literature giving diagnostic characters without describing new specimens:
Description and comments on observed material (Fig.
New undescribed characteristics: Ex-umbrella not smooth, showing small concavities and warts; the radial canals departing from the manubrium bend downwards, extending over four small perradial mesogleal convexities with ovoid yellowish nodules, before bending back up again to run over the ectodermal cavity of the sub-umbrella to the bell rim. These perradial mesogleal convexities are similar to those seen in the Leptothecate medusa Modeeria rotunda Quoy & Gaimard, 1827 (
Characteristics differing from previous descriptions: mesogleal thickness between the ex- and sub-umbrella on the top of the bell ca. one fourth of the height of the ex-umbrella, similar to the drawing of the New Zealand specimen of
Leuckartiara brownei
-
scientificName: Leuckartiara brownei; kingdom:Animalia; phylum:Cnidaria; class:Hydrozoa; order:Anthoathecata; family:Pandeidae; genus:Leuckartiara; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2019-11-16; individualID:MCMEC2019_Leuckartiara_brownei_a; lifeStage:adult; associatedMedia:"http://morphobank.org/permalink/?P3993", "https://youtu.be/QkFIkgJPmto", "https://youtu.be/fRwpi5KAhWQ", "https://youtu.be/dEIbVYlF_TQ", "https://youtu.be/liqjNkGn3Sk"; identifiedBy:Dhugal Lindsay; type:StillImage, Video; language:en; rightsHolder:Emiliano Cimoli
-
scientificName: Leuckartiara brownei; kingdom:Animalia; phylum:Cnidaria; class:Hydrozoa; order:Anthoathecata; family:Pandeidae; genus:Leuckartiara; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:NIKON D500 camera equipped with a TAMRON SP 90mm F2.8 Di Macro VC USD F017N lens; eventDate:2018-11-29; individualID:MCMEC2018_Leuckartiara_brownei_b; lifeStage:adult; associatedMedia:http://morphobank.org/permalink/?P3993; identifiedBy:Dhugal Lindsay; type:StillImage; language:en; rightsHolder:Emiliano Cimoli
-
scientificName: Leuckartiara brownei; kingdom:Animalia; phylum:Cnidaria; class:Hydrozoa; order:Anthoathecata; family:Pandeidae; genus:Leuckartiara; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:NIKON D500 camera equipped with a TAMRON SP 90mm F2.8 Di Macro VC USD F017N lens; eventDate:2018-11-29; individualID:MCMEC2018_Leuckartiara_brownei_c; lifeStage:adult; associatedMedia:http://morphobank.org/permalink/?P3993; identifiedBy:Dhugal Lindsay; type:StillImage; language:en; rightsHolder:Emiliano Cimoli
Southern Ocean, in the McMurdo Sound [described by Browne (1910) as a juvenile Perigonimus sp. according to
Original description after
Leuckartiara brownei. A. Line drawing of the holotype (bell height 10 mm) (
Additional information on specimens identified as same species from outside the Southern Ocean: from the Mediterranean Sea (
Literature giving diagnostic characters without describing new specimens:
Description of and comments on observed material (Fig.
Narcomedusae
Solmundella bitentaculata
-
scientificName: Solmundella bitentaculata; kingdom:Animalia; phylum:Cnidaria; class:Hydrozoa; order:Narcomedusae; family:Solmundaeginidae; genus:Solmundella; continent:Antarctica; waterBody:McMurdo Sound; maximumElevationInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:NIKON D500 camera equipped with a TAMRON SP 90mm F2.8 Di Macro VC USD F017N lens; eventDate:2018-11-27; individualID:MCMEC2018_Solmundella_bitentaculata_a; lifeStage:adult; associatedMedia:http://morphobank.org/permalink/?P3993; identifiedBy:Dhugal Lindsay; type:StillImage; language:en; rightsHolder:Emiliano Cimoli
Cosmopolitan (
Original description after
Solmundella bitentaculata. A. Drawing from the original description as Carybdea bitentaculata from Indonesia (
Additional information from specimens from the Southern Ocean: There is currently only one species of Solmundella, though historically they were long dissociated into the species S. bitentaculata (Quoy & Gaimard, 1833) and S. mediterranea (Müller, 1851), which were subsequently synonymised (
Additional information from specimens from outside the Southern Ocean: Solmundella bitentaculata is a cosmopolitan species, which may actually be composed of multiple cryptic species (
Characteristics of the observed material differing with previous descriptions (Fig.
Leptomedusae
Leptomedusa sp. A
-
kingdom: Animalia; phylum:Cnidaria; class:Hydrozoa; order:Leptomedusae; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2019-11-14; individualID:MCMEC2019_Leptomedusa_sp_A_a; lifeStage:adult; associatedMedia:http://morphobank.org/permalink/?P3993; identifiedBy:Dhugal Lindsay; type:StillImage; language:en; rightsHolder:Emiliano Cimoli
Description of and comments on observed material: N = 1 in 2019 (Fig.
Leptomedusa sp. B
-
kingdom: Animalia; phylum:Cnidaria; class:Hydrozoa; order:Leptomedusae; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2019-11-22; individualID:MCMEC2019_Leptomedusa_sp_B_a; lifeStage:adult; associatedMedia:https://youtu.be/hrufuPQ7F8U; identifiedBy:Dhugal Lindsay; type:StillImage, Video; language:en; rightsHolder:Emiliano Cimoli
Description of and comments on observed material: N = 1 in 2019 (Fig.
Siphonophorae
Pyrostephos vanhoeffeni
-
scientificName: Pyrostephos vanhoeffeni; kingdom:Animalia; phylum:Cnidaria; class:Hydrozoa; order:Siphonophorae; family:Pyrostephidae; genus:Pyrostephos; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2019-11-25; individualID:MCMEC2019_Pyrostephos_vanhoeffeni_a; lifeStage:adult; associatedMedia:"http://morphobank.org/permalink/?P3993", "https://youtu.be/R5E_HAW49DM", "https://youtu.be/o0XGpFavjyo", "https://youtu.be/QAADM0MERIo", "https://youtu.be/zEA6-7-qcYI", "https://youtu.be/SbuAA2nEVnU"; identifiedBy:Dhugal Lindsay; type:StillImage, Video; language:en; rightsHolder:Emiliano Cimoli
-
scientificName: Pyrostephos vanhoeffeni; kingdom:Animalia; phylum:Cnidaria; class:Hydrozoa; order:Siphonophorae; family:Pyrostephidae; genus:Pyrostephos; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2018-11-29; individualID:MCMEC2019_Pyrostephos_vanhoeffeni_b; lifeStage:adult; associatedMedia:"http://morphobank.org/permalink/?P3993", "https://youtu.be/tW2Ko92f3Bo", "https://youtu.be/2rrQCybEg0Q", "https://youtu.be/G9tev_gdUvQ", "https://youtu.be/NfJjKBRh5Hs", "https://youtu.be/1-aLzxLpzWs", "https://youtu.be/HnaIASH9yM0", "https://youtu.be/OSTJ3ldg63w", "https://youtu.be/d7OPyXn64g4", "https://youtu.be/YE50FZg8mpU", "https://youtu.be/csUoJl5Mapc", "https://youtu.be/uc6cP0YSrwc"; identifiedBy:Dhugal Lindsay; type:StillImage, Video; language:en; rightsHolder:Emiliano Cimoli
Antarctica: Ross Sea from under the ice (
Original description after
Pyrostephos vanhoeffeni. Drawing from the original account by
Description after
Description of and comments on observed material (Fig.
Semaeostomeae
Diplulmaris antarctica
-
scientificName: Diplulmaris antarctica; kingdom:Animalia; phylum:Cnidaria; class:Hydrozoa; order:Semaeostomeae; family:Ulmaridae; genus:Diplulmaris; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2019-11-16; individualID:MCMEC2019_Diplulmaris_antarctica_a; lifeStage:adult; associatedMedia:"http://morphobank.org/permalink/?P3993", "https://youtu.be/qKnd53wZVZo"; identifiedBy:Dhugal Lindsay; type:StillImage, Video; language:en; rightsHolder:Emiliano Cimoli
-
scientificName: Diplulmaris antarctica; kingdom:Animalia; phylum:Cnidaria; class:Hydrozoa; order:Semaeostomeae; family:Ulmaridae; genus:Diplulmaris; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2019-11-30; individualID:MCMEC2019_Diplulmaris_antarctica_b; lifeStage:juvenile; associatedMedia:"https://youtu.be/q9pcie-ri9M", "https://youtu.be/33EccdfSTh8", "https://youtu.be/kki0KyhFdUc"; identifiedBy:Dhugal Lindsay; type:StillImage; language:en; rightsHolder:Emiliano Cimoli
-
scientificName: Diplulmaris antarctica; kingdom:Animalia; phylum:Cnidaria; class:Hydrozoa; order:Semaeostomeae; family:Ulmaridae; genus:Diplulmaris; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2019-12-01; individualID:MCMEC2019_Diplulmaris_antarctica_c; lifeStage:juvenile; associatedMedia:"http://morphobank.org/permalink/?P3993", "https://youtu.be/pLlGoqwDZMs", "https://youtu.be/4PbHRjs4JVQ", "https://youtu.be/fh1rmQ_piZ8", "https://youtu.be/9MZ2BrZBLvE", "https://youtu.be/ce7Rvhf_8rw", "https://youtu.be/4XyQIQw04vs", "https://youtu.be/qDyH3_mnVBs", "https://youtu.be/NYDEDKs8PR0", "https://youtu.be/6EMBHjnJ7cU"; identifiedBy:Dhugal Lindsay; type:StillImage, Video; language:en; rightsHolder:Emiliano Cimoli
Southern Ocean: off Anvers Island, Antarctic Peninsula (
Original description after
Juvenile Diplulmaris antarctica. A. Drawing from the original description (modified from
Adult Diplulmaris antarctica. A. Drawing from the original description by
Additional information from specimens from the Southern Ocean: from Gauss Station as Ulmaropsis drygalskii (
Additional information from specimens from outside the Southern Ocean: to our knowledge, no specimens have been described outside the Southern Ocean. The records from Madagascar (
Comments on observed material: N = 3 in 2019 [two juveniles (Fig.
Beroida
Genus Beroe
Description of the genus Beroe: sac-like bodies without tentacles or tentacle sheaths, very large mouth and stomodaeum, eight meridional canals, connected orally and a row of branched papillae in a figure of eight at the aboral pole (
Morphological characteristics of Beroe species that have been reported for the Southern Ocean (the compared characters were chosen, based on those that were evident in our filmed specimens).
|
Species |
Body length (L) adults (mm) |
Body shape |
Comb row length vs. body length adult |
Inter-comb plate distance |
Branching from meridional canals |
Colour |
Illustration |
Type locality |
|
B. compacta Moser, 1909 |
2.5 |
cylindrical |
whole body length (based on drawing) |
short |
/ |
opaque, shimmering yellowish between white comb rows |
Fig. |
Gauss Station, Antarctica |
|
B. cucumis Fabricius, 1780 |
/ |
oblong shape, elongated towards the extremities |
whole body length |
/ |
/ |
whitish with pink/red dots |
/ |
Greenland |
|
B. ovatus Bosc, 1802 |
/ |
oval |
whole body length (based on drawing) |
same as comb plate width (based on drawing) |
/ |
transparent with nine uncoloured comb rows |
Fig. |
“all seas” |
|
B. ovata sensu Chun, 1880 |
< 160 |
body elongated, cylindrical, not very noticeably compressed, gradually tapering towards the aboral pole in a semicircular arc |
3/4 (based on drawing) |
short (space between three comb plates ca. equal to width of comb plate, based on drawing) |
numerous diverticula, no anastomoses |
young transparent; adults during period of increased reproduction pink or bright red, otherwise unpigmented, grey-white or light transparent red |
Fig. |
Gulf of Naples, Mediterranean Sea |
|
B. ovata sensu Mayer, 1912 |
70-115 |
mitre-shaped with lateral compression very marked and mouth a wide-gaping slit |
3/4 (1/2 in juveniles) |
wide (space between two comb plates ca. equal to width of comb plate, based on drawing) |
loose network of numerous diverticula with few anastomoses |
dull-milky (in Florida) to highly coloured, with deep pink or reddish-brown canals (in northern waters as in Chesapeake Bay) |
Fig. |
Atlantic Coast of North America |
|
Beroe sp. A |
/ |
oval (body length ca. 2.4 times body width) |
ca. 2/3 of body length |
short (space between four comb plates ca. equal to width of comb plate) |
diverticula without anastomoses |
brownish-orange stomodeum and diverticula |
Fig. |
Mc Murdo Sound, this study |
|
Beroe sp. B |
ca. 35 |
oval (body length ca. 1.5 times body width) |
ca. 1/4 of body length |
short (space between 5 comb plates ca. equal to width of comb plate) |
no diverticula |
transparent to milky white |
Fig. |
Mc Murdo Sound, this study |
Beroe species recorded from the Southern Ocean
b: Beroe ovata sensu Chun, 1880, drawing of an adult specimen from the Gulf of Naples, Mediterranean Sea (modified from
c: Beroe ovata sensu Mayer, 1912 drawing of a specimen from St. Mary’s River, Maryland (modified from
d: Beroe compacta Moser, 1909 drawing (length 2.5 mm) from Gauss Station, Antarctica (modified from
Beroe sp. A
-
kingdom: Animalia; phylum:Ctenophora; class:Nuda; order:Beroida; genus:Beroe; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2019-11-16; individualID:MCMEC2019_Beroe_sp_A_a; lifeStage:adult; associatedMedia:http://morphobank.org/permalink/?P3993; identifiedBy:Dhugal Lindsay; type:StillImage, Video; language:en; rightsHolder:Emiliano Cimoli
-
kingdom: Animalia; phylum:Ctenophora; class:Nuda; order:Beroida; genus:Beroe; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2019-11-30; individualID:MCMEC2019_Beroe_sp_A_b; lifeStage:juvenile; associatedMedia:"https://youtu.be/kGBUQ7ZtH9U", "https://youtu.be/Vbl_KEmPNmU"; identifiedBy:Dhugal Lindsay; type:Video; language:en; rightsHolder:Emiliano Cimoli
-
kingdom: Animalia; phylum:Ctenophora; class:Nuda; order:Beroida; genus:Beroe; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2018-11-16; individualID:MCMEC2018_Beroe_sp_A_c; lifeStage:adult; associatedMedia:"http://morphobank.org/permalink/?P3993", "https://youtu.be/trWsOI6g-9Y"; identifiedBy:Dhugal Lindsay; type:StillImage; language:en; rightsHolder:Emiliano Cimoli
-
kingdom: Animalia; phylum:Ctenophora; class:Nuda; order:Beroida; genus:Beroe; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2018-11-25; individualID:MCMEC2018_Beroe_sp_A_d; lifeStage:juvenile; associatedMedia:http://morphobank.org/permalink/?P3993; identifiedBy:Dhugal Lindsay; type:StillImage; language:en; rightsHolder:Emiliano Cimoli
-
kingdom: Animalia; phylum:Ctenophora; class:Nuda; order:Beroida; genus:Beroe; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2018-11-27; individualID:MCMEC2018_Beroe_sp_A_e; lifeStage:juvenile; associatedMedia:http://morphobank.org/permalink/?P3993; identifiedBy:Dhugal Lindsay; type:StillImage; language:en; rightsHolder:Emiliano Cimoli
-
kingdom: Animalia; phylum:Ctenophora; class:Nuda; order:Beroida; genus:Beroe; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2018-11-17; individualID:MCMEC2019_Beroe_sp_A_f; lifeStage:adult; associatedMedia:http://morphobank.org/permalink/?P3993; identifiedBy:Dhugal Lindsay; type:StillImage; language:en; rightsHolder:Emiliano Cimoli
-
kingdom: Animalia; phylum:Ctenophora; class:Nuda; order:Beroida; genus:Beroe; continent:Antarctica; waterBody:Little Razorback Island; eventDate:2010-12-02; individualID:LRISH2010_Beroe_sp_A_g; lifeStage:adult; associatedMedia:http://morphobank.org/permalink/?P3993; identifiedBy:Gerlien Verhaegen; type:StillImage; language:en; rightsHolder:Shawn Harper
A similar brownish-orange undescribed Beroe species has been observed in Antarctica, in the Ross Sea (
Specimens of Beroe sp. A observed in the Ross Sea. a-c and e-f photos courtesy: E. Cimoli; d photo courtesy: Shawn Harper.
b: Adult specimen in apico-lateral view observed on 16/11/2018 (MCMEC2018_Beroe_sp_A_c)
c: Adult specimen in lateral view observed on 16/11/2018 (MCMEC2018_Beroe_sp_A_c)
d: Adult specimen observed at Little Razorback Island, Ross Sea, on 02/12/2010 (LRISH2010_Beroe_sp_A_g).
e: Juvenile specimen MCMEC2019_Beroe_sp_A_b in stomodeal view observed on 30/11/2019.
f: Juvenile specimen MCMEC2019_Beroe_sp_A_b observed on 30/11/2019.
Description of and comments on observed material (Fig.
Beroe sp. B
-
kingdom: Animalia; phylum:Ctenophora; class:Nuda; order:Beroida; genus:Beroe; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2019-11-15; individualID:MCMEC2019_Beroe_sp_B_a; associatedMedia:"http://morphobank.org/permalink/?P3993", "https://youtu.be/VC-peoIaI0I"; identifiedBy:Dhugal Lindsay; type:StillImage, Video; language:en; rightsHolder:Emiliano Cimoli
First reported observation.
Description of and comments on observed material: N = 1 in 2019 (Fig.
Cydippida
Callianira cristata
-
scientificName: Callianira cristata; kingdom:Animalia; phylum:Ctenophora; class:Tentaculata; order:Cydippida; family:Cydippida incertae sedis; genus:Callianira; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2019-11-22; individualID:MCMEC2019_Callianira_cristata_a; lifeStage:adult; associatedMedia:"https://youtu.be/30g9CvYh5JE"; identifiedBy:Dhugal Lindsay; type:Video; language:en; rightsHolder:Emiliano Cimoli
-
scientificName: Callianira cristata; kingdom:Animalia; phylum:Ctenophora; class:Tentaculata; order:Cydippida; family:Cydippida incertae sedis; genus:Callianira; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2019-11-20; individualID:MCMEC2019_Callianira_cristata_b; lifeStage:adult; associatedMedia:http://morphobank.org/permalink/?P3993; identifiedBy:Dhugal Lindsay; type:StillImage; language:en; rightsHolder:Emiliano Cimoli
Antarctica: Ross Sea [photographed by ©Shawn Harper in
Original description after Moser (1909) (Fig.
Callianira cristata. A-B. Drawing from the original description (specimen length 12 mm) (modified from
Description and comments of observed material: N = 1 in 2019 (Fig.
"fam. Mertensiidae" sp. A
-
kingdom: Animalia; phylum:Ctenophora; class:Tentaculata; order:Cydippida; family:Mertensiidae; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2019-11-15; individualID:MCMEC2019_Mertensiidae_sp_A_a; associatedMedia:"http://morphobank.org/permalink/?P3993", "https://youtu.be/dKELHUITnlg", "https://youtu.be/GE6WgN8VBdw", "https://youtu.be/0W2HHLW71Pw"; identifiedBy:Dhugal Lindsay; type:StillImage, Video; language:en; rightsHolder:Emiliano Cimoli
-
kingdom: Animalia; phylum:Ctenophora; class:Tentaculata; order:Cydippida; family:Mertensiidae; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Sony Alpha 7 III camera equipped with a FE 90mm F2.8 Macro G OSS lens; eventDate:2019-11-29; individualID:MCMEC2019_Mertensiidae_sp_A_b; associatedMedia:"https://youtu.be/pvXYlQGZIVg"; identifiedBy:Dhugal Lindsay; type:Video; language:en; rightsHolder:Emiliano Cimoli
-
kingdom: Animalia; phylum:Ctenophora; class:Tentaculata; order:Cydippida; family:Mertensiidae; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:NIKON D500 camera equipped with a TAMRON SP 90mm F2.8 Di Macro VC USD F017N lens; eventDate:2018-11-27; individualID:MCMEC2018_Mertensiidae_sp_A_c; associatedMedia:http://morphobank.org/permalink/?P3993; identifiedBy:Dhugal Lindsay; type:StillImage; language:en; rightsHolder:Emiliano Cimoli
-
kingdom: Animalia; phylum:Ctenophora; class:Tentaculata; order:Cydippida; family:Mertensiidae; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:NIKON D500 camera equipped with a TAMRON SP 90mm F2.8 Di Macro VC USD F017N lens; eventDate:2018-11-29; individualID:MCMEC2018_Mertensiidae_sp_A_d; associatedMedia:http://morphobank.org/permalink/?P3993; identifiedBy:Dhugal Lindsay; type:StillImage; language:en; rightsHolder:Emiliano Cimoli
-
kingdom: Animalia; phylum:Ctenophora; class:Tentaculata; order:Cydippida; family:Mertensiidae; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:Seabotix LBV-300 ROV equipped with a GoPro Hero 5; eventDate:2018-12-01; individualID:MCMEC2018_Mertensiidae_sp_A_e; associatedMedia:http://morphobank.org/permalink/?P3993; identifiedBy:Dhugal Lindsay; type:StillImage; language:en; rightsHolder:Emiliano Cimoli
-
kingdom: Animalia; phylum:Ctenophora; class:Tentaculata; order:Cydippida; family:Mertensiidae; continent:Antarctica; waterBody:McMurdo Sound; maximumDepthInMeters:1; decimalLatitude:-77.637; decimalLongitude:166.401; samplingProtocol:NIKON D500 camera equipped with a TAMRON SP 90mm F2.8 Di Macro VC USD F017N lens; eventDate:2018-11-27; individualID:MCMEC2018_Mertensiidae_sp_A_f; associatedMedia:http://morphobank.org/permalink/?P3993; identifiedBy:Dhugal Lindsay; type:StillImage; language:en; rightsHolder:Emiliano Cimoli
first time observation.
Description of the family Mertensiidae: according to the key to Ctenophora by
Comments on observed material: N = 4 in 2018 and N = 2 in 2019 (Fig.
Mertensiidae sp. A. Lateral views (A-D), oral view (E) and aboral view (F) of specimens observed on 27/11/2018 (MCMEC2018_Mertensiidae_sp_A_c) (A) and 15/11/2019 (MCMEC2019_Mertensiidae_sp_A_a) (B-F). Abbreviations: ad-c., adradial canal; a.p., anal pore; c.g., ciliary grove; m., mouth; o.t.s.o., oral end opening of tentacle sheath; p.p., polar plate; s. o., tentacle sheath opening; st., statocyst; ss-cr., substomodeal comb row; st-cr., subtentacular comb row; t.b., tentacle bulb. C-H photos courtesy: E. Cimoli.
Other Phyla
Phylum Mollusca
Mollusca was the third most-observed phylum (20% of all observations): Clione limacina antarctica (N = 2 in 2018, N = 1 in 2019) (Fig.
Order
Analysis
Synopsis of observed species
A total of 49 individuals were observed during the summer of 2018 (N = 25) and 2019 (N = 24). The majority of observed specimens belonged either to the phylum Cnidaria (36.7%) or Ctenophora (30.6%), whereas the remaining observed phyla, namely Mollusca (22.4%), Arthropoda (8.1%) and Annelida (2.0%), were less represented. The observed species are summarised in Table
|
Phylum |
Taxa |
Species |
N (2018) |
N (2019) |
First time report for the Ross sea? |
Figures |
|
|
Cnidaria |
Hydrozoa (class) |
Anthoathecata (order) |
Koellikerina maasi |
2 |
1 |
no |
Fig. 2 |
|
Leuckartiara brownei |
2 |
1 |
no |
Fig. 3 |
|||
|
Leptothecata (order) |
Leptomedusa sp. A |
0 |
1 |
yes |
Fig. 5 |
||
|
Leptomedusa sp. B |
0 |
1 |
no (if our proposed species assignment is correct) |
Fig. 6 |
|||
|
Narcomedusae (order) |
Solmundella bitentaculata |
1 |
0 |
no |
Fig. 4 |
||
|
Siphonophorae (order) |
Pyrostephos vanhoeffeni |
4 |
2 |
no |
Fig. 7 |
||
|
Scyphozoa (class) |
Semaeostomeae (order) |
Diplulmaris antarctica |
0 |
3 |
no |
Figs. 8-9 |
|
|
Ctenophora |
Beroida (order) |
Beroe sp. A |
3 |
4 |
yes (previously only images erroneously assigned to Beroe cucumis were published, online) |
Fig. 10 |
|
|
Beroe sp. B |
0 |
1 |
yes |
Fig. 11 |
|||
|
Cydippida (order) |
Callianira cristata |
0 |
1 |
yes (previously only images assigned to Mertensiidae were published, online) |
Fig. 12 |
||
|
Mertensiidae sp. A |
4 |
2 |
yes |
Fig. 13 |
|||
|
Mollusca |
Pteropoda (order) |
Clione limacina antarctica |
2 |
1 |
no |
Fig. 14 |
|
|
Spongiobranchaea australis |
1 |
0 |
no |
Fig. 15 |
|||
|
Limacina helicina antarctica |
0 |
2 |
NA |
Fig. 16 |
|||
|
incertae sedis |
Gastropoda larvae |
3 |
2 |
NA |
Fig. 17 |
||
|
Arthropoda |
Amphipoda (order) |
Eusiridae |
3 |
1 |
NA |
Fig. 18 |
|
|
Annelida |
Polychaeta (class) |
Syllidae |
0 |
1 |
NA |
Fig. 19 |
|
|
Total |
25 |
24 |
|||||
Discussion
In this study, we conducted an in situ /in aqua optical survey of gelatinous zooplankton from under the ice in the McMurdo Sound, Antarctica. Our study represents the first formal optics-based survey of gelatinous zooplankton in the Ross Sea and the first study to use in situ /in aqua observations to describe taxonomic and a few trophic and behavioural characteristics of gelatinous zooplankton from the Southern Ocean. The Ross Sea has seen numerous net sampling surveys of gelatinous zooplankton in the past (e.g.
Our study demonstrates how valuable optical in situ observations are to investigate gelatinous zooplankton. Nonetheless, our study also encountered a few limitations. For example, despite most of the studied species being transparent, the observation of internal morphological characters is difficult without the collection and dissection of the specimens. A second limitation lies in the identification of certain species, based on morphological traits alone, especially for genera with numerous species, such as Beroe spp., where the type specimens are no longer extant. For one leptomedusan and three ctenophore species, their morphology did not match that of any species previously reported from the Southern Ocean. They could potentially be undescribed species, although this needs to be confirmed through DNA barcoding of all the described species from their type localities and further morphological comparisons. The future of gelatinous zooplankton studies lies in the integration of different methodologies, including appropriate collection methods, optical survey tools and molecular genetic comparisons.
Acknowledgements
Fieldwork was supported by the New Zealand Antarctic Research Institute (NZARI) grant under project code K043. We are grateful for the support of Antarctica New Zealand and the rest of the K043 team for field-based operation and logistics involved in the acquisition of the imagery. Particular thanks go to Dr. Vanessa Lucieer and Dr. Zbyněk Malenovský of the University of Tasmania (Australia) for support in acquiring the images. We thank Shawn Harper of the North Carolina Aquarium on Roanoke Island (USA) for providing one of his Beroe pictures from the Ross Sea. Dr. Naoto Jimi of the National Institute of Polar Research (Japan) is thanked for the identification of the Polychaete family and Svenja Halfter of the University of Tasmania (Australia) for the Amphipod family. We are also grateful to George Branch (University of Cape Town, South Africa) for literature support. The following reviewers are thanked for their constructive comments on the previous version of the manuscript: Dr. Horia Galea (Hydrozoan Research Laboratory, France), Dr. Steven Haddock (Monterey Bay Aquarium Research Institute, USA), Dr. Gillian Mapstone (The National History Museum, UK) and Dr. Otto Oliveira (Universidade Federal do ABC, Brazil). This publication was within the scope of the Research Fellowship project VE 1192/1-1 to GV, funded by the Deutsche Forschungsgemeinschaft (DFG) and a contribution to the Belmont Forum Project “World Wide Web of Plankton Image Curation”, (Belmont Forum grant 18076935 to DL).
Author contributions
GV wrote the main manuscript, described the specimens, prepared the figures, and annotated the images and videos to obtain the Common Objects in Context (COCO) dataset for machine learning. EC conducted the fieldwork and wrote the methods part. DL identified the species, revised the taxonomy and aided in the conceptualisation of the study. GV, DL and EC reviewed and edited the manuscript.
References
- Fishing down the food web of the Antarctic continental shelf and slope.Polar Record50(1):92‑107. https://doi.org/10.1017/S0032247412000757
- Antarctic Siphonophores from Plankton Samples of the United States Antarctic Research Program: Eltanin Cruises for Spring, Summer, Fall, and Winter (Cruises 3-5, 8-23, 25-28, 30, 35, and 38).In:Biology of the Antarctic Seas XX.Antarctic Research Series,49.American Geophysical Union,Washington, DC,436pp.
- Siphonophorae.In:Atlas del zooplancton del Atlántico Sudoccidental y métodos de trabajo con el zooplancton marino.58pp.
- Beroe abyssicola Mortensen, 1927: a redescription.Contributions to Natural Science of The Royal British Columbia Museum9:1‑7.
- Pyrostephos vanhoeffeni (Cnidaria, Siphonophora): New data on zooid development and an updated review on its distribution.XIth SCAR Biology Symposium(May 2014).
- Chapter 14. Sea ice as a habitat for primary producers. In:Sea ice.3rd.John Wiley & Sons, Ltd Chichester, UK,352-369pp.
- Long-term decline in krill stock and increase in salps within the Southern Ocean.Nature432(November):100‑103. https://doi.org/10.1038/nature02950.1.
- Southern Ocean in-situ temperature trends over 25 years emerge from interannual variability.Nature Communications12(514):1‑9. https://doi.org/10.21203/rs.3.rs-36449/v1
- RMT Trawl catch from the 1980/81 V5 FIBEX voyage.OBIS. URL: https://obis.org/dataset/82e3523b-d12b-4403-8276-a7b4ee69c49e
- RMT Trawl catch from1985/86 V1 ADBEX III voyage.OBIS. URL: https://obis.org/dataset/75e2a2b6-f58f-4c43-aefd-878cee7865b1
- RMT Trawl catch from the 1992/93 V6 KROCK voyage.OBIS. URL: https://obis.org/dataset/944da8c9-dce6-4f17-885c-976a5d831209
- RMT Trawl catch from the 1984/85 V5 SIBEX2 voyage. URL: https://data.aad.gov.au/ipt/resource?r=rmt_sibex2
- An evaluation of acoustic and video methods to estimate the abundance and vertical distribution of jellyfish.Journal of Plankton Research25(11):1307‑1318. https://doi.org/10.1093/plankt/fbg084
- The status of Callianira hexagona (Slabber, 1778) (Ctenophora).Zoologische Mededelingen Leiden85(11):825‑833.
- Some Medusae and Siphonophorae from the western Atlantic.Bulletin of the Museum of Comparative Zoölogy at Harvard CollegeLXII(8):365‑442.
- Reports on the scientific results of the expedition to the eastern tropical Pacific, in charge of Alexander Agassiz, by the U.S. Fish Commission steamer "Albatross", from October, 1904, to March, 1905. XVI. The Medusae.Memoirs of the Museum of Comparative Zoology at Harvard College37:1‑243. https://doi.org/10.1017/CBO9781107415324.004
- Medusae and Siphonophorae collected by the U.S. fisheries steamer "Albatross" in the Northwestern Pacific, 1906.Proceedings of the United States National Museum44(1946):1‑119. https://doi.org/10.5479/si.00963801.44-1946.1
- Trachymedusae and Narcomedusae of South-East Australian Waters.Marine and Freshwater Research6(3):410‑428. https://doi.org/10.1071/MF9550410
- Histoire naturelle des vers : contenant leur description et leurs moeurs. Avec figures dessinées d'après nature.2.Déterville,Paris,300pp.
- Notes additionnelles sur les Hydroméduses de la Mer de Bismarck (Hydrozoa-Cnidaria) II*.Indo-Malayan Zoology5:87‑99.
- Hydromedusae of the New Zealand Oceanographic Institute (Hydrozoa, cnidaria).New Zealand Journal of Zoology22(2):223‑238. https://doi.org/10.1080/03014223.1995.9518038
- Deep-water Hydromedusae from the Lacaze-Duthiers submarine canyon (Banyuls, northwestern Mediterranean) and description of two new genera, Guillea and Parateclaia.Scientia Marina64(SUPPLEMENT 1):87‑95. https://doi.org/10.3989/scimar.2000.64s187
- Fauna of the Mediterranean Hydrozoa.68 (Supplement 2).Scientia Marina,Barcelona,438pp.
- An introduction to Hydrozoa.Mémoires du Muséum national d'Histoire naturelle194:1‑591.
- Towards the acoustic estimation of jellyfish abundance.Marine Ecology Progress Series295:105‑111. https://doi.org/10.3354/meps295105
- XLVI. A preliminary report on Hydromedusae from the Falkland Islands.Journal of Natural History9(52):272‑284. https://doi.org/10.1080/00222930208678586
- Coelenterata V. Medusae. National Antarctic Expedition, 1901-1904.Natural History5:1‑62.
- Medusae from the Indian Ocean.Second Series- ZoologyXVII:171‑210.
- Underwater Field Guide to Ross Island & McMurdo Sound, Antarctica. http://peterbrueggeman.com/nsf/fguide/index.html. Accessed on: 2021-4-01.
- Toward a global reference database of COI barcodes for marine zooplankton.Marine Biology168(78):1‑26. https://doi.org/10.1007/s00227-021-03887-y
- Phylum Cnidaria: corals, medusae, hydroids, myxozoans. In:New Zealand Inventory of Biodiversity.Canterbury University Press,Christchurch,42pp.
- Phylogenetic relationships of the endemic Antarctic benthic hydroids (Cnidaria, Hydrozoa): What does the mitochondrial 16S rRNA tell us about it?Polar Biology33(1):41‑57. https://doi.org/10.1007/s00300-009-0683-5
- Cost considerations for long-term ecological monitoring.Ecological Indicators1(2):123‑134. https://doi.org/10.1016/s1470-160x(01)00015-2
- Defining Southern Ocean fronts and their influence on biological and physical processes in a changing climate.Nature Climate Change10(3):209‑219. https://doi.org/10.1038/s41558-020-0705-4
- Die Ctenophoren des Golfes von Neapel und der Angrenzenden Meeres-Abschnitte.1.W. Engelmann,Leipzig,313pp.
- An under-ice hyperspectral and RGB imaging system to capture fine-scale biophysical properties of sea ice.Remote Sensing11(23):1‑35. https://doi.org/10.3390/rs11232860
- Phylogenetics of Trachylina (Cnidaria: Hydrozoa) with new insights on the evolution of some problematical taxa.Journal of the Marine Biological Association of the United Kingdom88(8):1673‑1685. https://doi.org/10.1017/S0025315408001732
- Climate change and Southern Ocean ecosystems I: How changes in physical habitats directly affect marine biota.Global Change Biology20(10):3004‑3025. https://doi.org/10.1111/gcb.12623
- Multi-jet propulsion organized by clonal development in a colonial siphonophore.Nature Communications6:1‑6. https://doi.org/10.1038/ncomms9158
- RMT Trawl catch from the 1995/96 V4 BROKE voyage.Australian Antarctic Data Centre. URL: https://data.aad.gov.au/ipt/resource?r=rmt_broke
- Mnemiopsis leidyi (Ctenophora) in Narragansett Bay, 1975-1979: abundance, size composition and estimation of grazing.Estuarine, Coastal and Shelf Science15(2):121‑134. https://doi.org/10.1016/0272-7714(82)90023-3
- Biogeographic Atlas of the Southern Ocean.Scientific Committee on Antarctic Research,Cambridge,498pp.
- Trade-offs between sampling effort and data quality in habitat monitoring.Biodiversity and Conservation28(1):55‑73. https://doi.org/10.1007/s10531-018-1636-5
- Preservation of the invasive ctenophore Mnemiopsis leidyi using acidic Lugol's solution.Journal of Plankton Research31(8):917‑920. https://doi.org/10.1093/plankt/fbp030
- Duobrachium sparksae (incertae sedis Ctenophora Tentaculata Cydippida): A new genus and species of benthopelagic ctenophore seen at 3,910 m depth off the coast of Puerto Rico.Plankton and Benthos Research15(4):296‑305. https://doi.org/10.3800/pbr.15.296
- Time and depth comparisons of sub-ice zooplankton in McMurdo Sound, Antarctica.Polar Biology9(7):431‑435. https://doi.org/10.1007/BF00443229
- JellyMonitor: Automated detection of jellyfish in sonar images using neural networks.International Conference on Signal Processing Proceedings, ICSP2018-August:406‑412. https://doi.org/10.1109/ICSP.2018.8652268
- Argentina–Chile National Geographic Pristine Seas Expedition To The Antarctic Peninsula - Deep Sea Cam Data.2.1.SCAR - AntOBIS. URL: https://ipt.biodiversity.aq/resource?r=natgeo_prist0cean_wap_deepseacam_2020&v=1.2
- Warming of the Southern Ocean since the 1950s.Science295(5558):1275‑1277. https://doi.org/10.1126/science.1065863
- Sphaeronectes pughi sp. nov., a new species of sphaeronectid calycophoran siphonophore from the subantarctic zone.Polar Science6(2):196‑199. https://doi.org/10.1016/j.polar.2011.11.001
- The end of an enigmatic taxon: Eudoxia macra is the eudoxid stage of Lensia cossack (Siphonophora, Cnidaria).Systematics and Biodiversity11(3):381‑387. https://doi.org/10.1080/14772000.2013.825658
- A study of the gelatinous mesozooplankton (Cnidaria and Ctenophora) of Eastern Antarctica, summer 2008.Université Pierre et Marie Curie,Paris,51pp.
- Biodiversity and distribution patterns of planktonic cnidarians in San Matías Gulf, Patagonia, Argentina.Marine Ecology34(Supplement 1):71‑82. https://doi.org/10.1111/maec.12027
- Das system der Medusen: erster theil einer Monographie der Medusen.1.G. Fischer, vormals F. Mauke,360pp.
- On the natural history and distribution of oceanic ctenophores.Deep-Sea Research25:233‑256. https://doi.org/10.1016/0146-6291(78)90590-8
- The plankton of the South Georgia whaling grounds and adjacent waters, 1926-1932.Discovery Reports11:1‑456.
- A paradigm shift in the trophic importance of jellyfish?Trends in Ecology and Evolution33(11):874‑884. https://doi.org/10.1016/j.tree.2018.09.001
- Gelatinous zooplankton community around a hydrothermally active deep-sea caldera : results from ROV video records.Plankton and Benthos Research16(1):1‑19. https://doi.org/10.3800/pbr.16.
- The Zooplankton community of Croker Passage, Antarctic Peninsula.Polar Biology4(3):161‑170. https://doi.org/10.1007/BF00263879
- Abundance, distribution and diversity of gelatinous predators along the northern Mid-Atlantic Ridge: A comparison of different sampling methodologies.PLoS One12(11):1‑18. https://doi.org/10.1371/journal.pone.0187491
- AAMBER 86/87 cruise krill/zooplankton sampling data.Australian National Antarctic Research Expedition Research Notes 79.
- Aurora Australis Voyage 7 (KROCK) 1992-93 Zooplankton Data.1.Australian Antarctic Data Centre. URL: https://data.aad.gov.au/metadata/records/AADC-00071
- Aurora Australis Voyage 6 (AAMBER2) 1990-91 Zooplankton Data.1.Australian Antarctic Data Centre. URL: https://data.aad.gov.au/metadata/records/AADC-00083
- Nella Dan: AAMBER Cruise - Zooplankton and Krill data.1.Australian Antarctic Division. URL: https://data.aad.gov.au/metadata/records/AAMBER_zoo-krill
- Nella Dan: SIBEX II Cruise - Krill and zooplankton data.1.Australian Antarctic Data Centre. URL: https://researchdata.edu.au/nella-dan-sibex-krill-zooplankton/701558
- Antarctic ecosystem responses following ice-shelf collapse and iceberg calving: Science review and future research.Wiley Interdisciplinary Reviews: Climate Change12(1):1‑28. https://doi.org/10.1002/wcc.682
- Machine Learning for the study of plankton and marine snow from images.Annual Reviews in Marine Science.
- Composition and structure of macrozooplankton and micronekton communities in the vicinity of free-drifting Antarctic icebergs.Deep-Sea Research Part II: Topical Studies in Oceanography58(11-12):1469‑1484. https://doi.org/10.1016/j.dsr2.2010.11.026
- Salp distribution and size composition in the Atlantic sector of the Southern Ocean.Deep-Sea Research Part II: Topical Studies in Oceanography51:1369‑1381. https://doi.org/10.1016/j.dsr2.2004.06.017
- Image-based monitoring of Jellyfish using deep learning architecture.IEEE Sensors Journal16(8):2215‑2216. https://doi.org/10.1109/JSEN.2016.2517823
- On ctenophores of the neighbourhood of Misaki.The Zoological Society of Japan9(4):451‑474.
- Notes on the two Japanese ctenophores, Lampetia pancerina Chun and Beroe ramosa n. sp.The Zoological Society of Japan10(2):15‑18.
- Hydromedusae collected in the south-western part of the North Sea and in the eastern part of the Channel in 1903-1914.43.Musée Royal d'Histoire Naturelle de Belgique,55pp.
- Medusae collected by the Swedish Antarctic Expedition, 1901-03.4.PA Norstedt & Söner,Stockholm,16pp.
- Medusae and Siphonophora. In:Scientific Results of the Norwegian Antarctic Expeditions 1927–1928.Oslo,65pp.
- Medusae collected by the Lund University Chile Expedition 1948-49. Reports of the Lund University Chile Expedition 1948–49.Acta Universitatis Lundensis47(7):1‑19.
- Hydromedusae.In:Great Barrier Reef Expedition 1928-29 Scientific Reports Volume 6 1938-1958.63pp.
- The medusae of the Tropical West Coast of Africa.Danish Science Press3:239‑324.
- Hydromedusae from the Discovery collections.University Press,Cambridge,339pp.
- Some Jellyfish from macquarie Island and Heard Island.Australian National Antarctic Research Expeditions ReportsI(Zoology):1‑5.
- Medusae mainly from the West Coast of Africa. In:Expédition océanographique belge dans les eaux côtières africaines de l'Atlantique Sud (1948-49).3.33pp.
- Synopsis of the Medusae of the World.Journal of the Marine Biological Association of the United Kingdom40:7‑382. https://doi.org/10.1017/S0025315400007347
- The Hydromedusae of the Pacific and Indian Oceans.Dana-Report68:1‑164.
- The Hydromedusae of the Pacific and Indian Oceans. Sections II and III.Dana-Report72:1‑200.
- Die Beroe (Ctenophora) der südlichen Nord-see, Beroe gracilis n. sp.Zoologischer Anzeiger127(5-6):172‑174.
- Pelagic scyphomedusae (Scyphozoa: Coronatae and Semaeostomeae) of the Southern Ocean. In:Biology of the Antarctic Seas, XVI. Antarctic Research Series.41.106pp. https://doi.org/10.1029/ar041p0059
- Medusae from Mcmurdo Sound, Ross Sea including the descriptions of two new species, Leuckartiara brownei and Benthocodon hyalinus.Polar Biology11(1):19‑25. https://doi.org/10.1007/BF00236517
- Biogeography of jellyfish in the North Atlantic, by traditional and genomic methods.Earth System Science Data7(2):173‑191. https://doi.org/10.5194/essd-7-173-2015
- Ctenophora. In:Marine plankton: A practical guide to ecology, methodology, and taxonomy.1.Oxford University Press,251-263pp. https://doi.org/10.1093/oso/9780199233267.003.0020
- Chapter 6.3. Southern Ocean gelatinous zooplankton. In:Biogeographic Atlas of the Southern Ocean.Cambridge,266-275pp. [ISBN978-0-948277-28-3].
- DNA barcoding of pelagic cnidarians: current status and future prospects.Bulletin of the Plankton Society of Japan62(1):39‑43. https://doi.org/10.24763/bpsj.62.1_39
- Plankton in the spotlight.Australian Antarctic Magazine18.
- The perils of online biogeographic databases: a case study with the ‘monospecific’ genus Aegina (Cnidaria, Hydrozoa, Narcomedusae).Marine Biology Research13(5):494‑512. https://doi.org/10.1080/17451000.2016.1268261
- Distribution and abundance of Larvaceans in the Southern Ocean.University of Tasmania,350pp.
- Studies on Chinese Hydrozoa. I. On some Hydromedusae from the Chekiano Coast.Peking Natural History Bulletin2(4):351‑365.
- Die craspedoten Medusen.Lipsius & Tischer,Kiel & Leipzig,107pp.
- Die Craspedoten Medusen der Siboga-Expedition.Siboga- Expeditie10:1‑84.
- Méduses. In:Expédition Antarctique Française (1903-1905).17pp. [ISBN1290195000315].
- Analysis of locomotion in a siphonophore colony.Proceedings of the Royal Society of London. Series B. Biological Sciences159(975):366‑391. https://doi.org/10.1098/rspb.1964.0008
- Distribution of the Macroplankton in the Atlantic Sector of the Antarctic.Discovery Reports9:65‑160.
- Siphonophora from the Indian Sector of the Antarctic.The Antarctic. The Committee Reports.(30)125‑134.
- Towards a phylogenetic classification of Leptothecata (Cnidaria, Hydrozoa).Scientific Reports6:1‑23. https://doi.org/10.1038/srep18075
- Jellytoring: Real-time jellyfish monitoring based on deep learning object detection.Sensors (Switzerland)20(6):1‑21. https://doi.org/10.3390/s20061708
- Antarctic sea ice change and variability - Physical and ecological implications.Polar Science4(2):149‑186. https://doi.org/10.1016/j.polar.2010.05.001
- Medusae of the World. Volume I The Hydromedusae.Carnegie Institution of Washington109:1‑230.
- Ctenophores of the Atlantic Coast of North America.Carnegie Institution of Washington162:1‑58.
- The Hydromedusae of Madras.Bulletin of the Madras Government Museum, new series, Natural History Section3(2):1‑32.
- Embryologische Studien an Medusen: Ein Beitrag zur Genealogie der Primitiv-organe.A. Holder,Vienna,154pp.
- Medusae, siphonophores and ctenophores of the Alboran Sea, south western Mediterranean.Scientia Marina60(1):145‑163.
- Environmental DNA reflects spatial and temporal jellyfish distribution.PLOS One12(2):1‑15. https://doi.org/10.1371/journal.pone.0173073
- Die Ctenophoren der Siboga-Expedition.EJ Brill,34pp.
- Neues über Cenophoren.Zoologischer Anzeiger31:786‑790.
- Die Ctenophoren der Deutschen Südpolar-Expedition 1901-1903.Deutsch Südpolar-Expedition11(3):117‑192.
- Die Siphonophoren der Deutschen Südpolar-Expedition, 1901-1903.Zoologie IX Band17:1‑33.
- Über eine eigenthümliche Meduse des Mittelmeeres und ihren Jugendzustand.Archiv für Anatomie, Physiologie und wissenschaftliche Medicin1851:272‑277.
- Effect of formaldehyde on the gelatinous zooplankton (Pleurobrachia pileus, Aurelia aurita) during preservation.Turkish Journal of Zoology20:423‑426.
- Antarctic Hydromedusae and Water Masses.Pesquisa Antártica Brasileira2(1):101‑141.
- Die Hydromedusen des Golfes von Triest. In:Arbeiten aus dem Zoologischen Instituten der Universität Wien und der Zoologischen Station in Triest.20.69pp. [ISBN0355851000281].
- Ocean Biodiversity Information System. Intergovernmental Oceanographic Commission of UNESCO. www.iobis.org. Accessed on: 2020-11-11.
- International Polar Year and census of Antarctic Marine Life Ross Sea voyage (TAN0802) biodiversity data.Southwestern Pacific OBIS, National Institute of Water and Atmospheric Research, Wellington, New Zealand. Release date:2013-12-12. URL: https://nzobisipt.niwa.co.nz/resource?r=ipy_caml
- A guide to the Hydromedusae of the Southern Ocean and Adjacent Waters.Australian National Antarctic Research Expedition Research Notes5:1‑136.
- A guide to the Ctenophores of the Southern Ocean and adjacent waters.Australian National Antarctic Research Expedition Research Notes36:1‑43.
- Medusae (Hydrozoa, Scyphozoa, Cubozoa) of the Benguela current (southeastern Atlantic).Scientia Marina56(1):1‑64.
- Vertical distribution and abundance of mesoplanktonic medusae and siphonophores from the Weddell Sea, Antarctica.Polar Biology14(4):243‑251. https://doi.org/10.1007/BF00239172
- Macro and megaplanktonic cnidarians collected in the eastern part of the weddell gyre during summer 1979.Journal of the Marine Biological Association of the United Kingdom74(4):873‑894. https://doi.org/10.1017/s0025315400090111
- Distribution patterns of the mesozooplankton, principally siphonophores and medusae, in the vicinity of the Antarctic Slope Front (eastern Weddell Sea).Journal of Marine Systems9(3-4):231‑248. https://doi.org/10.1016/S0924-7963(96)00047-4
- The gelatinous zooplankton in the pelagic system of the Southern Ocean: a review.Annales de l'Institute Oceanographique73(2):139‑158.
- Fuseudoxid: The elusive sexual stage of the calycophoran siphonophore Crystallophyes amygdalina (Clausophyidae: Crystallophyinae).Acta Zoologica83(4):329‑336. https://doi.org/10.1046/j.1463-6395.2002.00124.x
- Gelatinous zooplankton net-collected in the Gulf of Maine and adjacent submarine canyons: New species, new family (Jeanbouilloniidae), taxonomic remarks and some parasites.Scientia Marina70(3):363‑379. https://doi.org/10.3989/scimar.2006.70n3363
- Distribución y abundancia de zooplancton en canales y fiordos australes. In:Advances in the oceanographic knowledge of Chilean inner waters, Puerto Montt to Cape Horn.Oceanographic Committee National-Pontifical Catholic University of Valparaiso,107-113pp.
- Genetic identity of two physonect siphonophores from Southern Ocean waters-the enigmatic taxon Mica micula and Pyrostephos vanhoeffeni.Journal of the Marine Biological Association of the United Kingdom99(2):303‑310. https://doi.org/10.1017/S0025315418000218
- Cnidaria from the Croker Passage (Antarctic Peninsula) with a special focus on Siphonophorae.Polar Biology33(8):1131‑1143. https://doi.org/10.1007/s00300-010-0802-3
- Vertical migration of siphonophora (cnidaria) and their productivity in the Croker Passage, the Antarctic.Polish Polar Research35(1):115‑131. https://doi.org/10.2478/popore-2014-0007
- Antarctic sea ice variability and trends, 1979-2010.Cryosphere6(4):871‑880. https://doi.org/10.5194/tc-6-871-2012
- Jellyfish in ecosystems, online databases, and ecosystem models.Hydrobiologia616(1):67‑85. https://doi.org/10.1007/s10750-008-9583-x
- Krill vs salps: dominance shift from krill to salps is associated with higher dissolved N:P ratios.Scientific reports10(1). https://doi.org/10.1038/s41598-020-62829-8
- Vertical distribution and abundance of pelagic cnidarians in the Eastern Weddell Sea, Antarctica.Journal of the Marine Biological Association of the United Kingdom77(2):341‑360. https://doi.org/10.1017/s002531540007171x
- Two new species of Bargmannia (Pyrostephidae, Physonectae, Siphonophorae).Zootaxa4686(1):74‑82. https://doi.org/10.11646/zootaxa.4686.1.3
- Metabarcoding of zooplankton diversity within the Chukchi Borderland, Arctic Ocean : improved resolution from multi-gene markers and region-specific DNA databases.Marine Biodiversity51(4):1‑19.
- Des Zoophytes ou Animaux rayonnés.In:Voyage autour du monde, entrepris par ordre du roi. Exécuté sur les corvettes de S.M. l'Uranie et la Physicienne, pendant les années 1817, 1818, 1819 et 1820.32pp.
- Zoologie IV: Zoophytes. In:Voyage de la corvette l'Astrolabe: exécuté par ordre du roi, pendant les années 1826-1827-1828-1829.Paris,389pp.
- The jellyfish joyride: causes, consequences and management responses to a more gelatinous future.Trends in Ecology and Evolution24(6):312‑322. https://doi.org/10.1016/j.tree.2009.01.010
- A guide to the seashores of Eastern Africa and the Western Indian Ocean islands.Sida/Department for Research Cooperation, SAREC,448pp.
- Segmentation methods for visual tracking of deep-ocean jellyfish using a conventional camera.IEEE Journal of Oceanic Engineering28(4):595‑608. https://doi.org/10.1109/JOE.2003.819315
- The medusae of the British Isles: anthomedusae, leptomedusae, limnomedusae, trachymedusae and narcomedusae.1.Cambridge University Press,Cambridge,530pp.
- Rapid sea level rise along the Antarctic margins driven by increased glacial discharge.Nature Geoscience7:732‑735. https://doi.org/10.1038/NGEO2230.1
- Southern ocean warming.Oceanography31(2 Special Issue):52‑62. https://doi.org/10.5670/oceanog.2018.215
- Using unmanned aerial vehicles (UAVs) to measure jellyfish aggregations.Marine Ecology Progress Series591:29‑36. https://doi.org/10.3354/meps12414
- Overview of the comb jellies (Ctenophora) from the South-western Atlantic and Sub Antarctic region (32–60°S; 34–70°W).New Zealand Journal of Marine and Freshwater Research55(2):286‑310. https://doi.org/10.1080/00288330.2020.1775660
- Automated pattern recognition of phytoplankton–procedure and results.Internationale Revue der gesamten Hydrobiologie und Hydrographie65(3):427‑437. https://doi.org/10.1002/iroh.19800650311
- The zooplankton of the Antarctic Peninsula region.In:Southern Ocean ecology: the BIOMASSE perspective.Cambridge University Press,79-92pp.
- Athecate hydroids and their medusae (Cnidaria: Hydrozoa).New Zealand Oceanographic Institute Memoir(106)1‑159.
- The European athecate hydroids and their medusae (Hydrozoa, Cnidaria): Filifera Part 2.Revue suisse de zoologie.114(2):195‑396. https://doi.org/10.5962/bhl.part.80453
- COI DNA barcode of the paratype of Glaciambulata neumayeri (Hydrozoa, Trachymedusae, Ptychogastriidae).KY426133.1.GenBank. URL: https://www.ncbi.nlm.nih.gov/nuccore/KY426133
- Feeding ecology and metabolism of the Antarctic cydippid ctenophore Callianira antarctica.Marine Ecology Progress Series317(July):111‑126. https://doi.org/10.3354/meps317111
- Near-field zooplankton, ice-face biota and proximal hydrography of free-drifting Antarctic icebergs.Deep-Sea Research Part II: Topical Studies in Oceanography58(11-12):1457‑1468. https://doi.org/10.1016/j.dsr2.2010.11.025
- Polar ocean ecosystems in a changing world.Nature437(7057):362‑368. https://doi.org/10.1038/nature04161
- Polar Zooplankton. In:Polar Oceanography, Part B: Chemistry, Biology, and Geology.SECOND EDITION.Academic Press, Inc.,71pp. [ISBN9780080925950]. https://doi.org/10.1016/B978-0-08-092595-0.50008-8
- Trends in Antarctic annual sea ice retreat and advance and their relation to El Niño–Southern Oscillation and Southern Annular Mode variability.Journal of Geophysical Research113(C3):1‑20. https://doi.org/10.1029/2007jc004269
- Recent Southern Ocean warming and freshening driven by greenhouse gas emissions and ozone depletion.Nature Geoscience11(11):836‑841. https://doi.org/10.1038/s41561-018-0226-1
- Impact of formalin preservation on Pleurobrachia bachei (Ctenophora).Journal of Experimental Marine Biology and Ecology303(1):11‑17. https://doi.org/10.1016/j.jembe.2003.10.017
- On the significance of Antarctic jellyfish as food for Adélie penguins, as revealed by video loggers.Marine Biology163(5):1‑8. https://doi.org/10.1007/s00227-016-2890-2
- Jellyfish and other gelata as food for four penguin species – insights from predator-borne videos.Frontiers in Ecology and the Environment15(8):437‑441. https://doi.org/10.1002/fee.1529
- Systematische Studien an den Trachylinae der Meteorexpedition, zugleich ein Beitrag zu einer Revision der Trachylinae.Zoologische Jahrbucher : Abteilungfur Systematik, Geographie und Biologie der Tiere69:1‑92.
- Impacts of local human activities on the Antarctic environment.Antarctic Science21(1):3‑33. https://doi.org/10.1017/S0954102009001722
- Structure of the pelagic cnidarian community in Lützow-Holm Bay in the Indian sector of the Southern Ocean.Polar Science4(2):387‑404. https://doi.org/10.1016/j.polar.2010.05.007
- Community structure of pelagic cnidarians off Adélie Land, East Antarctica, during austral summer 2008.Polar Biology37(2):269‑289. https://doi.org/10.1007/s00300-013-1430-5
- A synopsis of the Siphonophora.British Museum (Natural History),London,230pp. https://doi.org/10.1542/peds.2011-2107C
- A mesopelagic ctenophore representing a new family, with notes on family-level taxonomy in Ctenophora: Vampyroctena delmarvensis gen. nov. sp. nov. (Vampyroctenidae, fam. nov.).Marine Biodiversity50(3):1‑12. https://doi.org/10.1007/s12526-020-01049-9
- Antarctic climate change and the environment: An update.Polar Record50(3):237‑259. https://doi.org/10.1017/S0032247413000296
- Studies on Japanese Hydromedusae. 2. Trachomedusae and Narcomedusae.Japanese Journal of Zoology2(1):73‑97.
- UNESCO-IOC Register of Marine Organisms (URMO). http://www.marinespecies.org/urmo/. Accessed on: 2021-1-01.
- Die Lucernariden und Skyphomedusen der Deutschen Südpolar-Expedition 1901-1903.Deutsche Südpolar-Expedition10:25‑49.
- Die Craspedoten Medusen der Deutschen Südpolar-Expedition 1901-1903.Deutsche Südpolar-Expedition 1901–1903Zoologie 5(I):351‑395.
- Organism life cycles, predation, and the structure of marine pelagic ecosystems.Marine Ecology Progress Series130(1-3):277‑293. https://doi.org/10.3354/meps130277
- Ctenophore relationships and their placement as the sister group of other animals.Nature Ecology & Evolution1:1737‑1746. https://doi.org/10.1038/s41559-017-0331-3
- La stucture des palpons de Apolemia uvaria Esch., et les phénomènes de l'absorption dans les organes.Bulletin de l'Académie Royale des Sciences, des Lettres et des Beaux-Arts de Belgique. Third Series27:354‑363.
- World Register of Marine Species. http://www.marinespecies.org. Accessed on: 2021-1-07.
- Pacific coast pelagic invertebrates: a guide to the common gelatinous animals.Sea Challengers,Monterey,108pp.
- A method of jellyfish detection based on high resolution multibeam acoustic image.MATEC Web of Conferences283(2019). https://doi.org/10.1051/matecconf/201928304008
Supplementary materials
Licence number: 5119250419357